Abstract
In this research, a sol–gel process was used to synthesize zirconia-alumina (ZrO2-Al2O3) composite nanopowders containing 10, 20, and 50 mol% alumina (Al2O3) without any stabilizer. Zirconium acetate hydroxide and aluminum isopropoxide were employed as precursors. The produced composite nanopowders were characterized using a Brunauer-Emmett-Teller (BET) surface area analyzer, Fourier transform infrared (FTIR) spectroscopy, thermogravimetric/differential thermal analysis, X-ray diffraction analysis (XRD), and scanning electron microscopy (SEM). The BET results showed a decrease in surface area and average particle size and an increase in pore size with increasing Al2O3 content. FTIR, in corroboration with XRD, confirms the crystallization of corundum (α-Al2O3) as one of the alumina phases. The X-ray studies showed that an increase in Al2O3 content shifted the tetragonal zirconia (t-ZrO2)-phase crystallization to higher temperatures. Microstructural characterization by SEM depicted that the particles tend to be more agglomerated with increasing Al2O3 content.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Zirconia (ZrO2) has numerous unique properties such as good mechanical strength due to its high toughness, excellent resistance against crack propagation, good thermal resistance, relatively high thermal expansion coefficient, and low thermal conductivity at high temperature[1], which have proven to be superior to other ceramics. Based on these properties, ZrO2 nanopowders not only have many industrial applications as ceramic bodies, but also can be dispersed as reinforcement in various composite matrixes in order to improve their mechanical properties such as hardness and wear resistance. Studies on the dispersion of ZrO2 nanopowders, which are well known to be present at a low specific surface area, in various matrixes have been reported in literatures[2–6]. In some cases, wear resistance and also barrier properties of sol–gel-derived coatings were improved by dispersing ZrO2 nanopowders in the hybrid matrixes[2]. Wang et al.[3] incorporated the ZrO2 nanopowders into poly methyl methacrylate to overcome some of its drawbacks such as poor heat resistance and weak mechanical surface. Yang et al.[4] reported the highest hardness and the maximum resistance for cracking in Ce-TZP/Al2O3 composites (TZP (tetragonal ZrO2 polycrystal)) containing 20% ZrO2 nanoparticles. Moreover, several attempts have been made for the combination of hydroxyapatite with yttria-stabilized ZrO2 nanopowders in order to improve its mechanical properties[5, 6].
It is well known that the magnitude of improvement of the mechanical properties depends strongly on the composition, size, and specific surface area of the nanopowders. Therefore, the combination of a hard nanostructured material like Al2O3 with a high-toughness ZrO2 matrix looks as a promising way to produce an excellent composite reinforcement and improve the mechanical properties of the matrix such as hardness, wear resistance, and scratch resistance.
The composite nanopowders have been synthesized via various methods such as chemical vapor synthesis, microwave-hydrothermal route, inert gas condensation, laser ablation, and sol–gel processing. The sol–gel method is one of the most promising methods because it offers many advantages for synthesizing high homogeneity and well-controlled properties of nanopowders. Excellent control of the stoichiometry of the precursor solution, customizable microstructure, ease of compositional modification, ease of introducing various function groups of materials, relatively low annealing temperature, and simple and inexpensive equipment are some more advantages of this process[7].
It is also worth mentioning that the ZrO2 has commonly three polymorphs: monoclinic (m-ZrO2, below 1,170°C), tetragonal (t-ZrO2, between 1,170°C and 2,370°C), and cubic (c-ZrO2, above 2,370°C). The high-temperature cubic and tetragonal phases can be stabilized at room temperature by incorporating some dopants such as Y3+ and Ca2+ in the lattice[1]. Whereas, according to our previous studies[8, 9], tetragonal is the stable crystalline phase, without any stabilizer, at low temperatures in nano and submicron structures of ziconia. Moreover, Garvie et al.[1, 10–12] have reported a critical size in ZrO2 powders in which the tetragonal is the stable crystalline phase below that size.
The main aim of the present research was to gain an understanding of the effect of Al2O3 content on the mentioned crystallization and phase transformation in the nanopowders. In addition, the influence of the Al2O3 content was investigated on the size and morphology of the composite powders which are determinative factors for particles as reinforcement of the composite matrix. The ZrO2-Al2O3 unstabilized composite nanopowders were synthesized via a sol–gel process and then were characterized using a Brunauer-Emmett-Teller (BET) surface area analyzer, Fourier transform infrared (FTIR), thermogravimetric/differential thermal analysis (TG/DTA), X-ray diffraction analysis (XRD), and scanning electron microscopy (SEM) techniques. An evaluation on the operation of these composite nanopowders as reinforcement in zirconia matrixes produced via a sol–gel process is planned for future work.
Methods
Materials and processing
The ZrO2 sol was prepared using 3 g of zirconium acetate hydroxide ((CH3CO2)x Zr(OH)y; Sigma-Aldrich Corporation, St. Louis, MO, USA) in 50-ml ethanol (C2H5OH) as precursor. In order to prevent the formation of zirconium hydroxide, 2.5 ml of acetone (CH3COCH3) was added to the solution. A transparent ZrO2 sol was obtained after homogenizing the solution with a magnetic stirrer for 1 h at room temperature.
For preparation of the Al2O3 sol, 4.39 g of aluminum isopropoxide ((C3H7O)3Al; Merck & Co., Inc., Whitehouse Station, NJ, USA) was mixed with 50-ml ethanol at a constant temperature of 80°C, followed by adding 2-ml concentrated nitric acid and 1.5-ml ethyl acetoacetate with stirring for 1 h. The prepared sol also was transparent at room temperature.
The alumina sol was added into the zirconia to prepare ZrO2-m mol% Al2O3 (ZmA hereafter) composite sols, where m was equal to 10, 20, and 50. According to the zeta potential changes of the Z20A composite solution versus pH (Figure1), the pH of the final solution is set to 2.3 by dripping nitric acid. Finally, the composite solutions were homogenized with a magnetic stirrer for 1 h at room temperature. In the end, they were filtered using a 0.22-μm filter.
No precipitate or turbidity was observed after 24 h of aging; then, gelation processes were carried out by instilling commercial ammonia (NH3) at pH = 4.5 (for the Z20A solution). The obtained gels were dried at 120°C for 24 h. The resulting composite powders were milled in air media in a high-density polythene bottle for 48 h using high-purity zirconia balls with a diameter of 5 mm. The heat treatment procedures were applied with a heating rate of 5°C/min up to 1,100°C and a soaking time of 2 h under an air atmosphere and then cooled over night inside the furnace. The summarized process flowchart of nanopowder preparation is illustrated in Figure2.
Characterization
Thermal decomposition and structural evolution of the composite nanopowders after annealing were investigated by TG/DTA (PL-STA-1640, Polymer Laboratories, Stanton Redcraft, UK) and FTIR (FTIR-8400 S with IRsolution, Shimadzu Corporation, Kyoto, Japan). Phase identification and crystallite size of ZmA nanocomposite powders were characterized using a Philips PW1800 X-ray diffractometer (FEI Co., Hillsboro, OR, USA) with nickel-filtered Cu Kα radiation (λ = 1.5406 Å) at 40 kV and 30 mA. The crystallite size of ZmA nanopowders was determined from the X-ray diffraction data using the following the Debye-Scherrer formula (Equation 1):
where d is the crystallite size (in nanometers), k = 0.9 is a correction factor to account for particle shapes, λ is the wavelength of Cu target equals 1.54056 Å, β is the full width at half maximum intensity, and θ is the diffraction peak angle (Bragg's angle). Before calculating the crystallite size, line broadening due to the equipment, βEquip, was subtracted from the obtained peak width, βOb, using Equation 2:
The BET surface area and the pore size distribution of the calcined nanopowders were determined by a BET surface area analyzer (Belsorp-mini 00165 series, BEL Japan, Inc., Osaka, Japan) using N2 as the adsorptive gas. The morphology changes of the composite powders with different Al2O3 contents were examined by a SE-SEM (Wega/Tescan, TESCAN, a.s., Brno, Czech Republic).
Results and discussion
Thermal analysis
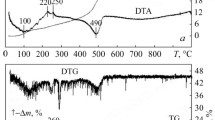
The TG-DT analyses for Z20A powders were carried out in air at a temperature range between 25°C and 1,200°C with a heating rate of 5°C/min, and the resulting profiles are shown in Figure3. The TGA data is compatible with the DTA data. In the first step of the TGA curve, about 8 wt.% is lost at 25°C to 180°C, which was taken to be physically adsorbed H2O and ethanol evaporation from the powders at the same time; the DTA curve shows a negative temperature differential, indicating endothermic reaction. The second step of the TGA curve shows about 32 wt.% loss at 180°C to 400°C; that is considered as the debonding of bonded H2O and the elimination of organic compounds through oxidation. The DTA curve becomes two exothermic reactions at 320°C and 370°C. As the temperature rises, in the last step of the TGA curve, 16 wt.% is lost at 400°C to 460°C. It shows a sharp weight loss accompanied with a significant exothermic peak at 390°C to 470°C in the DTA curve. Possibly, the oxidation of nitro compounds that may be formed by nitration of ethyl acetoacetate and HNO3 is the cause of this huge exothermic reaction. When the samples were annealed above 500°C, no further weight loss of the organics was observed. In the DTA curve, however, the shift of the base line at about 1,000°C shows that thermal energy was produced during the calcination process, which may correspond to form the crystallized t-ZrO2 and/or α-Al2O3 phases in Z20A composite powders, as will be shown later by XRD analysis.
The FTIR at 4,000 to 400 cm−1 for Z20A powders as a function of heat treatment temperature was studied by FTIR spectroscopy, and the results are shown in Figure4. The broadening absorption peak at 3,450 cm−1 belongs to OH-stretching band vibrations. It can be seen that increasing the temperature up to 1,000°C causes a decrease in intensity of this broadening peak, which is due to evaporation of organic compounds and water from the powders. May be due to the moisture adsorbed during the test, the mentioned broad band has appeared even at 1,000°C. The obtained peak at 3,132 cm−1 is caused by the C-H vibration of hydrocarbon groups. All of the samples have an intense peak around 2,341 cm−1 that can be attributed to the vibrations of O = C = O bonds. Zr-OH-group bending band vibration peaks appeared at 1,517 and 1,487 cm−1. A phenomenon yielded from the repulsion of hydrated groups causes the intensity of these peak vibrations to decrease with increasing thermal treatment temperature. Also, the peaks between 1,070 and 612 cm−1 correspond to the Al-O vibration[13], whereas the absorption peaks below 670 cm−1 indicate Zr-O bond vibrations[14]. Heating the gel at 500°C causes broadening of peaks in the lower frequency range (400 to 1,000 cm−1), owing to the overlapping of Zr-O and Al-O infrared vibrations of alumina and zirconia phases[15]. Thus, these individual peaks could not be detected in the nanopowder calcined at 100°C and 500°C. However, the peaks corresponding to Al-O and Zr-O could be clearly observed in the sample calcined at 1,000°C.
Crystallization
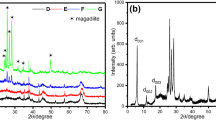
Figures5,6, and7 show XRD patterns of ZrO2-Al2O3 powders containing 10, 20, and 50 mol% Al2O3, respectively, that were synthesized at 500°C, 700°C, 900°C, and 1,100°C. Also, the crystallite size of zirconia and alumina phases calculated from the XRD patterns is listed in Table1.
The XRD patterns indicate that the dried composite gels existed as an amorphous mixture, and heating at temperatures lower than 500°C did not cause crystallization of the gels, except for the Z10A sample, in which some peaks of the t-ZrO2 phase has appeared (Figure5). In all samples, the higher intensity and much sharper peak of the t-ZrO2 peaks were observed with increasing temperature. As shown in Figure6, the t-ZrO2 and α-Al2O3 crystallized phases appeared in Z20A powders, but in the powders containing 50 mol% Al2O3, the t-ZrO2 phase disappeared, and the m-ZrO2 phase crystallized beside the α-Al2O3 phase when heat treatment process was carried out at 1,100°C (see Figure7). It seems that the ZrO2-phase crystallization temperature increases with increasing Al2O3 content, which is also reported by other researchers[16–18]. They suggested that the crystallization constraint by the added Al2O3 would result in higher activation energy for the crystallization process. The peaks of α-Al2O3 were found to appear above 900°C in the Z20A and Z50A powders. However, in these composite powders, the formation of α-Al2O3 was obviously detected at a temperature of 1,100°C (Figures6 and7), but it was not visible for Z10A (Figure5). It may suggest the formation of the tetragonal phase as a solid solution in Z10A nanopowders that has been previously reported by Yoshimura et al.[16] and other researchers[17, 19].
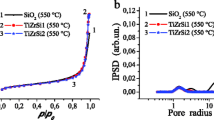
The nitrogen adsorption and desorption isotherm curves of the synthesized ZmA composite nanopowders are shown in Figure8a,b,c. These curves show type-IV hysteresis loops that characterize mesoporous adsorbents. The hysteresis loops in these curves result from wedge-shaped capillaries with a closed edge at the narrower side[20]. The decrease in the difference between the desorption and adsorption curves in the hysteresis loops and their shift to higher relative pressures (P/P0) with increasing amount of alumina may be ascribed to an increase in the pore size, which consequently leads to decreasing the capillary effect.
The specific surface BET areas were obtained from the isotherms for the specimens. Then, the mean pore radii were calculated by the BJH method[21], and the resulting pore size distribution curves are shown in Figure9.
The results obtained from Figures5,6,7,8, and9 are summarized in Table1. According to the data presented in this table, an increase in alumina content leads to a reduction in the surface area of the nanopowders and a decrease in the average crystallite sizes which have been obtained by substituting their XRD results, in Debye-Scherrer's formula (see Equation 1). The median pore size increases by adding alumina in composite nanopowders, which may be due to a decrease in the t-ZrO2 content with high surface area.
It should be pointed that according to Garvie's[8–10] studies, the smaller size particles of the t-ZrO2 phase are more stable at room temperature because of their larger specific surface area. This statement is in a good agreement with the results obtained from Table1. The large area of particles in the Z50A composite nanopowders has caused the disappearance of the t-ZrO2 phase and crystallization of m-ZrO2 beside the α-Al2O3 phase.
Microscopic observation
The SEM observations of ZmA (where m = 10, 20, and 50) composite nanopowders heat-treated at 1,100°C for 2 h are shown in Figure10a,b,c, respectively. From these micrographs, it can be seen that most of the particles are spherical in shape. They tend to be larger and agglomerate with increasing Al2O3 content that confirms the results obtained from BET analysis. The average agglomerated size of particles in Figure10c is about 242 nm which is above the critical size for the composite powders, so the monoclinic is the stable phase.
Conclusion
The sol–gel process has been employed to synthesize unstabilized ZmA (where m = 10, 20, and 50) composite nanopowders using zirconium acetate hydroxide and aluminum isopropoxide as precursors. The results obtained in the present study are as follows:
-
1.
Increasing the Al2O3 content in composite nanopowders shifts the t-ZrO2-phase crystallization toward higher temperatures. Finally, this phase disappeared in Z50A nanopowders, and the α-Al2O3 phase is observed beside the m-ZrO2.
-
2.
Increasing the Al2O3 content results in a decrease in the surface area and crystallite size and an increase in the pore size of nanopowders.
-
3.
The morphology of particles also was affected by Al2O3 content, and increasing that tends to form more agglomerated particles.
References
Shackelford JF, Doremus RH (Eds): Ceramic and glass materials: structure, properties and processing. New York: Springer; 2008.
Zheludkevich ML, Salvado IM, Ferreira MGS: Sol–gel coatings for corrosion protection of metals. J Mater Chem 2005, 15: 5099–5111. 10.1039/b419153f
Wang H, XuP ZW, Shen L, Du Q: Transparent poly (methyl methacrylate)/silica/zirconia nanocomposites with excellent thermal stabilities. Polym Degrad Stab 2005, 87: 319–328. 10.1016/j.polymdegradstab.2004.08.015
Yang G, Li J, Wang G, Yashima M, Min S: Influences of ZrO2 nanoparticles on the microstructure and mechanical behavior of Ce-TZP/Al2O3 nanocomposites. J Mater Sci 2005, 40: 6087–6090. 10.1007/s10853-005-2143-0
Adolfsson E, Shen Z: Densification of zirconia-hydroxyapatite ceramics without phase changes. Key Eng Mater 2006, 309: 1141–1144.
Mobasherpour I, Hashjin MS, Toosi SSR, Kamachali RD: Effect of the addition ZrO2-Al2O3 on nanocrystalline hydroxyapatite bending strength and fracture toughness. Ceram Int 2009, 35: 1569–1574. 10.1016/j.ceramint.2008.08.017
Sakka S, Kozuka H: Handbook of sol–get science and technology: processing, characterization and applications, vol. I sol–gel processing. New York: Kluwer Academic; 2005.
Nouri E, Shahmiri M, Rezaie HR, Talayian F: Investigation of structural evolution and electrochemical behaviour of zirconia thin films on the 316 L stainless steel substrate formed via sol–gel process. Surf Coat Technol 2011, 205: 5109–5115. 10.1016/j.surfcoat.2011.05.024
Nouri E, Shahmiri M, Rezaie HR, Talayian F: A comparative study of heat treatment temperature influence on the thickness of zirconia sol–gel thin films by three different techniques: SWE, SEM and AFM. Surf CoatTechnol 2012, 206: 3809–3815. 10.1016/j.surfcoat.2011.11.030
Garvie RC: The occurrence of metastable tetragonal zirconia as a crystallite size effect. Phys Chem 1965, 69: 1238–1243. 10.1021/j100888a024
Garvie RC, Goss MF: Intrinsic size dependence of the phase transformation temperature in zirconia microcrystals. J Mater Sci 1986, 21: 1253–1257. 10.1007/BF00553259
Garvie RC: Stabilization of the tetragonal structure in zirconia microcrystals. J Phys Chem 1978, 82: 218–224. 10.1021/j100491a016
Chandradass J, Jun B, Bae DS: Effect of different fuels on the alumina–zirconia nanopowder synthesized by sol–gel autocombustion method. J Non–Crystalline Solids 2008, 354: 3085–3087. 10.1016/j.jnoncrysol.2008.02.018
Quan ZW, Wang LS, Lin J: Synthesis and characterization of spherical ZrO2:Eu3+ phosphors by spray pyrolysis process. Mater Res Bull 2005, 40: 810–820. 10.1016/j.materresbull.2005.02.006
Sarkar D, Mohapatra D, Ray S, Bhattacharyya S, Adak S, Mitra N: Synthesis and characterization of sol–gel derived ZrO2 doped Al2O3 nanopowder. Ceram Int 2007, 33: 1275–1282. 10.1016/j.ceramint.2006.05.002
Yoshimura M, Oh ST, Sando M, Niihara K: Crystallization and microstructural characterization of ZrO2 (3 mol% Y2O3) nano-sized powders with various Al2O3 contents. J Alloys Compd 1999, 290: 284–289. 10.1016/S0925-8388(99)00213-3
Balmer ML, Lange FF, Levi CG: Metastable phase selection and partitioning for Zr(1-x)AlxO(2-x/2) materials synthesized with liquid precursors. J Am Ceram Soc 1994, 77: 2069–2075. 10.1111/j.1151-2916.1994.tb07098.x
Balmer ML, Lange FF, Jayaram V, Levi CG: Development of nano-composite microstructures in ZrO2-Al2O3 via the solution precursor method. J Am Ceram Soc 1995, 78: 1489–1494. 10.1111/j.1151-2916.1995.tb08842.x
Ferkel H, Naser J, Riehemann W: Laser-induced solid solution of the binary nanoparticle system Al2O3-ZrO2. Nanostruct Mater 1997, 8: 457–464. 10.1016/S0965-9773(97)00187-6
Jayaseelan DD, Rani DA, Nishikawa T, Awaji H: Powder characteristics, sintering behavior and microstructure of sol–gel derived ZTA composites. J Eur Ceram Soc 2000, 20: 267–275. 10.1016/S0955-2219(99)00165-X
Gregg SJ, Sing KSW: Adsorption, surface area and porosity. New York: Academic; 1982.
Acknowledgments
The authors wish to thanks the financial support of this work by the Iranian Nanotechnology Initiative Council and Iran Research Institute of Petroleum Industry.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
EN carried out the structural studies, participated in the sequence alignment, and drafted the manuscript. MS participated in the sequence alignment and drafted the manuscript. HRR participated in the design of the study and revision of the language of the manuscript. FT conceived the study and participated in its design and coordination. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Nouri, E., Shahmiri, M., Rezaie, H.R. et al. The effect of alumina content on the structural properties of ZrO2-Al2O3 unstabilized composite nanopowders. Int J Ind Chem 3, 17 (2012). https://doi.org/10.1186/2228-5547-3-17
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2228-5547-3-17