Abstract
Background
Pod of pigeon pea (Cajanus cajan) was used as a novel eco-friendly material to investigate its nickel binding efficiency. The influence of key physicochemical parameters such as contact time, initial metal ion concentration, adsorbent dosage, and pH on Ni(II) removal was studied. Optimum Ni(II) removal was obtained with a contact time of 45 min, pH of 8.0, and an adsorbent dose of 0.4.
Results
The removal of Ni(II) decreased from 95% to 85% as the initial metal concentration increased from 20 to 100 mg L−1. Under the optimal conditions selected, metal ion adsorption equilibrium was very well represented by the Freundlich isotherm model followed by the Langmuir isotherm and Temkin isotherm models. The adsorption process followed second-order kinetics, and the corresponding rate constants for initial Ni(II) concentration ranging from 20 to 100 mg L−1 were found to be 0.179 to 0.0035 g mg−1 min−1. Various thermodynamic parameters such as standard enthalpy (ΔH°), standard entropy (ΔS°), and standard free energy (ΔG°) were evaluated to predict the nature of adsorption.
Conclusions
The adsorbent prepared from the pod of pigeon pea appears to be a promising adsorbent for the removal of Ni(II) ions from aqueous solution. The study indicated that the pretreated adsorbent produced through chemical activation using NaOH is an effective material for the removal of Ni(II). Hence, the adsorbent prepared from the pigeon pea pod would be an economically useful tool and alternative to the commercially available activated carbon in the treatment of effluent containing Ni(II) ions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Industrial effluents from mining, electroplating, tanning, manufacture of fertilizers etc., resulted in huge deposits of toxic heavy metals in the environment. Owing to their persistence, bio-magnification, toxicity, and consistently increasing applications, they pose major environmental concerns [1]. Among these heavy metals, nickel exhibits remarkable toxicity, thereby causing irreversible damage to the environment and human health. Uptake of large doses of nickel affects human health adversely, causing lung, nose, larynx, and prostate cancers, birth defects, various allergic reactions, and heart disorders. Moreover, prolonged exposure to nickel may result in genotoxicity, neurotoxicity, nephrotoxicity, immunotoxicity, and reproductive toxicity [2]. Conventional technologies for the removal of metal ions from industrial effluents are less efficient and very costly and generate toxic sludge. The search for new techniques for the removal of toxic metals directed attention towards biosorption [3]. Due to the presence of surface functional groups such as acetamido, alcoholic, carbonyl, phenolic, amido, amino, and sulfydryl groups, cellulosic agricultural waste materials act as an important source for metal biosorption. Effective metal biosorption was demonstrated by microalgae, sea weeds, bacteria, fungi, animal residues, and agricultural residues [4]. Agricultural wastes are characterized by ready availability, affordability, eco-friendliness, and high uptake capacity for heavy metals. The husk of grains such as rice, wheat, Bengal gram, and corn had been studied extensively in the past, for their adsorption efficiency. The results showing high efficiencies at lower cost triggered further studies to estimate the adsorption characteristics of similar low-cost agricultural waste products. In this regard, a novel biosorbent was examined for nickel binding traits, and the combinations of key physical parameters that influence the extent of nickel removal were investigated. Dry matter yield of pigeon pea pod shells (PPP) is almost equal to that of the seed. The protein content in pods is also negligible. It contains low levels of digestible energy and sulfur and, hence, not a preferred livestock feed [5]. In the present study, the physical parameters that influence Ni(II) uptake in a batch system such as pH, contact time, initial metal ion concentration, and adsorbent dosage were optimized. The suitability of the Freundlich, Langmuir, and Temkin adsorption models was also investigated for each sorbent system. In order to understand the reaction mechanism, kinetic data have been studied using pseudo-first-order and pseudo-second-order kinetic models.
Methods
Batch adsorption studies
The biosorption capacity of PPP was evaluated by varying initial metal ion concentrations, pH, contact times, temperatures, and adsorbent doses while fixing the volume of metal solution at 100 mL. While varying one parameter, the other parameters were maintained constant. The mixture was agitated in a thermostatic orbital shaker at 120 rpm followed by filtration using Whatman (no. 1) filter paper (Whatman plc, Kent, UK). The filtrate containing the residual concentration of Ni(II) was determined spectrophotometrically (SL159, ELICO Ltd., Hyderabad, India) at 465 nm after complexation with dimethylglyoxime (DMG) [6, 7].
Metal analysis
Spectrophotometric analysis was adopted to estimate nickel(II) using DMG. Different standard solutions containing less than 100 mg L−1 of nickel(II) were prepared, and 2 mL of 20% (w/v) potassium sodium tartrate solution, 10 mL of 4% (w/v) ammonium per sulfate, 2.5 mL of 5 M sodium hydroxide solutions 15 ml of (1+30) HCl and 0.5 ml of 1% DMG solutions were added. The absorbance was measured after 30 min using UV–vis spectrophotometer at 465 nm. A reagent blank was also prepared for the Ni(II) solution. The amount of nickel present in the sample was obtained from the standard calibration curve [6, 7].
Removal efficiency
The percentage nickel removal, R (%), was determined after each run as follows:
where C i and C f were the initial and final concentrations of nickel, respectively, in the solution in milligrams per liter.
Adsorption isotherm model equations
Adsorption isotherms are useful in finding out the adsorption capacity of the adsorbent, the solute-solution interaction, and the degree of accumulation of adsorbate on the surface of the adsorbent. In the present study, the adsorption of Ni(II) onto the pretreated biosorbent was studied by adsorption isotherm models such as the Langmuir, Freundlich, and Temkin isotherms [8].
Langmuir isotherm
The Langmuir model is based on the assumptions that the uptake of metal ions occurs on a homogeneous surface by monolayer adsorption, that uniform energies of adsorption are involved, and that there is no transmigration of adsorbate in the plane of the surface [9].
The linearized form of Langmuir isotherm is represented by the following equation:
where q e (mg g−1) is the amount of adsorbed metal ions per unit weight of adsorbent at equilibrium concentration, C e (mg L−1). Q o (mg g−1), and k (L mg−1) are the Langmuir constants related to the maximum monolayer capacity and energy of adsorption, respectively. Langmuir parameters are obtained by plotting 1/q e versus 1/C e .
Freundlich isotherm
The Freundlich isotherm model assumes that the uptake of metal ions occurs on a heterogeneous surface by multilayer adsorption, and nonuniform energies of adsorption are involved [10]. The linearized Freundlich model isotherm was applied for the adsorption of Ni (II) and is expressed as follows:
The K F (mg g−1 (L−1 mg−1)1/n) and 1/n are Freundlich constants related to adsorption capacity and intensity of adsorption, respectively. Freundlich parameters were obtained by plotting ln q e versus ln C e .
Temkin isotherm
Temkin isotherm model assumes that the heat of adsorption of all molecules in the layer decreases linearly with coverage due to adsorbent-adsorbate interactions, and the adsorption is characterized by a uniform distribution of the binding energies up to some maximum binding energy [11]. The linearized form of Temkin model is represented by the following equation:
where, B T = (RT)/b T , T is the absolute temperature in Kelvin and R is the universal gas constant (8.314 J mol−1 K−1). The constant b T is related to the heat of adsorption (J mol−1), A T is the equilibrium binding constant (L−1 min−1) corresponding to the maximum binding energy. The slope and intercept from a plot of q e versus ln C e determines the isotherm constants A T and BT.
Adsorption kinetics
In order to investigate the controlling mechanism of adsorption processes such as mass transfer and chemical reaction, pseudo-first-order and pseudo-second-order kinetic equations were used to test the experimental kinetic data [12].
Pseudo-first-order/Lagergren kinetic model
The pseudo-first-order or Lagergren kinetic rate equation for the sorption of liquid–solid system was derived based on solid adsorption capacity. In most cases, this equation did not fit well throughout the whole contact time [13]. According to this model, the overall adsorption rate is directly proportional to the driving force, i.e., the difference between initial and equilibrium concentrations of the adsorbate (q e − q t ). Therefore, the pseudo-first-order kinetic equation can be expressed as follows:
where q e is the amount of solute adsorbed at equilibrium per unit mass of adsorbent (mg g−1), q t is the amount of solute adsorbed at any given time t, and k1 is the rate constant of the pseudo-first-order adsorption process (min−1). Using the boundary conditions and with simplification, it yields the following equation:
where k1 can be calculated from the slope of the linear plot between log (q e − q t ) versus t for different initial adsorbate concentrations.
Pseudo-second-order kinetic model
A pseudo-second-order reaction model is based on sorption equilibrium capacity. According to this model, the driving force for adsorption (q e − q t ) is proportional to the available fraction of active sites [14, 15]. Hence, the pseudo-second-order equation is expressed as follows:
On integration for boundary conditions when t = 0 to t > 0 and q = 0 to q > 0 and with further simplifications, it yields the following equation:
where h = k2 is the rate constant of the pseudo-second-order adsorption (g mg−1 min−1). The plot of t/q t versus t at different adsorption parameters will give a linear relationship, which allows for the computation of q e , k2, and h.
Results and discussion
Characterization of biosorbent
FT-IR analysis
The changes in vibrational frequencies of functional groups in the adsorbent were analyzed using FT-IR spectra. The spectra of the pretreated and loaded adsorbent measured within the range of 4,000 to 450 cm−1 wave number are presented in Figure 1A,B. FT-IR studies of various adsorbents have identified the presence of several surface functionalities with C=O (carboxylic, anhydride, lactone, and ketone) at 1,750 to 1,630 cm−1, C=C at 1,600 to 1,450 cm−1, C-O stretching and O-H bending (lactonic, ether, phenol, etc.) at 1,440 to 1,000 cm−1, C-H (900 to 600 cm−1), and OH, NH, CH stretching in alkyl or aryl at 3,488 to 3,100 cm−1[12].
It can be seen from Figure 1A,B that the absorbance of peaks in Ni(II)-loaded biosorbent is subsequently lower than those in the pretreated biosorbent. This indicates that bond stretching occurs to a lesser extent due to the presence of nickel, and subsequently, peak absorption is attenuated. Thus, the major functional groups involved in efficient removal of nickel are attributed to the presence of C=O, C-O, O-H, and C-H bonds which can coordinate with nickel.
SEM analysis
The surface morphology of the native, pretreated, and metal-loaded biosorbents was investigated by SEM. The SEM image of native biosorbent shows a highly porous morphology and a coarse surface texture with pores of different shapes and sizes. After adsorption, the effect of nickel binding to the adsorbent surface caused changes in morphology as shown in Figure 2B. It is observed that the pores on the surface are not distinct, and this is due to the adsorption of nickel into these structures. This implies that pigeon pea pod presents an adequate morphological profile to retain Ni(II) ions.
Batch adsorption studies
Effect of pH
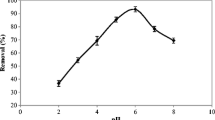
In heavy metal biosorption, pH is the most important parameter. The speciation of metals in the solution and the state of chemically active sites are pH dependent. As shown in Figure 3, at pH of 4.0, only low percentage removal of nickel was obtained. However, when the pH was increased to 8.0, higher values for metal removal was achieved, thus indicating better adsorption capacity of nickel related to the proposed adsorbent since the zeta potential of PPP was found to be 6.84. This phenomenon can be explained by the surface charge of pretreated adsorbent and H+ ions present in the solution. At high pH, the reaction sites are deprotonated and negatively charged, so they attract Ni(II) metals, and the removal increases [16]. As the pH is lowered, the overall surface charged on the biomass cells however becomes positive, which will inhibit the approach of positively charged metal cations [17]. It is likely that protons will then compete with metal ions for ligands and thereby decrease the interaction of metal ions with the cells [18]. When pH values of 9.0 or 10.0 were tested, only slight variations on the results were observed, indicating that good adsorption capacity was already obtained at pH of 8.0. For the biosorption of Ni(II), maximum removal was achieved at pH of 8.0. This could be due to an increasing negative charge density on the adsorbent surface. It is apparent that Ni(II) is strongly adsorbed at higher pH values. At pH greater than 8.0, Ni(II) removal was mostly due to precipitation, not by sorption. Similar results were obtained when modified pine bark and wood of Moringa oleifera were used as biosorbent [19, 20].
Effect of adsorbent dosage
The dosage of adsorbent is a key parameter to control both availability and accessibility of adsorption sites. The results as shown in Figure 4; percentage of removal for 100 mg L−1 Ni(II) was observed to increase gradually from 88% to 97% with the increase of adsorbent dosage from 0.1 to 0.5 g, and maximum removal was achieved at a dosage of 0.4 g. The observed trend may be due to the fact that the higher the dosage of adsorbent, the greater is the availability of surface area and functional groups for metal ions. These chemical groups play an important role in the formation of van der Waals bonding as the functional groups play the main role in binding metals with the adsorbent during the adsorption process [7]. This provides more possibilities for adsorption to occur since there was less competition between metals for the binding sites.
Effect of contact time
To establish an appropriate contact time between the pretreated adsorbent and metal ion solution, percentage removal of metal ion was measured as a function of time. The graph in Figure 5 indicates that the rate of the percentage of Ni(II) removal is higher at the beginning probably due to the larger surface area of the adsorbent, being available at beginning for the adsorption of metals. As the surface adsorption sites become exhausted, the uptake rate is controlled by the rate at which the adsorbate is transported from the exterior to the interior sites of the adsorbent particles. The equilibrium rate of Ni(II) removal was attained after agitating for 45 min. This is in agreement with the results obtained by Kehinde et al. [21], where maximum removal was demonstrated at a contact time of 30 min. Therefore, equilibrium time of 45 min was selected for all further studies.
Effect of initial metal concentration
Ni(II) removal as a function of initial metal ion concentration and contact time is given in Figure 5. The initial metal ion concentration was varied from 20 to 100 mg L−1, while time was varied from 15 to 120 min at a constant adsorbent dose of 0.4 g L−1, pH 8.0, and agitating speed of 120 rpm. The maximum Ni(II) removal was attained within 45 min after further removal was negligible, which may be due to quick exhaustion of adsorption sites after the process. After the formation of one molecule thick layer of metal ion on the adsorbent, the adsorbent capacity gets exhausted, and then the uptake rate is controlled by the rate at which the sorbate is transported from the exterior to the interior sites of the adsorbent. The data indicate that the initial metal ion concentration determines the equilibrium concentration and also determines the uptake rate of metal ion and its kinetic character. Ni(II) removal decreased as initial Ni(II) concentration increased from 20 to 100 mg L−1. This may be due to the saturation of sorption sites in the adsorbent for complexation of Ni(II) ions at higher concentration. A similar trend was observed for the adsorption of Ni(II) on crab shell particles [22] and in the case of Cr(VI) removal using sunflower biomass [23] where the percentage sorption decreased with the increase in initial nickel ion concentrations within the experimental operating conditions.
Equilibrium isotherms
The biosorption equilibrium is established when the concentration of sorbate in bulk solution is in dynamic balance with that on the liquid-sorbent interface. The degree of the sorbent affinity for the sorbate determines its distribution between the solid and liquid phases. Several models are often employed to interpret the equilibrium data. In the present study, the Langmuir, Freundlich, and Temkin models were utilized to explain the experimental data as given in Figure 6.
Figure 6a indicates the applicability of Langmuir isotherm. The Langmuir constants Q o and k evaluated from the slope and intercept are 19.23 mg g−1 and 0.0043 L mg−1, respectively. The Q o value is comparable to the adsorption capacity of the studied mosambi fruit peel [7]. The best fit equilibrium model was determined based on the linear regression correlation coefficient R2. From Table 1, it was observed that the sorption data were very well represented by the Freundlich isotherm (Figure 6b) with an average higher correlation coefficient of 0.993. The value of the Freundlich exponent n = 2.021 in the range of 1 to 10, indicates favorable adsorption. The results were comparable to that of the studies conducted by Yasemin and Zaki [13] using saw dust. The performance of the PPP was comparable with other low-cost absorbents cited in the literature which are given in Table 2.
Adsorption kinetics
The kinetic studies of Ni(II) adsorption on pretreated pigeon pea pod was carried out using the pseudo-first-order and pseudo-second-order models on experimental data. The effect of initial nickel concentrations was investigated to find the best fit kinetic model. The kinetic constants and correlation coefficients of pseudo-first-order kinetic model are given in Table 3. The experimental q e values were compared with the q e values determined by pseudo-first-order rate kinetic models. The Lagergren first-order rate constants k1 and q e determined from the model indicate that this model had failed to estimate q e since the experimental values differ from the estimated one (Figure 7).
The pseudo-second-order kinetic model was applied by plotting t/q t versus t, and this model gave high values of regression correlation coefficient as seen in Figure 8. This implies that the mechanism of adsorption of Ni(II) ion on the pod of pigeon pea follows the pseudo-second-order kinetics, as shown in Figure 8. This is compared with the kinetic studies of Ni(II) adsorption of coconut husk conducted by Kehinde et al. [21] where regression coefficients showed high values for the pseudo-second-order kinetic model (R2 = 0.999) indicating its applicability to adsorption. Both factors indicate that the adsorption of metal ions followed the second-order kinetic model, indicating that the rate-limiting step was a chemical adsorption process between the metal ion and pretreated pigeon pea pod [20]. Also, from Table 3, it was clear that the pseudo-second-order rate constant k2 decreased with the increase in the initial nickel concentrations.
Effect of temperature
To determine the effect of temperature on the adsorption of dye, experiments were also conducted at 303, 313, and 323 K. The degree of adsorption increases with increased temperature, indicating that the adsorption is endothermic [32]. The values of free-energy change (ΔG°), enthalpy change (ΔH°), and entropy change (ΔS°) for the adsorption process were calculated using the following equations [33]:
where R is the gas constant (8.314 J mol−1 K−1), K e is the equilibrium constant obtained from the Langmuir equation (C Ae /C e ) (where C Ae is the amount of Ni(II) ions adsorbed at equilibrium (mg L−1) and C e is the retained concentration of each ions at equilibrium (mg L−1), and T is the absolute temperature (K):
Thus, a plot of ln K e versus 1/T should be a straight line. The ΔH° and ΔS° values were obtained from the slope and intercept of this plot, respectively. The positive values of ΔH° confirm the endothermic nature of adsorption. The negative values of ΔG° at 30°C, 40°C, and 50°C indicate the spontaneous nature of adsorption for phosphate (Table 4). The positive values of ΔS suggest increased randomness at the solid/solution interface during the adsorption of nickel on PPP.
Advantage of pigeon pea pod as a biosorbent
The cost of Ni(II) removal from wastewater originating from various sources in general is expensive; for comparison sake, activated carbon-based removal of nickel costs around $750 (average) per ton [34] using various waste. Its carbon derivatives for nickel removal depend on availability, and its removal efficiency, which is PPP here, was obtained freely as the pod is usually discarded as a waste. The cost of this adsorbent material can be fixed at $40/ton after taking into account expenses on transportation, chemicals, electricity, and miscellaneous costs. The performance of the adsorbent was comparable with other low-cost absorbents cited in the literature (Table 2).
Experimental
Preparation of biosorbent from pigeon pea pod
Pigeon pea (Cajanus cajan) pod (PPP), which is usually discarded as waste, was collected from an agricultural land near Kovilur village of Dindigul district in the southern part of Tamil Nadu, India. The PPP was washed extensively in running tap water to remove dirt and other particulate matter. It was dried at 40°C for 24 h and was then finely ground and sieved with a mesh size of 150 μm. Fourier transform infrared (FT-IR) analysis was performed to obtain qualitative information on surface functional groups responsible for the metal uptake. The spectra of the adsorbent were measured within the range of 450 to 4,000 cm−1. The finely powdered biosorbent was pretreated using 1 M sodium hydroxide (NaOH) solution. The mixture was filtered, and the powdered residue was washed with distilled water, several times, to remove any base contents. This filtered biomass was dried in an oven at 60°C for 6 to 8 h. The dried biomass was stored in airtight containers to protect it from moisture. Further, surface properties before and after pretreatment of pod were analyzed using scanning electron microscopy (SEM); the results of which confirmed changes in the structural morphology of the adsorbents.
Preparation of synthetic single-metal solution
An aqueous stock solution (1,000 mg L−1) of Ni(II) ions was prepared using NiCl2 6H2O salt. The solution's pH was adjusted using 0.5 N HCl or NaOH. Fresh dilutions of 100 mg L−1 of synthetic solutions were used for each study. All the chemicals were procured from HiMedia (analytic grade; HiMedia Technology Limited, Shenzhen, China).
Conclusions
The following conclusions can be drawn based on the investigation of Ni(II) removal by pigeon pea pod. The adsorbent prepared from the pod of pigeon pea appears to be a promising adsorbent for the removal of Ni(II) ions from aqueous solution. The study indicated that the pretreated adsorbent produced through chemical activation using NaOH is an effective material for the removal of Ni(II). The maximum removal of Ni(II) is obtained at an alkaline pH of 8.0. It is found from kinetic studies that the adsorption rate of Ni(II) is faster for the initial 20 min, after which it is saturated. The equilibrium time for Ni(II) adsorption on the pigeon pea pod is obtained at 45 min. Based on the Langmuir isotherm, the maximum adsorption capacity was calculated to be 19.23 mg g−1 of adsorbent. Equilibrium studies reveal that the Langmuir model provides better solutions and correlation to the adsorption data when compared with the Freundlich and Temkin isotherm models. This suggests that adsorption is limited to the monolayer. The kinetics of biosorption was well represented by the pseudo-second-order kinetic model. The data obtained from adsorption isotherms at different temperatures were used to calculate thermodynamic quantities such as ΔG°, ΔH°, and ΔS°. In thermodynamic studies, the positive ΔH° value indicated the endothermic nature of the adsorption interaction, whereas the positive ΔS° value showed the increased randomness at the solid-solution interface during the adsorption process. The negative value of ΔG° indicated the feasibility and spontaneous nature of the adsorption of nickel(II) onto the pigeon pea pod. The pod of pigeon pea used to produce the adsorbent is a locally and abundantly available agricultural waste product. Hence, the adsorbent prepared from the pigeon pea pod would be an economically useful tool and alternative to the commercially available activated carbon in the treatment of effluent containing Ni(II) ions.
Authors’ information
AJ, SM, and KP are faculty members of Biotechnology Kumaraguru College of Technology, Coimbatore, India. LC and SHS are project students.
References
Das N, Vimala R, Karthika P: Biosorption of heavy metals - an overview. Indian J Biotechnol 2007, 7: 159–169.
Rao LN, Prabhakar G: Removal of heavy metals by biosorption - an overall review. J Eng Res Stud 2011, 2: 17–22.
Ahalya N, Ramachandra TV, Kanamadi RD: Biosorption of heavy metals. Res J Chem Environ 2003, 7: 71–79.
Zolgharnein J, Shahmoradi A, Sangi MR: Optimization of Pb (II) biosorption by Robinia tree leaves using statistical design of experiments. Talanta 2008, 76: 528–532. 10.1016/j.talanta.2008.03.039
Whiteman PC, Norton BW: Alternative uses of pigeon pea. In: proceedings of International Workshop on Pigeon peas, pp 1981, 365–382.
Bickerdike EL, Willard HH: Dimethylglyoxime for determination of nickel in large amounts. Anal Chem 1952, 24: 1026–1026. 10.1021/ac60066a031
Krishna RH, Swamy AVVS: Studies on the removal of Ni (II) from aqueous solutions using powder of mosambi fruit peelings as a low cost sorbent. Chem Sci J 2011, CSJ: 31.
Kumar PS, Kirthika K: Equilibrium and kinetic study of adsorption of nickel from aqueous solution onto bael tree leaf powder. J Eng Sci Technol 2009, 4: 351–363.
Langmuir I: The evaporation, condensation, and reflection of molecules and the mechanism of adsorption. Phys Rev 1917, 8: 149–176.
Freundlich H: Über die adsorption in lösungen (adsorption in solution). Z Phys Chem 1906, 57: 384–470.
Temkin MJ, Pyzhev V: Recent modifications to Langmuir isotherms. Acta Physiochim URSS 1940, 12: 217–222.
Anirudhan TS, Sreekumari SS: Adsorptive removal of heavy metal ions from industrial effluents using activated carbon derived from waste coconut buttons. J Environ Sci 2011, 23: 1989–1998. 10.1016/S1001-0742(10)60515-3
Yasemin B, Zeki T: Removal of heavy metals from aqueous solution by sawdust adsorption. J Environ Sci 2007, 19: 160–166. 10.1016/S1001-0742(07)60026-6
Ho YS, McKay G: Pseudo-second order model for sorption processes. Process Biochem 1999, 34: 451–465. 10.1016/S0032-9592(98)00112-5
Qiu H, Lv L, Zhang Q, Zhang W, Zhang Q: Critical review in adsorption kinetic models. J Zhejiang Univ Sci 2009, 10: 716–724. 10.1631/jzus.A0820524
Lohani MB, Singh A, Rupainwar DC, Dhar DN: Studies on efficiency of guava ( Psidium guajava ) bark as bioadsorbent for removal Hg (II) from aqueous solutions. J Hazard Mater 2008, 159: 626–629. 10.1016/j.jhazmat.2008.02.072
Arshad M, Zafar MN, Younis S, Nadeem R: The use of neem biomass for the biosorption of zinc from aqueous solutions. J Hazard Mater 2008, 157: 534–540. 10.1016/j.jhazmat.2008.01.017
Sag Y, Ozer D, Kutsal T: A comparative study of the biosorption of lead (II) ions to Z. remigera and R. arrhizus . Process Biochem 1995, 30: 169–174. 10.1016/0032-9592(95)80008-5
Gaballah I, Kilbertus G: Recovery of heavy metal ions through decontamination of synthetic solutions and industrial effluents using modified barks. J Geochem Explor 1998, 62: 241–286. 10.1016/S0375-6742(97)00068-X
Kalavathy MH, Miranda LR: Moringa oleifera - a solid phase extractant for the removal of copper, nickel and zinc from aqueous solutions. Chem Eng J 2010,2012(158):188–199.
Kehinde OO, Oluwatoyin TA, Aderonke OO: Comparative analysis of the efficiencies of two low cost adsorbents in the removal of Cr(VI) and Ni(II) from aqueous solution. Afr J Environ SciTechnol 2009, 3: 360–369.
Murugesan S, Rajiv S, Thanapalan M: Optimization of process variables for a biosorption of nickel (II) using response surface method. Korean J Chem Eng 2009, 26: 364–370. 10.1007/s11814-009-0061-6
Jain M, Garg VK, Kadirvelu K: Equilibrium and kinetic studies for sequestration of Cr(VI) from simulated wastewater using sunflower waste biomass. J Hazard Mater 2009, 171: 328–334. 10.1016/j.jhazmat.2009.06.007
Krishnani KK, Meng X, Dupont L: Metal ions binding onto lignocellulosic biosorbent. J Environ Sci Heal A 2009, 44: 688–699. 10.1080/10934520902847810
Reddad Z, Gerente C, Andres Y, Marie-Christine R, Thibault J-F, Le Cloirec P: Ni(II) and Cu(II) binding properties of native and modified sugar beet pulp. Carbohydr Polym 2001, 49: 23–31.
Krishnani KK, Meng X, Boddu VM: Fixation of heavy metals onto lignocellulosic sorbent prepared from paddy straw. Water Environ Res 2008, 80: 2165–2174. 10.2175/106143008X304785
Krishnani KK, Meng X, Christodoulatos C, Boddu VM: Biosorption mechanism of nine different heavy metals onto biomatrix from rice husk. J Hazard Mater 2008, 153: 1222–1234. 10.1016/j.jhazmat.2007.09.113
Kadirvelu K, Thamaraiselvi K, Namasivayam C: Adsorption of nickel (II) from aqueous solution onto activated carbon prepared from coir pith. Sep Purif Technol 2001, 24: 497–505. 10.1016/S1383-5866(01)00149-6
Gupta SS, Bhattacharyya KG: Adsorption of Ni(II) on clays. J Colloid Interface Sci 2006, 295: 21–32. 10.1016/j.jcis.2005.07.073
Coskun R, Soykan C, Sacak M: Adsorption of copper (II), nickel (II) and cobalt (II) ions from aqueous solution by methacrylic acid/acrylamide monomer mixture grafted poly (ethylene terephthalate) fiber. Sep Purif Technol 2006, 49: 107–114. 10.1016/j.seppur.2005.09.002
Panda GC, Das SK, Bandopadhyay TS, Guha AK: Adsorption of nickel on husk of Lathyrus sativus : behavior and binding mechanism. Colloids Surf B 2007, 57: 135–142. 10.1016/j.colsurfb.2007.01.022
Bhatnagar A, Jain AK: A comparative adsorption study with different industrial wastes as adsorbents for the removal of cationic dyes from water. J Colloid Interf Sci 2005, 281: 49–55. 10.1016/j.jcis.2004.08.076
Bulut Y, Aydın H: A kinetics and thermodynamics study of methylene blue adsorption on wheat shells. Desalination 2006, 194: 259–267. 10.1016/j.desal.2005.10.032
Krishna AK, Sreejalekshmi KG, Baiju RS: Nickel (II) adsorption onto biomass based activated carbon obtained from sugarcane bagasse pith. Bioresource Technol 2011, 102: 10239–10247. 10.1016/j.biortech.2011.08.069
Acknowledgments
The authors are thankful to the management of Kumaraguru College of Technology for the provided research facilities, SAIF-IIT Madras for SEM analysis, and STIC Cochin for FT-IR analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
The main idea of this work was suggested by AJ. He was the supervisor for this undergraduate project work and was also the corresponding author who prepared this manuscript. SM was the first advisor and an adsorption studies expert; he monitored the kinetics and isotherm studies. KP was the second advisor under whom the preliminary studies were carried out by LC. SHS carried out the thermodynamics study. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Aravind, J., Muthusamy, S., Sunderraj, S.H. et al. Pigeon pea (Cajanus cajan) pod as a novel eco-friendly biosorbent: a study on equilibrium and kinetics of Ni(II) biosorption. Int J Ind Chem 4, 25 (2013). https://doi.org/10.1186/2228-5547-4-25
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2228-5547-4-25