Abstract
The applicability of soft and hard modeling methods was used to determine the formation constants of complexes between 3,6-bis[(2-arsonophenyl)azo]-4,5-dihydroxy-2,7-naphtalenedisulphonic acid disodium salt (Arsenazo III) and 13 lanthanides. The results showed that all the 13 lanthanides (M) studied form a similar type of complexes and spectral profiles with Arsenazo III (L) with three formation constants corresponding to M/L ratios of 1:1, 1:2, and 2:2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Spectrophotometric titrations are important methods for the investigation of solution equilibria. The titration consists of a collection of spectra of a solution measured as a function of the reagent added which influences the equilibrium under the investigation [1, 2].
The proper chemometric algorithms can be used in evaluating the equilibrium information such as the stability constant through analysis of the spectroscopic data. Several soft and hard modeling algorithms have been developed to analyze bilinear data obtained from chemical systems.
Soft modeling methods range from very general approaches, such as evolving factor analysis (EFA) [3], which is particularly used to estimate the number of species involved in equilibrium studies and the kinetic process by repeated factor analysis of rationally selected subsets of spectra. Factor analysis investigates the rank of the subset of spectra by determining its number of significant eigenvalues [4].
Multivariate curve resolution-alternating least squares (MCR-ALS) [5] is an iterative soft modeling resolution method that has been successfully applied to solve many mixed dynamic processes monitored spectrometrically, such as chromatographic runs [6, 7], biomacromolecular reactions [8, 9], voltammetric data, and environmental data [10–12]. In fact, MCR is a self-modeling chemometric discipline that comprises several techniques in establishing an initial model on the data and extracting maximum amount of information from the data. These results are useful to validate hard modeling results and investigate the complex chemical systems [13].
Hard modeling approaches of fitting multivariate response data are based on mathematical relationships, which describe the measurements quantitatively [14]‐[16]. In chemical equilibria, the analysis is based on the equilibrium model which quantitatively describes the reaction and all concentrations in the solution under investigation. Within the hard modeling methods, analysis of spectral data has shown an optimal performance on many occasions [16–18].
Hard modeling or model-based method was used to analyze a given measurement based on a predetermined model. The model can be a simple mathematical function or a complex chemical system that is fitted to a data set. So, it needs to determine the right model to be fitted, such as the exact chemical reaction mechanism in kinetics or the correct equilibrium model in a titration experiment. Unfortunately, it is a very difficult one. There are no general applicable tools available to guide towards finding the model that correctly describes the chemical process under investigation because the model fitting is much easier than model finding. There is a good collection of methods available in performing model-free or soft modeling analyses. Typically, these methods (soft-model) deliver the shapes of the concentration profiles of all reacting components as well as the shapes of their absorption spectra. Such information can be very useful in supplying preliminary information about the system under investigation and ultimately could guide towards the correct model. In many instances, there is no model or mathematical function at all that could be used to quantitatively describe the process under investigation. Then, the concentration profiles, in conjunction with the pure component spectra, are all there which are to be extracted from the data.
In this work, the soft and hard modeling methods are suggested for the spectrophotometric study of three competitive complex formations. The applicability of the methodology serves as an example in the estimation of the complexation constants of lanthanide cations (M) by Arsenazo III (L).
Arsenazo III, with the common descriptive name of 3,6-bis[(2-arsonophenyl)azo]-4,5-dihydroxy-2,7-naphtalenedisulphonic acid disodium salt, is a disodium salt with the general formula of C22H16As2Na2N4O14S2. This bis-azo derivative of chromotropic acid forms stable complexes with large variety of different cations [19]. The chromophore groups in the ligand promote the spectrophotometric study in the visible field. Specifically, this multipurpose complexing ligand is extensively used for spectrophotometric analysis. Interactions of Arsenazo III with lanthanides were investigated in some detail [20–23]. In particular, Arsenazo III was shown to make the similar type of complex with lanthanides and is a very useful remark to minimize the number of models for this series.
Our interest in the behavior of this ligand is stimulated by at least two reasons. Firstly, formation constant values of the above complexes were reported for 11 lanthanides by Lu [24] which makes use of the data from the first stages of the complexometric titration and is based on the Taylor expansion of the concentration of the ligand as a function of the titrant concentration. This method is rather complicated and is not user-friendly.
The second reason was the fact that Arsenazo III was shown to build up a similar type of complex with lanthanides. In this context, two different methodologies were proposed to evaluate formation constants. These methodologies are based on soft and hard modeling that leads to the evaluation of stoichiometry of the main species and formation constants. Meanwhile, we have presented the spectral and concentration profiles for each species in the titration process.
Methods
Apparatus and software
An Agilent photodiode array spectrophotometer (model 8453, Santa Clara, CA, USA) equipped with a 1-cm path length glass cell was used in recording absorbance spectra. A Pentium 4 personal computer was used in controlling the spectrophotometer and collecting the data from its interface. All spectral measurements were performed using a blank solution as a reference. Measurements of pH were made with a Metrohm 632 pH meter (Labequip Ltd. Markham, Canada) using a combined glass electrode. The computations were made with a Pentium 4 computer. All the programs in the computing process were written in MATLAB 6.5 for Windows (MathWorks, Inc., Natick, MA, USA).
Reagents and solutions
All reagents were of analytical reagent grade, and distilled water was used throughout. All the 13 lanthanide stock solutions (0.01 M) were prepared by dissolving the following weight and kind of salts in 10 mL of distilled water.
The reagents were as follows: La, 43.30 mg lanthanum nitrate (Merck, Darmstadt, Germany); Ce, 56.84 mg cerium(III) sulfate (Reacton, Moffat, UK); Pr, 17.02 mg praseodymium oxide (Reacton); Nd, 16.82 mg neodymum oxide (Reacton); Sm, 25.67 mg samarium chloride (Reacton); Eu, 42.81 mg europium(III) nitrate (Reacton); Gd, 18.13 mg gadolinium oxide (Reacton); Tb, 18.69 mg terbium oxide (Reacton); Dy, 18.65 mg dysprosium oxide (Reacton); Ho, 18.89 mg holium oxide (Reacton); Er, 19.10 mg erbium oxide (Reacton); Yb, 19.70 mg ytterbium(III) oxide (Reacton); and Lu, 19.89 mg lutetium oxide (Reacton)
A stock solution of monochloroacetic acid (BDH, Dorset, UK) buffer solution (0.1 M) was prepared daily by dissolving 9.44 g of CH2ClCOOH in water and diluting to 1,000 mL after adjusting the pH to 3.2. A stock solution of Arsenazo III (0.001 M) was prepared by dissolving 0.0820 g of arsenazo chloride as a disodium salt (Merck) in water and diluting to 100 mL.
Procedure
A 5.0-mL Arsenazo III (0.001 M) solution and 50.0-mL monochloroacetic acid buffer (pH = 3.2; 0.1 M) were added in a 100-mL volumetric flask and diluted to the mark with distilled water after adjusting the pH to 3.2. A 3-mL Arsenazo III solution (5 × 10−5 M at pH = 3.2) was transferred to a spectrophotometric cell, and the titration was started by successive 0.2-μL additions of each standard stock lanthanide solution (0.01 M) to the cell. The absorbance data of this solution were recorded (at 380 to 780 nm) after each addition, against a reagent blank. The titration was followed until the absorbance variations became small and nearly constant.
Results and discussion
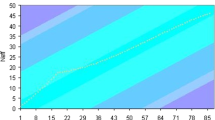
The three-dimensional absorbance plot (absorbance vs. wavelength vs. mole ratio) of Arsenazo III titrated with europium cation in a solution of pH = 3.2 is shown in Figure 1. This lanthanide is selected as a representative example because basically similar observations are made with all other 12 investigated lanthanides in this research. The λmax of Arsenazo III is 530 nm, and a shift with two narrow and distinct isosbestic points is observed in its spectrum to longer wavelengths upon addition of metal cations.
After building up the titration data matrices for Arsenazo III with each of the 13 lanthanide-Arsenazo III complex systems, the next step was to determine the number of species and models for the complexation to estimate the conditional constants for the complexes formed based on mole ratio data.
Determination of the number of efficient factors and species by factor analysis
In order to estimate the number of coexisting absorbing species in the reaction systems (i.e., the number of produced complexes), principal component analysis (PCA) was performed on the digitized absorbance mole ratio data matrices, which were obtained from the spectrophotometric titration of Arsenazo III with each lanthanide cation, separately.
According to Figure 2, the structure of the residuals shows that only noise has remained after the subtraction of the contributions of the first four eigenvectors. Therefore, according to this figure and some other methods based on PCA, there are four absorbing species created in the titration process.
Models for the complexation
Studies of multiple equilibria in solutions are essential for the solution of many analytical and speciation problems. There are different analytical procedures in this field [25, 26], using least-squares methods, to estimate the number of species present in the reaction system, their stoichiometries, and their stability constants. The experimental data needed usually arise from the successive steps of a potentiometric or spectrometric titration. Least-squares methods are hard models and require knowledge about the chemistry of the reaction system, i.e., an initial set of stoichiometric coefficients and stability constants for the postulated species. Moreover, these methods, using a systematic search of the possible models and with the aid of chemical reasoning, can determine the composition of the species and the stability constants, provided that mass-law and mass-balance equations are fulfilled. According to Figure 2, each solution at a particular mole ratio can be considered as a mixture of four components.
Therefore, based on the results obtained by factor analysis methods (‘Determination of the number of efficient factors and species by factor analysis’ section) and MCR-ALS (Figure 3), we confined our studies on the systems containing four absorbing species, and by using the mass balance equations and equilibrium expressions, a hard model was generated for lanthanide-Arsenazo III systems using the following simple equilibria (Equations 1 to 3) and ML, ML2, and M2L2 complexes were tested and defined.
So, a system which contains M, L, ML, ML2, and M2L2 species with three competitive conditional complex formations is defined by three different chemical equilibria (Equations 1 to 3).
Simulated data
In checking the potential of the proposed method, the algorithm was tested using the simulated data set that was created according to mole ratio method and was very similar to the real data set. In simulating the three different competitive complexation systems, four simulated spectra of species were formed according to the spectral profiles obtained by MCR-ALS (Figure 3) and summed together in a known proportion in such a way that the model of mole ratio method is mimicked using the equilibrium constants K11 = 105, K12 = 1010, and K22 = 1015 and the concentration of Arsenazo III, CL = 5.0 × 10−5 M.
Random error was added to the set of generated artificial data. The error is a set of noise drawn from a normal distribution with mean zero and standard deviation equal to 0.25% of absorbance value.
Experimental
The spectra measured from a chemical equilibrium process can form a second-order data matrix, which contains both the equilibrium information and the pure spectrum of each component involved in the chemical process. Using the initial concentration profiles estimated by EFA and MCR-ALS for the mole ratio method with a reasonable lack-of-fit error of 0.13802% has resolved the pure absorption spectra and concentration profiles and allowed the identification of some species equilibria (Equations 1 to 3).
Model-based fitting is readily explained as the direct consequence of the much smaller number of parameters to be fitted. In essence, the number of nonlinear parameters equals the number of unknown equilibrium constants. They are the only parameters that need to be fitted iteratively. The unknown molar absorptivities are linear parameters and are, as mentioned before, effectively eliminated from the iterative fitting process, and the parameters that should be changed are the equilibrium constants (K11, K12, and K22).
The simple equilibrium (Equations 1 to 3) models which were confirmed by previous methods (MCR-ALS) were first tested. They turn out to give simple provisional inequalities that can be used to predict the main complexes that will form.
Using the equilibrium models and calculated concentration profile (Figure 4) obtained by the fitting procedure, the formation constants of lanthanide-Arsenazo III complexes were measured and presented in Table 1.
In addition, the estimated concentration profile which was obtained by the MCR-ALS procedure was used to calculate the formation constants of lanthanides-Arsenazo III complexes (Table 2). Tables 1 and 2 show a good convergence of the results obtained by the two soft and hard modeling approaches, which confirms the previous equilibrium models (Equations 1 to 3).
Figure 5a,b,c illustrates the results obtained by two different methods: MCR-ALS and fitting, which were used separately to find the complex formation constants and to compare with the Taylor expansion results reported earlier [24].
Conclusions
The proposed work uses chemometric methods to extract complex formation constants from a system with several chemical equilibria from the analysis of the data obtained by spectrophotometric mole ratio titration method, and there are comparable results obtained by MCR-ALS and curve fitting method.
The knowledge about the chemical behavior of the analytes which was obtained by factor analysis and soft modeling method was used to implement a hard modeling equilibrium constraint in hard modeling that ensures the correct complex formation constants. The proposed method makes it possible to obtain the stability equilibrium constants, pure absorption spectra, and species concentration profiles in several ligand-metal ion complex formation systems by severe spectral overlapping. In addition, the proposed method was tested with simulated data sets which were created according to mole ratio method and were very similar to the real data set. The proposed method can be applied in resolving the real three competitive complex formation processes, with full spectral overlapping of three complex species. The results open up a new way to study this kind of competitive complexation processes based on the following achievements:
-
1.
The applicability of chemometric methods was shown to resolve complex systems with three competitive complex formation processes in extracting complex formation constants of species with severe spectral overlapping.
-
2.
MCR-ALS was used in the initial estimation of parameters related to the equilibrium concentration and spectra, and then they were confirmed using the hard model.
-
3.
Complexation constants were measured by two different methods (MCR-ALS and fitting) with satisfactory results and good agreement with the earlier published work [24].
References
Martell AE, Motekaitis RJ: Determination and use of stability constants. Weinheim, VCH; 1988.
Polster J, Lachmann H: Spectrometric titrations. Analysis of chemical equilibria, VCH, Weinheim; 1989.
Maeder M, Zuberbuhler AD: Anal Chim Acta. 1986, 181: 287.
Malinowski ER: Factor analysis in chemistry. 3rd edition. Wiley-VCH, New York; 2002.
de Juan A, Maeder M, Martinez M, Tauler R: Chemom Intell Lab Syst. 2000, 54: 123. 10.1016/S0169-7439(00)00112-X
Tauler R, Barceló D: Trends Anal Chem. 1993, 12: 319. 10.1016/0165-9936(93)88015-W
Johnson K, de Juan A, Rutan SC: J Chemom. 1999, 13: 331. 10.1002/(SICI)1099-128X(199905/08)13:3/4<331::AID-CEM551>3.0.CO;2-T
de Juan A, Izquierdo-Ridorsa A, Tauler R, Fonrodona G, Casassas E: Biophys J. 1997, 73: 2937. 10.1016/S0006-3495(97)78322-X
Vives M, Gargallo R, Tauler R: Anal Chem. 1999, 71: 4328. 10.1021/ac990131m
Mendieta J, Diaz-Cruz MS, Tauler R, Esteban M: Anal Biochem. 1996, 240: 34.
Salau JS, Tauler R, Bayona JM, Tolosa I: Environ Sci Technol. 1997, 37: 3482.
Nigam S, de Juan A, Cui V, Rutan SC: Anal Chem. 1999, 71: 5225. 10.1021/ac9904314
Hirschfeld T: Anal Chem. 1980, 52: 297A.
Gui M, Rutan SC: Anal Chem. 1994, 66: 1513. 10.1021/ac00081a025
Molloy KJ, Maeder M, Schumacher MM: Chemom Intell Lab Syst. 1999, 46: 221. 10.1016/S0169-7439(98)00173-7
Janssens LD, Boens N, Ameloot M, De Schryver FC: J Phys Chem. 1990, 94: 3564. 10.1021/j100372a039
Maeder M, Zuberbühler AD: Anal Chem. 1990, 62: 2220. 10.1021/ac00219a013
Bugnon P, Chottard JC, Jestin JL, Jung B, Laurenczy G, Maeder M, Merbach AE, Zuberbühler AD: Anal Chim Acta. 1994, 298: 193. 10.1016/0003-2670(94)00255-X
Basargin NN, Ivanov VM, Kuznetsov VV, Mikhailova AV: J Anal Chem. 2000, 55: 204. 10.1007/BF02757200
Rohwer H, Collier N, Hosten E: Anal Chim Acta. 1995, 314: 219. 10.1016/0003-2670(95)00279-9
Hosten E, Rohwer H: Anal Chim Acta. 1997, 345: 227. 10.1016/S0003-2670(97)00100-1
Rohwer H, Hosten E: Anal Chim Acta. 1997, 339: 271. 10.1016/S0003-2670(96)00471-0
Budesinsky B: Talanta. 1968, 15: 1063. 10.1016/0039-9140(68)80117-1
Lu YW, Laurent G, Pereira H: Talanta. 2004, 62: 959. 10.1016/j.talanta.2003.10.030
Leggett J: Computational methods for the determination of formation constants. Plenum, New York; 1985.
Gans P: Chem Rev. 1976, 19: 99.
Acknowledgement
The authors gratefully acknowledge Dr. Z. Zeinali for her theoretical support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Kakhki, J.F., Abedi, M.R. Application of soft and hard modeling methods to resolve the three competitive complex formation of 13 lanthanide-Arsenazo III complexes. Int J Ind Chem 3, 9 (2012). https://doi.org/10.1186/2228-5547-3-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2228-5547-3-9