Abstract
Background
The carob seed oil (CO) was tested as inhibitor of the corrosion of C38 steel in 1 M HCl by weight loss and electrochemical measurements. The extract was found to inhibit the corrosion of C38 steel in 1 M HCl.
Results
The results of the study reveal that the inhibition efficiency of CO depends on its concentration and attains approximately 86.7% at 0.5 g/L. Polarization curves reveal that CO is a mixed-type inhibitor. Changes in impedance parameters (charge transfer resistance, Rt, and double-layer capacitance, Cdl) were indicative of CO adsorption on the metal surface, leading to the formation of a protective film. The effect of temperature on the corrosion behavior with the addition of 0.5 g/L of carob seed oil was studied in the temperature range of 298 to 328 K.
Conclusions
Results show that the inhibition efficiency of the plant extract increases with increasing temperature, and the adsorption of the latter on the C38 steel surface is found to obey the Langmuir adsorption isotherm. Some thermodynamic functions of dissolution processes were also determined.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Acid solutions generally used for the removal of rust and scale in industrial processes and the deterioration of metal due to these processes are very significant. Inhibitors are used in these processes to control metal dissolution. Hydrochloric acid is widely used in the pickling of steel and different steel-based alloys[1, 2]. One way of protecting steel from corrosion is to use corrosion inhibitors. Organic compounds containing heteroatoms are commonly used to reduce the corrosion attack on steel in acidic media[3–16]. The recent trend is towards environmentally friendly inhibitors. Most of the natural products are non-toxic, biodegradable, and readily available in plenty. These advantages have incited us to draw a large part of our laboratory program to examine natural substances as corrosion inhibitors such as fennel oil[17], prickly pear seed oil[18], argan extract[18–21], argan oil[19, 22], rosemary oil[23–25], Thymus oil[26, 27], pennyroyal mint oil[28], lavender oil[29], jojoba oil[30], and Artemisia[31–33].
Carob fruits are among the most import tree fruit crops in the Mediterranean countries, and their production and consumption have increased considerably in recent years. They are widely used in the boiled juice ‘pekmez’ production and powder drink industry. The seeds of the carob are utilized in the food industry for their gum content[34]. The present work was established to study the corrosion inhibition of C38 steel in 1 M HCl solution by carob seed oil as corrosion inhibitor using different techniques: weight loss, potentiodynamic polarization, and electrochemical impedance spectroscopy (EIS).
Methods
Fatty acid composition
The fatty acid composition was determined following the ISO standard ISO 5509:2000 (ISO 2000)[35]. In brief, one drop of the oil was dissolved in 1 mL of n-heptane, 50 μg of sodium methylate was added, and the closed tube was agitated vigorously for 1 min at room temperature. After adding 100 μL of water, the tube was centrifuged at 4,500×g for 10 min, and the lower aqueous phase was removed. Then, 50 μL of HCl (1 mol with methyl orange) was added, the solution was shortly mixed, and the lower aqueous phase was rejected. About 20 mg of sodium hydrogen sulfate (monohydrate, extra pure; Merck, Darmstadt, Germany) was added, and after centrifugation at 4,500×g for 10 min, the top n-heptane phase was transferred to a vial and injected in an Agilent Technologies 6890 N gas chromatograph equipped with a capillary column (30 m × 0.32 mm; Supelco, Bellefonte, PA, USA) and flame ionization detection. The column was programmed to increase from 135°C to 160°C at 2°C/min and from 160°C to 205°C at 1.5°C/min; the detection temperature was maintained at 220°C, injector temperature 220°C. The vector gas was helium at a pressure of 5,520 Pa. Peaks were identified by comparing retention times with those of standard fatty acid methyl esters.
Weight loss measurements
Coupons cut into 2 cm × 2 cm × 0.08 cm dimensions (having composition of 0.179% C, 0.165% Si, 0.439% Mn, 0.203% Cu, 0.034% S, and Fe balance) are used for weight loss measurements. Prior to all measurements, the exposed area was mechanically abraded with 180, 320, 800, and 1,200 grades of emery papers. The specimens are washed thoroughly with bidistilled water, degreased, and dried with ethanol. Gravimetric measurements are carried out in a double-walled glass cell equipped with a thermostated cooling condenser. The solution volume is 80 cm3. The immersion time for the weight loss is 6 h at 298 K.
Electrochemical tests
The electrochemical study was carried out using a potentiostat PGZ100 piloted by Voltamaster software. This potentiostat is connected to a cell with three electrode thermostats with double wall (Tacussel Standard CEC/TH). A saturated calomel electrode and platinum electrode were used as reference and auxiliary electrodes, respectively. The material used for constructing the working electrode was the same with that used for the gravimetric measurements. The surface area exposed to the electrolyte is 0.04 cm2.
Potentiodynamic polarization curves were plotted at a polarization scan rate of 0.5 mV/s. Before all experiments, the potential was stabilized at free potential during 30 min. The polarization curves are obtained from −800 to −400 mV at 298 K. The solution test is thereafter de-aerated by bubbling nitrogen. Gas bubbling is maintained prior and throughout the experiments. In order to investigate the effects of temperature and immersion time on the inhibitor performance, some tests were carried out in a temperature range of 298 to 328 K.
The EIS measurements are carried out with the electrochemical system (Tacussel), which included a digital potentiostat model Voltalab PGZ100 computer (Radiometer Analytical, Lyon, France) at Ecorr after immersion in solution without bubbling. After the determination of steady-state current at a corrosion potential, sine wave voltage (10 mV) peak to peak at frequencies between 100 kHz and 10 mHz are superimposed on the rest potential. Computer programs automatically controlled the measurements performed at rest potentials after 0.5 h of exposure at 298 K. The impedance diagrams are given in the Nyquist representation. Experiments are repeated three times to ensure the reproducibility.
Inhibition efficiencies EI% were calculated as follows:
For weight loss measurement:
where W0corr and Wcorr are the corrosion rates of steel due to the dissolution in 1 M HCl in the absence and the presence of definite concentrations of the inhibitor, respectively.
For impedance measurements:
where Rt and Rt0 are the charge transfer resistance values with and without the inhibitor, respectively.
For potentiodynamic polarization measurements:
where I0corr and Icorr are the corrosion current densities in the absence and the presence of the inhibitor.
Results and discussion
Fatty acid composition
Gas chromatography analysis of dried plant extract showed that the plant extract contains ten different fatty acid methyl esters (Table1). The main constituents were linoleic acid (45.05%), oleic acid (33.66%), palmitic acid (14.84%), and stearic acid (3.50%).
From this table, it is evident that the carob seed oil could serve as an effective green corrosion inhibitor. It is interesting to see here that all the identified compounds from the plant extracts contained oxygen and/or p-electrons in their molecules.
It is a known fact that adsorption of the inhibitors is the main process affecting the corrosion rate of metals. Inhibition adsorption can affect the corrosion rate in two possible ways[36]. In the first way, inhibitors decrease the available reaction area through adsorption on the metal, which is called geometric blocking effect. In the second way, inhibitors modify the activation energy of the cathodic and/or anodic reactions occurring in the inhibitor-free metal in the course of the inhibited corrosion process, which is called energy effect[37].
Effect of concentration
Gravimetric measurements
Values of the inhibition efficiency and corrosion rate obtained from the weight loss measurements of C38 steel for different concentrations of carob seed oil in 1 M HCl at 298 K after 6 h of immersion are given in Table2. This shows that the inhibition efficiency increases with the increasing inhibitor concentration. At this purpose, one observes that the optimum concentration of the inhibitor required to achieve efficiency is found to be 0.50 g/L (EI% = 81.6%). The inhibition of C38 steel corrosion by carob seed oil (CO) can be explained in terms of adsorption on the metal surface. This compound can be adsorbed on the metal surface by the interaction between the pairs of oxygen electrons and the insaturation of the inhibitor molecules and the metal surface. This process is facilitated by the presence of vacant orbitals of low energy in iron atom, as observed in the transition group metals[38].
With and without carob seed oil at various concentrations (Wcorr) and the corresponding inhibition efficiency (EW).
Electrochemical impedance spectroscopy measurements
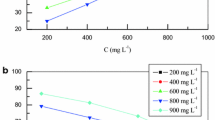
The corrosion behavior of C38 steel in acidic solution in the presence of CO was investigated by EIS methods at 298 K. Nyquist plots obtained for frequencies ranging from 100 kHz to 10 mHz at open circuit potential for C38 steel in 1 M HCl in the presence of various concentrations of CO are shown in Figure1. The impedance diagrams obtained are not perfect semicircles, and the difference was attributed to frequency dispersion[38]. The fact that impedance diagrams have a semicircular appearance shows that the corrosion of steel is controlled by a charge transfer process. The equivalent circuit model employed for this system is presented in Figure2. The resistance Rs is the resistance of the solution; Rt reflects the charge transfer resistance, and Cdl is the double-layer capacitance.
Values of the charge transfer resistance Rt were obtained from these plots by determining the difference in the values of impedance at low and high frequencies as suggested by Tsuru and Haruyama[39]. Values of the double-layer capacitance Cdl were calculated from the frequency at which the impedance imaginary component -Zi is maximum using the equation:
Table3 gives the values of the charge transfer resistance Rt, double-layer capacitance Cdl, and inhibition efficiency obtained from the above plots. It can be seen that the presence of carob seed oil enhances the values of Rt and reduces the Cdl values. The decrease in Cdl, which can result from a decrease in local dielectric constant and/or an increase in the thickness of the electric double layer[40], suggested that CO molecules function by adsorption at the metal/solution interface. Thus, the decrease in Cdl values and the increase in Rt values and consequently of the inhibition efficiency may be due to the gradual replacement of water molecules (volumes of the water molecules is 27.19 A°3) by the adsorption of the extract molecules on the metal surface, decreasing the extent of dissolution reaction[41, 42].
Polarization measurements
Figure3 shows the polarization curves of C38 steel in 1 M HCl and in the presence of different concentrations (0.02 to 0.5 g/L) of carob seed oil. With the increase of CO concentrations, both anodic and cathodic currents were inhibited. This result shows that the addition of CO reduces anodic dissolution and also retards the hydrogen evolution reaction.
Table4 gives the values of kinetic corrosion parameters as the corrosion potential Ecorr, corrosion current density Icorr, Tafel slope bc, and inhibition efficiency for the corrosion of C38 steel in 1 M HCl with different concentrations of carob seed oil. The corrosion current densities were estimated by Tafel extrapolation of the cathodic curves to the open circuit corrosion potential. From Table4, the following can be concluded:
The Icorr values decrease with increasing inhibitor concentration.
The addition of the carob seed oil produces slight changes in the values of Ecorr and bc. This indicates[43] that the adsorbed molecules of oil seed do not affect the mechanism of hydrogen evolution.
The values of inhibition efficiency (EI%) increase with inhibitor concentration, reaching a maximum value (86.7%) at 0.5 g/L.
These polarization curve measurements were in good agreement with the corrosion weight loss and impedance tests.
Effect of temperature
To investigate the mechanism of inhibition and to calculate the activation energies of the corrosion process, EIS measurements were taken at various temperatures in the absence and presence of different concentrations of carob seed oil. Figures4 and5 give the Nyquist plots of C38 steel in the absence and presence of 0.5 g/L of carob seed oil at different temperatures.
Corresponding data are given in Table5. In the studied temperature range (298 to 328 K), the values of Rt decrease with increasing temperature both in uninhibited and inhibited solutions, and the values of the inhibition efficiency of carob seed oil increase with increasing temperature. The corrosion current density of C38 steel decreases more rapidly with temperature in the absence of the inhibitor; these results confirm that the plant extract acts as an efficient inhibitor in the temperature range studied.
The activation parameters for the corrosion process were calculated from Arrhenius-type plot according to the following equation:
where Ea is the apparent activation energy, A is the pre-exponential factor, R is the universal gas constant, and T is the absolute temperature.
The variations of the 1/Rt logarithm of C38 steel in HCl containing 0.5 g/L of carob seed oil used with the reciprocal of the absolute temperature are presented in Figure6. Straight lines with coefficients of correlation higher than 0.99 are obtained.
Kinetic parameters such as enthalpy and entropy of the corrosion process may be evaluated from the temperature effect. An alternative formulation of Arrhenius equation is[44–46].
where h is Plank's constant, N is Avogrado's number, and ΔS°a and ΔH°a are the entropy and enthalpy of activation, respectively.
Straight lines are obtained with a slope (−ΔH°a/R) and intercept (Ln R/Nh + ΔS°a/R), from which the ΔH°a and ΔS°a values are calculated (Table6 and Figure7). The positive sign of the enthalpy (ΔH°a) reflects the endothermic nature of the steel dissolution process. The entropy of activation ΔS°a in the absence of the inhibitor is positive, and this value increases positively with the CO concentration. The increase of ΔS°a implies that an increase in disordering takes place in going from the reactants to the activated complex[47].
The linear regression coefficients are close to one, indicating that the corrosion of C38 steel in 1 M HCl solution may be elucidated using the kinetic model. A close inspection of the data in Table6 shows that the activation energy is lower in the presence of carob seed oil. The decrease of Ea is typical of the chemisorption process[15]. According to Equation 5, low values of A and high values of Ea lead to lower corrosion rates.
Adsorption isotherm and mechanism of inhibition
Adsorption isotherms are very important to understand the mechanism of corrosion inhibition reactions. The most frequently used isotherms are the Langmuir[48], Frumkin[49], and Temkin[50]. The Langmuir isotherm (C/θ vs C) assumes that there is no interaction between the adsorbed molecules on the surface. The Frumkin adsorption isotherm (θ vs C) assumes that there is some interaction between the adsorbates, and the Temkin adsorption isotherm (θ vs lgC) represents the effect of multiple layer coverage[51]. Figure8 shows the dependence of C/θ as a function of the of CO concentration. The curve obtained clearly showing that the data fit well with the Langmuir adsorption isotherm was found to be the best description of the adsorption behavior of the studied inhibitor, which obeys:
where C is the concentration of the inhibitor, K is the equilibrium constant of the adsorption process, and θ is the surface coverage.
This suggests that extract in present study obeyed the Langmuir isotherm and there is negligible interaction between the adsorbed molecules.
For the mechanism of adsorption, from the literature and in hydrochloric acid solution, the following mechanism is proposed for the corrosion of C38 steel[52, 53].
The anodic dissolution mechanism of mild steel is as follows:
Fe + Cl− ↔ (FeCl−)ads,
(FeCl−)ads ↔ (FeCl+)ads + e−,
(FeCl+) ↔ Fe2+ + Cl−.
The cathodic hydrogen evolution mechanism is as follows:
Fe + H+↔ (FeH+)ads,
(FeH+)ads + e− ↔ (FeH)ads,
(FeH)ads + H+ + e− ↔ Fe + H2.
Generally, the corrosion inhibition mechanism in an acid medium is adsorption of the inhibitor on the metal surface. The process of adsorption is influenced by different factors like the nature and charge of the metal, the chemical structure of the organic inhibitor, and the type of aggressive electrolyte[53].
Considering the inhomogeneous nature of metallic surfaces resulting from the existence of lattice defects and dislocations, a corroding metal surface is generally characterized by multiple adsorption sites having activation energies and heats of adsorption. Inhibitor molecules may thus be adsorbed more readily at surface active sites having suitable adsorption enthalpies. According to the detailed mechanism above, the displacement of some adsorbed Cl− water molecules on the metal surface by the inhibitor species to yield the adsorbed intermediate (FeCl−)ads reduces the amount of the species (FeCl+)ads available for the rate-determining steps and consequently retards Fe anodic dissolution.
Experimental
Oil extraction
The carob seeds were collected from Talioune (Taroudant) in Morocco in the summer of 2011. The seeds were removed from the carob fruits, finally washed with water, and left to air dry for 4 days. The previously cleaned seeds were comminuted into pieces. The seed pieces were then stored in an air-tight container in a refrigerator (−20°C) prior to analysis. Carob seed oil was obtained by extraction of the meal with petroleum ether (50°C) in a soxhlet extractor for 12 h. After extraction of the oil, the solvent was evaporated under reduced pressure. The obtained oil was kept in sealed glass bottles under deep-freezing (−18°C) for further analysis.
Solution preparation
The 1 M HCl solution was prepared by dilution of analytical-grade 37% HCl with double-distilled water. The test solutions were freshly prepared before each experiment by adding the oil directly to the corrosive solution. The test solution is thereafter de-aerated by bubbling nitrogen. Gas pebbling is maintained prior and throughout the experiments. The experiments were carried out in triplicate to ensure reproducibility.
Conclusions
Inhibitors play a vital role in providing protection against corrosion. The selection of the inhibitor is important for environmental protection. Not all inhibitors are eco-friendly.
There is a growing trend to use plant extracts and pharmaceutical compounds as corrosion inhibitors. Most of these compounds are environmentally friendly. Carob seed oil proved to be an eco-friendly inhibitor for C38 steel in 1 M HCl solution.
From the overall experimental results, the following conclusions can be deduced:
-
Chemical analysis shows that the plant extract contains ten different fatty acid methyl esters.
-
Carob seed oil mainly acts as a good inhibitor for the corrosion of C38 steel in 1 M HCl.
-
Inhibition efficiency increases with both the concentration of the inhibitor and the temperature.
-
The data obtained from the three different methods, potentiodynamic polarization, EIS, and weight loss, are in good agreement.
-
The studied CO was adsorbed chemically on the steel surface according to the Langmuir isotherm model. Thermodynamic parameters are determined.
References
Bentiss F, Gassama F, Barbry D, Gengembre L, Vezin H, Lagreneée M, Traisnel M: Appl Surf Sci. 2006, 252: 2684. 10.1016/j.apsusc.2005.03.231
Khaled KF, Hackerma N: Electrochim Acta. 2003, 48: 2715. 10.1016/S0013-4686(03)00318-9
Mihit M, El Issami S, Bouklah M, Bazzi L, Hammouti B, AitAddi E, Salghi R, Kertit S: Appl Suf Sci. 2006, 252: 2389. 10.1016/j.apsusc.2005.04.009
Hammouti B, Salghi R, Kertit S: J Electrochem Soc India. 1998, 47: 31.
Zarrok H, Oudda H, Zarrouk A, Salghi R, Hammouti B, Bouachrine M: Der Pharma Chemica. 2011, 3: 576.
El Issami S, Bazzi L, Mihit M, Hilali M, Salghi R, El Ait A: J Phys IV. 2005, 123: 307.
El I, Bazzi L, Mihit M, Hammouti B, Kertit S, AitAddi E, Salghi R: Pig Res Tech. 2007, 36: 161. 10.1108/03699420710749027
Mihit M, Laarej K, Abou El Makarim H, Bazzi L, Salghi R, Hammouti B: Arab J Chem. 2010, 3: 55. 10.1016/j.arabjc.2009.12.009
Mihit M, Salghi R, El Issami S, Bazzi L, Hammouti B, El Ait A, Kertit S: Pig Res Tech. 2006, 35: 151. 10.1108/03699420610665184
Barouni K, Bazzi L, Salghi R, Mihit M, Hammouti B, Albourine A, El Issami S: Mater Lett. 2008, 62: 3325. 10.1016/j.matlet.2008.02.068
El Issami S, Bazzi L, Hilali M, Salghi R, Kertit S: Ann Chim Sci Mat. 2002, 27: 63. 10.1016/S0151-9107(02)80019-8
Salghi R, Bazzi L, Hammouti B, Kertit S: Bull Electrochem. 2000, 16: 272.
Benali O, Larabi L, Tabti B, Harek Y: Anti-corr Meth Mater. 2005, 52: 280. 10.1108/00035590510615776
Benali O, Larabi L, Mekelleche SM, Harek Y: J Mater Sci. 2006, 41: 7064. 10.1007/s10853-006-0942-6
Benali O, Larabi L, Harek Y: J Appl Electrochem. 2009, 39: 769. 10.1007/s10800-008-9717-x
Merah S, Larabi L, Benali O, Harek Y: Pig Res Tech. 2008, 37: 291. 10.1108/03699420810901963
Lahhit N, Bouyanzer A, Desjobert JM, Hammouti B, Salghi R, Costa J, Jama C, Bentiss F, Majidi L: Port Electrochim Acta. 2011, 29: 127. 10.4152/pea.201102127
Ben Hmamou D, Salghi R, Bazzi L, Hammouti B, Al-Deyab SS, Bammou L, Bazzi L, Bouyanzer A: Int J Electrochem Sci. 2012, 7: 1303.
Afia L, Salghi R, El B, Bazzi L, Errami M, Jbara O, Al-Deyab SS, Hammouti B: Int J Electrochem Sci. 2011, 6: 5918.
Afia L, Salghi R, Bammou L, Bazzi E, Hammouti B, Bazzi L: Acta Metall Sin. 2012, 25: 10.
Afia L, Salghi R, Bazzi E, Zarrouk A, Hammouti B, Bouri M, Zarrouk H, Bazzi L, Bammou L: Res Chem Intermed. 2012. 10.1007/s11164-012-0496-y
Afia L, Salghi R, Bammou L, El B, Hammouti B, Bazzi L, Bouyanzer A: J. Saudia Chem Soc. 2011. 10.1016/j.jscs.2011.05.008
Chetouani A, Hammouti B, Benkaddour M: Res Pig Tech. 2004, 33: 26. 10.1108/03699420410512077
El Ouariachi E, Paolini J, Bouklah M, Elidrissi A, Bouyanzer A, Hammouti B, Desjobert J-M: J. Costa, Acta Metall Sin. 2010, 23: 13.
Bendahou M, Benabdallah M, Hammouti B: Pigm Res Techn. 2006, 35: 95. 10.1108/03699420610652386
Bouyanzer A, Hammouti B: Bull Electrochem. 2004, 20: 63.
Bammou L, Chebli B, Salghi R, Bazzi L, Hammouti B, Mihit M, El Idrissi H: Green Chem Lett Rev. 2010, 3: 173. 10.1080/17518251003660121
Bouyanzer A, Hammouti B, Majidi L: Mater Lett. 2006, 60: 2840. 10.1016/j.matlet.2006.01.103
Zerga B, Sfaira M, Rais Z, Ebn Touhami M, Taleb M, Hammouti B, Imelouane B, Elbachiri A: Mater. Tech. 2009, 97: 297. 10.1051/mattech/2009045
Bouyanzer A, Hammouti B: Pigm. Resin Techn.. 2004, 33: 287. 10.1108/03699420410560489
Benabdellah M, Hammouti B, Benkaddour M, Bendahhou M, Aouniti A: Appl Surf Sci. 2006, 252: 6212. 10.1016/j.apsusc.2005.08.030
Bammou L, Mihit M, Salghi R, Bazzi L, Bouyanzer A, Hammouti B: Int J Electrochem Sci. 2011, 6: 1454.
Ouachikh O, Bouyanzer A, Bouklah M, Desjobert J-M, Costa J, Hammouti B, Majidi L: Surf Rev Lett. 2009, 6: 49.
Ozcan MM, Arslan D, Gokcalık H: Int J Food Sci Nutr. 2007, 58: 652. 10.1080/09637480701395549
International Standard ISO 5509: Animal and vegetable fats and oils - preparation of methyl esters of fatty acids. 2nd edition. 2000.
Riggs OL Jr: Corrosion inhibitors. 2nd edition. Edited by: Nathan CC. Houston, NACE; 1973.
Al-Otaibi MS, Al-Mayouf AM, Khan M, Mousa AA, Al-Mazroa SA, Alkhathlan HZ: Arab. J. Chem. 2012. 10.1016/j.arabjc.2012.01.015
Benali O, Larabi L, Traisnel M, Gengembre L, Harek Y: Appl Surf Sci. 2007, 253: 6130. 10.1016/j.apsusc.2007.01.075
Tsuru T, Haruyama S, Gijutsu B: J Jpn Soc Corros Eng. 1978, 27: 573.
McCafferty E, Hackerman N: J Electrochem Soc. 1972, 119: 146. 10.1149/1.2404150
Bentiss F, Traisnel M, Lagrenée M: Corros Sci. 2000, 42: 127. 10.1016/S0010-938X(99)00049-9
Muralidharan S, Phani KLN, Pitchumani S, Ravichhandran S, Iyer SVK: J Electrochem Soc. 1995, 142: 1478. 10.1149/1.2048599
Ateya BG, Abo El-Khair BM, Abdel Hamid IA: Corros Sci. 1976, 16: 163. 10.1016/0010-938X(76)90057-3
Benabdellah M, Tounsi A, Khaled KF, Hammouti B: Arab J Chem. 2011, 4: 17. 10.1016/j.arabjc.2010.06.010
Elouali I, Hammouti B, Aouniti A, Ramli Y, Azougagh M, Essassi EM, Bouachrine M: J Mater Environ Sci. 2010, 1: 1.
Dahmani M, Et-Touhami A, Al-Deyab SS, Hammouti B, Bouyanzer A: Int J Electrochem Sci. 2010, 5: 1060.
Ouici HB, Benali O, Harek Y, Al-Deyab SS, Larabi L, Hammouti B: Int J Electrochem Sci. 2012, 7: 2304.
Langmuir I: J Am Chem Soc. 1947, 39: 1848.
Frumkin ANZ: Phys Chem. 1925, 116: 466.
De Boer JH: The dynamical character of adsorption. 2nd edition. Claredon Press, Oxford, UK; 1968.
Masel RI: Principles of adsorption and reaction on solid surfaces. Wiley, New York; 1996.
Bavarian B, Yeob K, Reiner L: Corrosion protection of steel rebar in concrete by migrating corrosion inhibitors. NACE, Corrosion, Houston; 2003.
Shahid M: Adv Nat Sci: Nanosci Nanotechnol. 2011, 2: 043001. 10.1088/2043-6262/2/4/043001
Acknowledgements
The authors wish to thank the Volubilis MA/10/226 for supporting this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
OB carried out the data treatment and redaction of the manuscript. FF carried out the oil extraction and fatty acid composition. HZ carried out the gravimetric measurements. DBH and AZ studied the effect of temperature. RS and BH carried out the thermodynamic parameters and redaction of the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Hmamou, D.B., Salghi, R., Zarrouk, A. et al. Carob seed oil: an efficient inhibitor of C38 steel corrosion in hydrochloric acid. Int J Ind Chem 3, 25 (2012). https://doi.org/10.1186/2228-5547-3-25
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2228-5547-3-25