Abstract
Background
The present study is aimed at investigating the degradation of azo dye solution of AR 274 by wet air oxidation conditions. The central composite design matrix and response surface methodology were applied in designing the experiments to evaluate the interactive effects of the three most important operating variables. Thus, the interactive effects of oxygen pressure (3.0 to 5.0 MPa), temperature (100°C to 250°C), and time (30 to 90 min) on the degradation of dye were investigated.
Results
The predicted values were found to be in good agreement with the experimental values (R2 = 0.9981 and Adj-R2 = 0.9965), which define the propriety of the model and the achievement of CCD in the optimization of WAO process.
Conclusions
Intermediates of dye degradation were detected by GC-MS, the possible degradation mechanism for the WAO of dye was discussed, and the probable degradation pathway was deduced.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Dye pollutants from textile and dyestuff industries are a major hazardous source of environmental contamination. The large quantity of dye wastewater has become a serious environmental problem owing to the characteristics of high color, high chemical oxygen demand, and fluctuating pH. The direct discharge of this wastewater into water bodies such as lakes and rivers causes pollution of the water and affects the flora and fauna. Effluent from textile industries contains different types of dyes, which show very low biodegradability owing to their high molecular weight and complex structures[1–3]. Some dyes, especially azo dyes, are known to be biorefractory pollutants even with carefully selected microorganism and under favorable conditions. Azo dyes are characterized by the presence of one or more azo bonds (−N = N-) and account for 60% to 70% of all textile dyes used. It is estimated that approximately 8 × 105 tons (t) of dyes are produced annually worldwide, and about 50% of them are azo dyes[4–8]. Thus, azo dyes constitute a significant portion of dyes that are used in industries nowadays. The product obtained from dye degradation could be mutagenic and carcinogenic, thereby causing long-term health concerns. Therefore, the treatment of effluents containing such compounds is important for the protection of natural waters as well as the environment[9–12]. Conventional methods of dyeing wastewater treatment include adsorption, flocculation, ozonation, advanced oxidation using UV/H2O2 or UV/TiO2, and biological oxidation. Other advanced oxidation treatments for dyeing wastewater treatment are wet air oxidation (WAO) and catalytic wet air oxidation (CWAO), which are operated at subcritical water and pressures of water. Previous researches have shown that the treatment efficiencies for various dyes using WAO and CWAO are in the range of 50% to 90% at the operating times of 30 to 240 min in different types of reactors[13–17].
A variety of advanced oxidation process (AOPs) have been attempted for the degradation of dyes, among which WAO seems to be a clean method as it does not involve the use of any harmful chemicals and uses only the clean reagent of air[7, 18]. By using WAO, organic pollutants are either partially oxidized into biodegradable intermediates or mineralized to carbon dioxide, water, and innocuous end products under elevated temperatures (100°C to 300°C) and pressures (0.5 to 20 MPa) using an oxidant such as oxygen. The enhanced solubility of oxygen in aqueous solutions at elevated temperatures and pressures provides a strong driving force for oxidation. The elevated pressures are required to keep water in the liquid state. Water also acts as a moderant by providing a medium for heat transfer and removing excess heat by evaporation. WAO has been demonstrated to oxidize organic compounds to CO2 and other innocuous end products. Carbon is oxidized to CO2; nitrogen is converted to NH3, NO3, or elemental nitrogen; and halogen and sulfur are converted to inorganic halides and sulfates. The degree of oxidation is mainly a function of temperature, oxygen partial pressure, residence time, and the oxidizability of the pollutants under consideration[19–22]. WAO is not only eco friendly but also economical when compared to other AOPs that use harmful and expensive oxidizing agents like ozone and hydrogen peroxide[23–25]. In this research, the aqueous solution of Acid Red 274 (AR 274) was selected as a model for textile wastewaters for evaluation under WAO conditions. Another part of this study involved the use of response surface methodology (RSM) and finding an applicable approximating function for predicting and determining the further response, and studying the optimum working state. The factors (variables) of oxygen pressure, temperature, and experimental time were investigated[26].
RSM is a kind of mathematical and statistical technique for designing experiments, building models, evaluating the relative significance of several independent variables, and determining the optimum conditions for desirable responses[5, 27, 28]. The two most common designs extensively used in RSM are the central composite design (CCD) and the Box-Behnken design (BBD). The CCD is ideal for sequential experimentation and allows a reasonable amount of information for testing lack of fit while not involving an unusually large number of design points[29–31].
Methods
Analysis methods
In the present study, AR 274 dye concentration was analyzed spectrophotometrically on a UV–vis spectrometer (Shimadzu UV-160A, Shimadzu Corporation, Kyoto, Japan) at 527 nm by measuring the absorbance of the untreated samples at maximum wavelength, and the percentage of AR 274 degradation efficiency percentage was calculated using the following formula:
where Co and Ct represent the initial and remaining AR 274 concentration at given time (t), respectively. The mineralization of AR 274 solution was monitored through the diminishment of the TOC, measured on a Tekmar-Dorhmann Apollo 9000 TOC analyzer (Teledyne Technologies, Inc., OH, USA).
The mass analysis process, which is the same as the previous method, was performed for intermediates of dye degradation[9]. The gas chromatography–mass spectrometry (GC-MS) analysis was performed using the 5890A Agilent model gas chromatograph (Agilent Technologies, Inc., CA, USA), interfaced with the ECD, NPD, and 5975C mass selective detector. The aqueous solutions were extracted three times with 15 mL dichloromethane. A 3-μL sample was analyzed on GC-MS. A HP5-MS capillary column (30 m × 0.25 mm × 0.25 μm) was used as the analytical column. Helium was used as the carrier gas with a flow rate of 2 mL/min. The GC injection port temperature was set at 250°C (split mode = 1/5), and the column temperature was fixed at 70°C for 5 min. Subsequently, the column was sequentially heated at a rate of 5°C/min to 120°C and held for 1 min, at a rate of 8°C/min to 200°C and held for 5 min, and at a rate of 10°C/min to 280°C and held for 10 min. The MS detector was operated in the EI mode (70 eV).
Experimental design and optimization
In this study, RSM was used for the optimization of process variable to enhance the degradation of AR 274 dye combined with the factorial experimental design of CCD. RSM is a useful method for studying the effect of several variables influencing the responses by varying them simultaneously and carrying out a limited number of experiments. A very limited number of studies employing the experimental design and optimization modeling approach for WAO process have been reported in the literature[32, 33]. The CCD is an effective design that is ideal for sequential experimentation and allows a reasonable amount of information for testing the lack of fit while not involving an unusually large number of design points. It was first announced by Box and Wilson in 1951, and is well suited for fitting a quadratic surface, which usually works well for the process optimization[31, 34–36].
In the present study, a CCD was employed for determining the optimum conditions for dye removal in WAO. The experimental results were analyzed using Design Expert 8.1, and the regression model was proposed. Oxygen pressure, temperature, and reaction time were chosen as three independent variables in the degradation process. Accordingly, the CCD matrixes of 20 experiments covering the full design of five factors were used for building quadratic models as shown in Table1[37]. The experimental data obtained from the CCD model experiments can be represented in the form of the following equation:
where Y is the predicted response; n is the number of factors; x i and x j are the coded variables; bo is the offset term; b i , b ii , and b ij are the first-order, quadratic, and interaction effects, respectively; i and j are the index numbers for factor; and ei is the residual error[35, 38].
The quality of the polynomial model was expressed by the coefficient of determination, namely, R2 and Adj-R2. The statistical significance was verified with adequate precision ratio and by the F test[26]. According to the obtained experimental data, the levels of the three main parameters investigated in this study are presented in Table1. For statistical calculations, the variables Xi (the real value of an independent variable) were coded as x i (dimensionless value of independent variable) according to the following equation:
where Xo is the value of Xi at the center point, and δX represents the step change[9, 30, 35].
Results and discussion
Optimization of degradation conditions using the RSM approach
In the present study, RSM was employed for identifying the simple and interactive effects of operating variables of dye degradation for WAO process. On the basis of the CCD, there are four important stages for the optimization of the experiments: (1) to perform statistically designed experiments for the experimental plan, (2) recommend the mathematical model basis of the experimental data and focus on the data of analysis of variance, (3) control the efficiency of the model directly with diagnostic plots, and (4) estimate the response and verify the model[5, 27].
Thus, RSM was used for obtaining a relationship between factors and the response and for optimizing the response. Table2 depicts a complete 23 factorial design with four center points in cube, and six axial points and two center points in axial. The experiments were carried out in three replicates and six blocks in order to fit the second-order polynomial model[39].
According to the RSM results, polynomial regression modeling was performed on the responses of the corresponding coded values of the three different process variables, and the results were evaluated. The predicted response (Y) for the percent TOC of samples treated was obtained using Equation 4:
A statistical approach using a CCD was used for efficiency degradation of dye and for determining the interaction between these factors. For the response surface methodology involving CCD, a total of 20 experiments was conducted for three factors at five levels with three replicates at center point. Table1 provides a list of independent variables and coded factor levels. The number of experiments required (N) is given by the expression:
2k (23 = 8; star points) + 2 k (2 × 3 = 6; axial points) + 6 (center points; 6 replications).
An RSM is appropriate when the optimal region for running the process has been identified. The design used for the optimization and observed responses for 20 experiments are given in Table2 for WAO[40]. In Equation 4, Y is the TOC removal percent of dye; and x1, x2, and x3 are the corresponding coded variables of oxygen pressure, temperature, and time, respectively.
Analysis of variance
Analysis of variance (ANOVA) values for the quadratic regression model obtained from CCD employed in the optimization of dye degradation are listed in Table3. On the basis of the experimental values, statistical testing was carried out using Fisher's test for ANOVA. The statistical significance of the second-order equation revealed that the regression is statistically significant (P < 0.0001); however, the lack of fit is not statistically significant at 99% confidence level. Table3 depicts the significance of the regression coefficients and ANOVA for the regression model, respectively. The results indicate that the response equation proved to be suitable for the CCD experiment[41, 42].
The model's F value of 595.49 in these tables implies that the model is significant for the degradation of the dye. If the model has a very high degree of adequacy for predicting the experimental results, the computed F value should be greater than the tabulated F value at a level of significance α. Thus, the calculated F value (Fmodel = 595.49) was compared with the tabulated F value (F0.05, df, (n − df + 1)) at a significance level of 0.05, when the df for the model was 9 and n = 20. It can be observed that the tabular F value (F0.05,9,10 = 3.02) is clearly less than the calculated F value of 595.49. Adequate precision measures the signal to noise ratio, and a ratio value greater than 4 is desirable. Therefore, in the quadratic model degradation of dye, an adequate precision of 90.78 indicates an adequate signal for WAO. P values less than 0.05 indicate that the model terms are significant, whereas values greater than 0.1 are not significant. The fit of the models were controlled by the coefficient of determination R2. Based on the ANOVA results, the models report high R2 value of 99.81% for dye degradation using WAO. Also, an acceptable agreement with the adjusted determination coefficient is necessary. In this study, the Adj-R2 value of 99.65% was found. The values of R2 and Adj-R2 are close to 1.0, which is very high and advocates a high correlation between the observed values and the predicted values. This indicates that the regression model provides an excellent explanation of the relationship between the independent variables and the response. The diagnostic plots given in Figures1,2, and3 were used for estimating the adequacy of the regression model. The calculated chi-square value of the model (χ2 = 0.25) was found to be less than the tabulated value (χ0.052 = 30.14), which revealed that there is no significant difference between the observed data and the model response[5, 9, 32–37]. The actual and the predicted TOC removal percentage values are given in Figure1. It can be observed that there are tendencies in the linear regression fit, and the model adequately explains the experimental range studied. The actual TOC removal percentage value is the measured result for a specific run, and the predicted value is evaluated from the independent variables in the CCD model[5, 9]. The normal percentage probability and studentized residual plot are shown in Figure2. The data points indicate that neither response transformation was required nor there was any apparent problem with normality. Figure3 depicts the studentized residual and predicted TOC removal percentage of dye degradation.
Interactive effect of processes of independent variables
To understand the impact of each variable, three dimensional (3D) plots were made for the estimated responses, which were the bases of the model polynomial function for analysis to investigate the interactive effect of the two factors on the TOC removal percentage within the experimental ranges given in Figures4,5, and6. The inferences so attained are discussed below[43].
Interactive effect of oxygen pressure and temperature
To investigate the integrated effect of temperature and oxygen pressure, RSM was used and the result was given in the form of 3D plots. As indicated in Figure4, the temperature and pressure conditions have considerable influence on the extent of degradation achieved. Figure4 indicates that the increasing pressure of oxygen partially speeded up the dye degradation, and the dye degradation rate increased until the oxygen was exhausted in the reaction medium. The oxygen pressure is part of the driving force for mass transfer; therefore, the degradation rate increases with the increasing oxygen amount. However, with the oxygen amount below the stoichiometric amount, it contributes little to the concentration of the dissolved oxygen[4]. The degradation rate is found to be affected by this situation. The TOC removal percentage of dye is found to increase with rising temperature for degradation processes. At the lowest temperature of 100°C, the removal of TOC is rather insignificant. At temperatures above 150°C, TOC removal after 30 min is almost dependent on temperature. For example, as shown in Figure4 (at 3.0 MPa oxygen pressure, temperature 100°C), the percentage of TOC removal was 7.18%, which increased to 50.0% at 3.0 MPa and 250°C temperature when using the WAO process. The increased percentage TOC removals were also monitored for oxygen pressure ranging from 3.0 to 5.0 MPa for the WAO process. With oxygen pressure reaching up to 5.0 MPa from 3.0 MPa at 250°C, the TOC removal percentage was found to increase until to 65.6% from 50.0 using WAO process. The degradation of dye would increase if the oxygen pressure is kept high; however, after the stoichiometric quantity is achieved, the oxygen quantity is not an effective factor for dye degradation[4, 16]. As can be seen in Figure4, TOC removal percentage increased remarkably with increasing temperature at all applied oxidant concentrations, and temperature has a significant impact on the dye degradation. Thus, with the increase of temperature, an improvement in dye degradation performance was determined[4].
At wet air oxidation conditions, the free radical easily occurs at high temperature and oxygen-rich conditions. The free-radical generation consists of O2 attacking the C-H bond of the organic molecule. The following reactions could occur by the addition of molecular oxygen[4, 44–49].
These reactions generate organic radicals and other free radicals, which in turn initiate the chain reactions of dye degradation with the help of dissolved oxygen. Several operating parameters influence the aforementioned formation of free radicals, and thereby the degradation rate of pollutants, among which temperature plays the most important role in the case of sufficient oxygen supply[7, 50, 51].
Interactive effect of temperature and time
Figure5 presents 3D plots indicating the effect of temperature and time on percentage TOC removal under the predefined conditions given by Design Expert. As indicated in Figure5, the maximum 80.3% TOC removal occurs at 60 min of experimental time and at 300°C ± 2°C in WAO conditions. The degradation rate slightly increases with the increase in the experimental time at moderate temperature; however, when the temperature increases, the effect of time is almost insignificant, and temperature becomes the main factor owing to the interaction between the oxidant and temperature. Thus, the degradation rate is almost primarily controlled by the temperature, the details of which are described above. Therefore, it appears that time might be less important for the degradation rate of dye. The same effect was observed in the study reported previously[9].
Interactive effect of oxygen pressure and time
As seen in Figure6, the response surface and 3D plots were enhanced as a function of oxidant pressure and experimental time. In addition, the TOC removal percentage rate increased with increased oxygen pressure and time at constant temperature. With the increasing experimental time, the interaction probability of formed radicals with dye, according to Equations 5 to 11, increased. The dye degradation by WAO was regarded as a free radical mechanism. With increasing time, the free radicals such as R· and ROO· would be increased, which promotes the degradation rate[7]. However, regardless of the experimental time, this interaction would be limited to the amount of oxygen. As free radical occurs easily at oxygen-rich conditions, depending on the time decrease, the amount of oxygen in the radicals will reduce and the degradation rate will gradually slow down.
Identification of degradation products
The degradation products obtained in the present study were similar to that obtained from the previous study wherein the degradation of AR 274 was realized using H2O2 in subcritical water[9]. This result is normal as common reagents were occurring in both studies. In this process, the attack of the azo bond would be the initial step of dye degradation, which pioneers the fast removal of color[7]. Figure7 depicts the GC-MS analysis of short-time oxidized solutions in WAO condition revealing the formation of benzamide (A1), benzoic acid (A2), hydroquinone (A3), benzoquinone (A4), 1-naphthol (B1), naphthalene-1,6-diol (B2), naphthalene-1,2,4,6-tetraol (B3), phthalic anhydride (B4), 2,6-dihydroxynaphthalene-1,4-dione (B5), phthalic acid (B6), o-cyclohexylphenol (C1), and catechol (C2). In the process, C-N and C-O bonds were cleavaged owing to temperature effects and attacks of hydroxyl radicals. The main intermediates identified during WAO treatment of AR 274 by GC-MS was shown on GC chromatogram in Figure8. For a longer duration of oxidation, all aromatic compounds would be further transformed into small molecules.
Experimental
Materials
The azo dye C.I. Acid Red 274, commercial name Supranol Red 3BW (CAS no: 61931-18-8), was obtained from Dystar Colours Distribution GmbH (Germany) and was used without further purification. The solution of AR 274 was prepared in 1,000 mg/L initial concentration with distilled water for all treatments. The molecular structure of AR 274 (C35H28N3O9Na2S2, Mw = 744 g/mol) was shown in Figure9. Oxygen was used as the oxidant in the WAO process, which was supplied by Linde Gas (Linde Group Turkey, Adana, Turkey) with 99.9% purity.
Wet air oxidation process
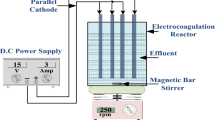
The experimental process is described in detail in Kayan et al.[44]. A brief overview of the process is as follows: Wet air oxidation process was carried out at temperature ranging from 100°C to 250°C with oxygen pressure of 3.0 to 5.0 MPa in a 150-mL stainless steel reactor with magnetic stirrer and heater as shown in Figure10. The glass vessel was placed in the reactor and filled with 1,000 mg/L of AR 274 solution, and subsequently the reactor was placed on a magnetic stirrer and heater. Oxygen was directly supplied through a tube into the liquid phase. Ordinarily, the reactor was filled with 150 mL of reaction solution. The reaction solution was heated to the targeted temperature, and all the valves of the reactor were tightly closed during preheating. Upon reaching the targeted temperature, the amount of pure oxygen gas fed in the reactor in the liquid phase and its partial pressure were maintained with a gas relief valve. Samples were taken out periodically and analyzed for AR 274 degradation using a UV–vis spectrophotometer. The reaction temperature was measured using a thermocouple and controlled using a regulator. After the sample collection treatment, the reactor was cooled to room temperature using a water bath and sampled for total organic carbon (TOC) analysis of the final effluent in 90-min reaction time. The quantity of dissolved oxygen in water was calculated using an oxygen solubility model for the required degradation of AR 274 in distilled water[52].
Conclusions
The degradation of the azo dye was obtained by wet air oxidation system. Wet air oxidation seems a very attractive technique for the treatment of waste streams that are toxic and dilute. When molecular oxygen was used as an oxidant in WAO system, effective degradation or mineralization efficiency was obtained at high temperature. This clearly indicated that temperature had an effect on dye degradation. An increase in the pressure of oxygen partially speeded up the dye degradation and increased the dye degradation rate until the oxygen was exhausted in the reaction medium. This result indicated that temperature was the main factor, and the interactions of other parameters were discussed in detail. The degradation process was optimized using RSM based on CCD. The optimum values of oxygen pressure, temperature, and reaction time were 3.3 MPa, 255°C, and 111 min, respectively, where 67.02% TOC removal could be obtained from the proposed model. The ANOVA showed high coefficient of the determination values (R2 = 0.9981 and Adj-R2 = 0.9965). In this way, the degradation mechanism of WAO of Acid Red 274 dye was discussed, and the probable degradation pathway was deduced.
Abbreviations
- ANOVA:
-
analysis of variance
- AR 274:
-
Acid Red 274
- AOPs:
-
advanced oxidation processes
- CCD:
-
central composite design
- CWAO:
-
catalytic wet air oxidation
- GC-MS:
-
gas chromatography–mass spectrometry
- RSM:
-
response surface methodology
- WAO:
-
wet air oxidation
- TOC:
-
total organic carbon.
References
Wang A, Qu J, Liu H, Ge J: Degradation of azo dye acid red 14 in aqueous solution by electrokinetic and electrooxidation process. Chemosphere 2004, 55: 1189–1196. 10.1016/j.chemosphere.2004.01.024
Verma AK, Dash R, Bhunia P: A review on chemical coagulation/flocculation technologies for removal colour from textile wastewaters. J Environ Manage 2012, 93: 154–168. 10.1016/j.jenvman.2011.09.012
Martinez SS, Uribe EV: Enhanced sonochemical degradation of azure B dye by the electro-Fenton process. Ultrason Sonochem 2012, 19: 174–178. 10.1016/j.ultsonch.2011.05.013
Lei L, Dai Q, Zhou M, Zhang X: Decolorization of cationic red X-GRL by wet air oxidation: performance optimization and degradation mechanism. Chemosphere 2007, 68: 1135–1142. 10.1016/j.chemosphere.2007.01.075
Zhang Z, Zheng H: Optimization for decolorization of azo dye acid green 20 by ultrasound and H2O2 using response surface methodology. J Hazard Mater 2009, 172: 1388–1393. 10.1016/j.jhazmat.2009.07.146
Muruganandham M, Swaminathan M: Solar photocatalytic degradation of a reactive azo dye in TiO2–suspension. Sol. Energy. Mat. Sol. Cells 2004, 81: 439–457. 10.1016/j.solmat.2003.11.022
Zhou M, He J: Degradation of azo dye by three clean advanced oxidation processes: wet oxidation, electrochemical oxidation and wet electrochemical oxidation-A comparative study. Electrochem. Acta. 2007, 53: 1902–1910. 10.1016/j.electacta.2007.08.056
Zollinger H: Color chemistry—synthesis, properties and application of organic dyes and pigments. VCH Publisher, New York; 1991.
Kayan B, Gözmen B: Degradation of Acid Red 274 using H 2 O 2 in subcritical water: application of response surface methodology. J Hazard Mater 2012, 201–202: 100–106.
Devi LG, Kumar SG, Reddy KM, Munikrishnappa C: Photo degradation of methyl orange an azo dye by advanced Fenton process using zero valent metallic ion: influence of various reaction parameters and its degradation mechanism. J Hazard Mater 2009, 164: 459–467. 10.1016/j.jhazmat.2008.08.017
Erdemoglu S, Karaaslan S, Sayılkan F, Izgi B, Asiltürk M, Sayılkan H, Frimmel F, Güçer Ş: Photocatalytic degradation of Congo Red by hydrothermally synthesized nanocrystalline TiO 2 and identification of degradation products by LC–MS. J Hazard Mater 2008, 155: 469–476. 10.1016/j.jhazmat.2007.11.087
Özcan A, Oturan MA, Oturan N, Şahin Y: Removal of Acid Orange 7 from water by electrochemically generated Fenton's reagent. J Hazard Mater 2009, 163: 1213–1220. 10.1016/j.jhazmat.2008.07.088
Söğüt OÖ, Akgün M: Removal of CI basic blue 41 from aqueous solution by supercritical water oxidation in continuous-flow reactor. J of Industrial and Eng Chem 2009, 15: 803–808. 10.1016/j.jiec.2009.09.003
Tang C, Chen V: The photocatalytic degradation of reactive black 5 using TiO2/UV in an annular photoreactor. Water Res 2004, 38: 2775–2781. 10.1016/j.watres.2004.03.020
Bhargava SK, Tardio J, Prased J, Folger K, Akolekar DB, Grocott SC: Wet oxidation and catalytic wet oxidation. Ind. Eng. Chem. Res. 2006, 45: 1221–1258. 10.1021/ie051059n
Kim SC, Park HH, Lee DK: Pd-Pt/Al2O3 bimetallic catalysts for the advanced oxidation of reactive dye solutions. Catal. Today 2003, 87: 51–57. 10.1016/j.cattod.2003.10.008
Chang DJ, Chen IP, Chen MT, Lin SS: Wet air oxidation of a reactive dye solution using CoAlPO 4 -5 and CeO 2 catalysts. Chemosphere 2003, 52: 943–949. 10.1016/S0045-6535(03)00302-3
Rajeshwar K, Ibanez JG, Swain GM: Electrochemistry and the environment. J Appl Electrochem 1994, 24: 1077–1091.
Mishra VS, Mahajani VV, Joshi JB: Wet air oxidation. Ind. Eng. Chem. Res. 1995, 34: 2–48. 10.1021/ie00040a001
Copa WM, Gitchel WB: Wet oxidation standard handbook of hazardous waste treatment and disposal. Mc Graw-Hill, New York; 1989.
Laughlin RGW, Gallo T, Robey H: Wet air oxidation for hazardous waste control. J Hazard Mater 1983, 8: 1–9. 10.1016/0304-3894(83)80033-8
Zimmermann FJ: Wet oxidation of hazardous organics in wastewaters. US Patent 1950. 2665249 2665249
Kim KH, Ihm SK: Heterogeneous catalytic wet air oxidation of refractory organic pollutants in industrial wastewaters: a review. J Hazard Mater 2011, 186: 16–34. 10.1016/j.jhazmat.2010.11.011
Luck F: A review of industrial catalytic wet air oxidation processes. Catal. Today 1996, 27: 195–202. 10.1016/0920-5861(95)00187-5
Luck F: Wet air oxidation: past, present and future. Catal. Today 1999, 53: 81–91. 10.1016/S0920-5861(99)00112-1
Rauf MA, Marzouki N, Körbahti BK: Photolytic decolorization of Rose Bengal by UV/H 2 O 2 and data optimization using response surface method. J Hazard Mater 2008, 159: 602–609. 10.1016/j.jhazmat.2008.02.098
Box GEP, Draper NR: Empirical model-building and response surfaces. Wiley, New York; 1978.
Draper N, John JA: Response surface design for quantitative and qualitative variables. Technometrics 1988, 30: 423–428. 10.1080/00401706.1988.10488437
Somayajula A, Asaithambi P, Susree M, Matheswaran M: Sonoelectrochemical oxidation for decolorization of reactive red 195. Ultrasonics Sonochem 2011. 10.1016/j.ultsonch.2011.12.019
Myers RH: Response surface methodology. Allyn and Bacon, Boston; 1971.
Montgomery DC: Design and analysis of experiments. 4th edition. Wiley, New York; 1996.
Singh KP, Gupta S, Singh AK, Sinha S: Optimizing adsorption of crystal violet dye from water by magnetic nanocomposite using response surface modeling approach. J Hazard Mater 2011, 186: 1462–1473. 10.1016/j.jhazmat.2010.12.032
Sahu JN, Acharya J, Meikap BC: Response surface modeling and optimization of chromium (VI) removal from aqueous solution using tamarind wood activated carbon in batch process. J Hazard Mater 2009, 172: 818–825. 10.1016/j.jhazmat.2009.07.075
Özer A, Gürbüz G, Çalımlı A, Körbahti BK: Biosorption of copper(II) ions on Enteromorpha prolifera: application of response surface methodology (RSM). Chem. Eng. Journal 2009, 146: 377–387. 10.1016/j.cej.2008.06.041
Moghaddam SS, Alavi Moghaddam MR, Arami M: Coagulation/flocculation process for dye removal using sludge from water treatment plant: optimization through response surface methodology. J Hazard Mater 2010, 175: 651–657. 10.1016/j.jhazmat.2009.10.058
Myers RH, Montgomery DC: Response surface methodology: process and product optimization using designed experiments. 2nd edition. Wiley, New York; 2002.
Moghaddam SS, Alavi Moghaddam MR, Arami M: Response surface optimization of acid red 119 dye from simulated wastewater using Al based waterworks sludge and polyaluminium chloride as coagulant. J. Environ. Manag. 2011, 92: 1284–1291. 10.1016/j.jenvman.2010.12.015
Körbahti BK, Tanyolaç A: Continuous electrochemical treatment of simulated industrial textile wastewater from industrial components in a tubular reactor. J Hazard Mater 2009, 170: 771–778. 10.1016/j.jhazmat.2009.05.032
Lim CL, Morad N, Teng TT, Norli I: Chemical oxygen demand (COD) reduction of a reactive dye wastewater using H 2 O 2 /pyridine/Cu(II) system. Desalination 2011, 278: 26–30. 10.1016/j.desal.2011.04.069
Brereton RG: Chemometrics: data analysis for the laboratory and chemical plant. Wiley, New York; 2003.
Guaracho VV, Kaminari NMS, Ponte MJJS, Ponte HA: Central composite experimental design applied to removal of lead and nickel from sand. J Hazard Mater 2009, 172: 1087–1092. 10.1016/j.jhazmat.2009.07.100
Olmez-Hanci T, Kartal Z, Arslan-Alaton İ: Electrocoagulation of commercial naphthalene sulfonates: process optimization and assessment of implementation potential. J Environ Manage 2012, 99: 44–51.
Kasiri MB, Khataee AR: Photooxidative decolorization of two organic dyes with different chemical structures by UV/H 2 O 2 process: experimental design. Desalination 2011, 270: 151–159. 10.1016/j.desal.2010.11.039
Kayan B, Gözmen B, Demirel M, Gizir AM: Degradation of acid red 97 dye in aqueous medium using wet oxidation and electro-Fenton techniques. J Hazard Mater 2010, 177: 95–102. 10.1016/j.jhazmat.2009.11.076
Joklegar HS, Samant SD, Joshi JB: Kinetics of wet air oxidation of phenol and substituted phenols. Water Res 1991, 25: 135–145. 10.1016/0043-1354(91)90022-I
Chang S, Liu Y: Degradation mechanism of 2,4,6-trinitrotoluene in supercritical water. J Environ Sci 2007, 19: 1430–1435. 10.1016/S1001-0742(07)60233-2
Legrini O, Oliveros E, Braun AM: Photochemical process for water treatment. Chem. Rev. 1993, 93: 671–698. 10.1021/cr00018a003
Mantzavinos D, Hallenbrand R, Livingston AG, Metcalfe IS: Wet oxidation as a pretreatment method for wastewaters contaminated by bioresistant organics. Water. Sci. Techol. 1997, 36: 109–116.
Suresh KB, James T, Jaidev P, Karl F, Deepak BA, Stephen CG: Wet oxidation and catalytic wet oxidation. Ind. Eng. Chem. Res. 2006, 45: 1221–1258. 10.1021/ie051059n
Gözmen B, Kayan B, Gizir AM, Hesenov A: Oxidative degradation of reactive blue 4 by different advanced oxidation methods. J Hazard Mater 2009, 168: 129–136. 10.1016/j.jhazmat.2009.02.011
Alaton IA: A review of effects of dye-assisting chemicals on advanced oxidation of reactive dyes in wastewater. Color. Technol. 2003, 119: 345–353. 10.1111/j.1478-4408.2003.tb00196.x
Tromans D: Modelling oxygen solubility in water and electrolyte solutions. Ind. Eng. Chem. Res. 2000, 39: 805–812. 10.1021/ie990577t
Acknowledgments
We wish to thank Dr. Belgin Gözmen for GC-MS analysis and other supports. We also would like to thank Prof. Dr. A. Murat Gizir for the ever positive support and for the continuous encouragement.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
BK conceived of the study, coordinated in the research, and participated in the experimental design and optimization. MD participated in the degradation experiments, the preparation of graphs and tables, and in the statistical analysis of the data. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Demirel, M., Kayan, B. Application of response surface methodology and central composite design for the optimization of textile dye degradation by wet air oxidation. Int J Ind Chem 3, 24 (2012). https://doi.org/10.1186/2228-5547-3-24
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2228-5547-3-24