Abstract
Background
Benzene, toluene, ethylbenzene and xylenes (BTEX) are a group of volatile aromatic compounds. The human exposure to these compounds can comprise serious health consequences. Due to the toxicological properties of BTEX, there is an interest in the chemical control and consequently analytical procedures to measure these compounds. The objective of the present study was to develop a novel liquid-phase microextraction (LPME) based on solidification of floating organic microdrop coupled with gas chromatography flame ionization detection for pre-concentration and determination of BTEX in the aqueous samples.
Results
Microextraction efficiency factors, such as the effects of type and volume of extraction solvent, stirring rate, extraction time, temperature, and ionic strength, have been investigated and optimized: 15.0-μL 1-undecanol microdrop exposed for 15 min floated on the surface of a 10-mL water sample at 45°C, stirred at 1,200 rpm without adding salt. Under the selected conditions, a pre-concentration factor of 211 to 417, limit of detection of 0.07 to 0.18 μg L−1 (S/N = 3), limit of quantification of 0.22 to 0.49 μg L−1 (S/N = 10), and linearity range of 1.00 to 300 μg L−1 for benzene, 0.10 to 300 μg L−1 for toluene, 0.05 to 150 μg L−1 for ethylbenzene, and 0.02 to 150 μg L−1 for xylenes, with coefficient of determination (0.9979 < r2 < 0.9992), were observed. Relative standard deviation (RSD %) for analysis of 2.0 μg L−1 BTEX compounds in water samples was in the range of 0.9% to 6.4% (n = 5). The relative recovery of different water samples was higher than 79.8%.
Conclusions
The LPME method is a simple, rapid, economic and environmentally friendly technique that can be applied to the trapping of BTEX compounds from aqueous samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
Benzene, toluene, ethylbenzene and xylenes (BTEX) are a group of volatile aromatic compounds. BTEX are made up of naturally occurring chemicals that are found mainly in petroleum products and are widely utilized in fuels, plastics, paints, pesticides, chemical intermediates, and solvents [1]. Therefore, they can be emitted to the environment from an extensive variety of sources. The human exposure to these compounds can comprise serious health consequences like central nervous system problems, cancer, and damaging effects on the respiratory system, kidney, and heart [2, 3]; in order to decrease the human intake of these perilous compounds, a chemical control and, consequently, analytical methods are desirable.
Gas chromatography (GC) is the general technique used for the analysis of BTEX in environmental samples. For the quantification of BTEX in aqueous systems, gas chromatography combined with mass spectrometry (MS) [4–10] and flame ionization detection (FID) [11–15] has been employed in the past. Prior to analysis, for extraction and pre-concentration of target compounds from the aqueous samples, techniques such as liquid-liquid extraction (LLE) and solid-phase extraction have been utilized [16, 17]. However, these methods are time-consuming and need large volumes of organic solvents, which are toxic to human health and the environment, and they are also expensive. Solvent-free sample preparation methods or those employing less organic solvent are becoming increasingly important and have induced a major advance in analytical methodology. Purge and trap [4, 7, 8], headspace solid-phase dynamic extraction [5], headspace solid-phase microextraction [6, 12–14], headspace [9, 18], or direct aqueous injection [10, 15] have been reported as alternative to traditional methods for sample preparation. Liquid-phase microextraction (LPME) has been reported as a new method in which a low amount of solvent as extractant is used [19, 20]. This technique is a simple, low-cost, and rapid procedure requiring lower sample and extractant volumes [21]. Different operational modes have developed from LPME; the most commonly used of which are as follows: single-drop microextraction (SDME) [22], headspace solvent microextraction [11], hollow fiber-based LPME (HF-LPME) [23, 24], directly suspended droplet microextraction [25], ionic liquid-based single-drop microextraction [26], and dispersive liquid-liquid microextraction [27].
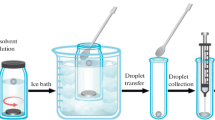
A modified LPME method [28] has been recently employed for pre-concentration of organic compounds in aqueous samples [29–32]. In this technique, a microdrop of the organic solvent is floated on the surface of an aqueous sample while being agitated by a stirring bar in the bulk of the solution. After the completion of the extraction, the sample vial is inserted into an ice bath to freeze the organic phase. The solidified microdrop is then transferred into a conical vial, where it melts immediately. Finally, the GC can be utilized to determine the species in the extractant.
The objective of the present study was to investigate the feasibility of the novel LPME technique based on solid organic drop combined with gas chromatography with flame ionization detection (GC-FID) for analysis of BTEX in water samples. Effects of various influential parameters, such as the type and volume of extraction solvent, stirring rate, extraction time, sample temperature, and ionic strength, have also been examined. The optimized method, after validation, has been applied to different real water samples.
Results and discussion
The suggested LPME method is established on equilibrium between the analyte concentration in the sample and that in the extractant solvent. The thermodynamic and kinetic equations of LLE are previously demonstrated [33]. To optimize the affecting factors in the analysis of BTEX in aqueous samples by the proposed method, the different parameters such as type and volume of solvent, sample stirring rate, sample solution temperature, extraction time, and salt addition were investigated.
Type of extraction solvent
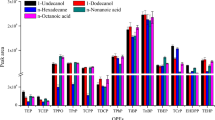
Selection of an appropriate solvent is a crucial parameter in LPME methods. The range of proper solvents for use with the above mentioned method is limited as the solvents must have low volatility and low solubility in water to be immiscible with water and as the analytes must be soluble in it. The solvent's melting point should be near the room temperature [34]. In this study, five different solvents were evaluated, i.e., 1-undecanol, 1-dodecanol, 2-dodecanol, n-hexadecane, and 1-bromohexadecane. Solvent selectivity was evaluated for the extraction of 10 mL of sample containing 2.0 μg L−1 of each BTEX. The stirred solution (400 rpm) was kept at 25°C for 20 min using 15 μL of proper organic solvent and without salt addition. The results depicted in Figure 1 reveal that among the solvents studied, 1-undecanol and 1-dodecanol provide higher extraction efficiency for most of the analytes. Because of low melting point, low vapor pressure, and low water solubility, 1-undecanol was selected as the optimized extraction solvent for further investigations. Moreover, its chromatographic peak was easily separated from the analyte peaks. The extraction efficiency was found on the average peak area of each analyte for three replicate analyses.
Volume of extraction solvent
The influence of microdrop volume on the analytical signal was studied in the range of 10.0 to 30.0 μL. Figure 2 shows that there was a corresponding increase in the analytical signal of target compounds from 10.0 until 20.0 μL of solvent volumes. Based on LLE equations, rate of the analyte migration into the microdrop is directly related to the surface area between the two liquid phases and is inversely related to the organic-phase volume [35]. Thus, an increase in the drop volume raises the interfacial area following the analytical signals. However, further increase in the microdrop volume results in decrease in the analytical signals [36]. Hence, the volume of 15.0 μL was chosen as the optimal solvent volume.
Stirring rate
Stirring intensity is one of the important parameters that improves the extraction efficiency and lowers extraction time. The agitation can regenerate a new sample solution surface, accelerating the mass transfer from the donor phase to the acceptor phase [37, 38]. The optimum stirring rate was evaluated at different stirring rates between 0 and 1,200 rpm. Higher stirring rates were not used because the microdrop was spattered on the vial wall and damaged. Therefore, an optimum stirring rate of 1,200 rpm was selected (Figure 3).
Sample temperature
The effect of sample temperature on extraction efficiency was also investigated between 25°C and 65°C by floating a 1-undecanol microdrop (15.0 μL) for 20 min on the surface of the water samples. It was expected that an increase in temperature leads to an enhancement of the mass transfer coefficients of the goal compounds from the aqueous sample into the microdrop and, consequently, to higher extraction efficiencies. Nevertheless, the results achieved were in incongruity with this anticipation. In fact, a significant reduction in sensitivity was observed for all the analytes at higher temperature values (>45°C). This behavior, which is disclosed in Figure 4, is likely to be the result of two effects. On one side, it has been reported that high temperatures can decrease the partition coefficients of analytes in the microdrop, resulting in a decrease of the rate of analyte adsorption on the outer surface of the microdrop. On the other hand, the sample temperature affects not only the kinetic and thermodynamic of the analyte extraction, but also those of the water molecules in the sample [39, 40]. Thus, optimum extraction temperature was set at 45°C.
Extraction time
As described above, LLE is a type of equilibrium extraction, and the optimal extraction efficiency is obtained when equilibrium is established. Hence, the extraction time performs a very essential role in the whole process. The range of the extraction time investigated was 5 to 35 min with other extraction conditions being constant. Figure 5 shows that there was a corresponding increase in analytical signal from 5 to 15 min, followed by a period of stability. An extraction time of 15 min was selected as a reasonable compromise between enrichment factor and analysis time.
Ionic strength
The ionic strength can be one of the effective parameters on the extraction efficiency. Depending on the target compounds, the presence of salt in aqueous sample can potentially result in contrary effects: it may improve [41], not influence [42], or diminish extraction [24, 43]. Apart from the salting-out effect which improves extraction efficiency, it also leads to alter the physical properties of the Nernst diffusion film in the interface of aqueous and organic phases; consequently, the diffusion rate of analytes into the solvent was reduced [44, 45]. To investigate this effect on the proposed LPME, the extraction was performed with a 10-mL spiked water sample containing various concentrations of NaCl (0% to 30%, w/v). The results show that the peak area of BTEX decreased with increasing salt concentration in the aqueous sample (Figure 6). Therefore, it was decided not to alter the salt content of the sample solutions in the following extractions. Hence, salt was not added in this study.
Method validation
Under the above optimum experimental conditions, method validation in terms of linearity, precision, and limits of detection (LOD) and quantifications (LOQ) for each analyte were determined by the accepted procedure of the U.S. Environmental Protection Agency [46]. The results have been presented in Table 1. The calibration curve was found to be linear in the range of 1.00 to 300 μg L−1 for benzene, 0.10 to 300 μg L−1 for toluene, 0.05 to 150 μg L−1 for ethylbenzene, and 0.02 to 150 μg L−1 for xylenes with a coefficient of determination (r2) of >0.9979 (n = 3). Precision was assessed by determination of repeatability, which expresses the precision of the method under the same operating conditions over a short interval of time. It also identified intraday precision and is explained as relative standard deviation (RSD%). The method repeatability was in the range of 3.8% to 8.6% (n = 5). The LOD (S/N = 3) and LOQ (S/N = 10) of target compounds were in the range of 0.07 to 0.18 μg L−1and 0.22 to 0.49 μg L−1, respectively. Furthermore, the pre-concentration factor was calculated as the ratio of the peak area after LPME method to the peak area of standard solution at the same concentrations [45, 47], which was from 211 to 417 (Table 1).
Application to real samples
Finally, the applicability of the extraction method was evaluated by the analysis of the real samples including tap, mineral, and river water. The results indicated that no BTEX have been detected in analyzed samples using the proposed method. All the real water samples were spiked at two different concentration levels (4.0 and 10.0 μg L−1) to assess the matrix effect. The relative recovery, defined as the peak area ratio of a natural water sample and ultrapure water sample spiked with analytes at the same level, was applied [35]. The relative recoveries of the analytes are given in Table 2, which varies from 79.8% to 101.7%; it shows that the influence of the matrix is not significant on the extraction recoveries. The chromatograms obtained by GC-FID of unspiked river water and those spiked at two concentrations of each analyte after the developed method at optimum conditions are shown in Figure 7.
Conclusion
A comparison of the proposed LLME method and some of the published methods for extraction and determination of BTEX are summarized in Table 3. The recommended LPME provides adequate pre-concentration factor and linear dynamic range, being the least time-consuming method. Precision is similar to that provided by other microextraction techniques reported in the literature. In addition, the LOD is comparable to that reported in most procedures. In conclusion, the LPME method is a simple, rapid, economic, and environmentally friendly technique that can be applied to the trapping of BTEX compounds from aqueous samples.
Experimental
Reagents and chemicals
The BTEX standards (benzene, toluene, ethylbenzene, p-xylene, m-xylene and o-xylene), the organic solvents (HPLC-grade or suprasolv for chromatography), and sodium chloride (analytical reagent grade) were purchased from Merck (Darmstadt, Germany) and Fluka (Buchs, Switzerland). Ultrapure water was prepared by a Milli-Q system (Bedford, MA, USA). An individual stock standard solution of 1,000 mg L−1 for each compound was prepared in methanol and stored at 4°C in the darkness. Stock standard mixture of the BTEX compounds (10 mg L−1) was prepared weekly by diluting the stock solutions with methanol. The working standard solutions, with concentration levels ranging from 0.01 to 400 μg L−1, were made daily by diluting the mixed stock solutions with Milli-Q water to the required concentration.
The tap water sample was freshly collected from our laboratory; the bottled mineral water sample was available at the supermarket, packed in polymeric container (Bisheh); and the river water sample was obtained from the upstream of Jajrood River (a nonresidential and mountainous area at the northeast of Tehran, Iran) in July 2011. The tap and river water samples were collected in glass bottles. The river water sample was filtered through a filter paper before analysis. The water samples were stored in a refrigerator at 4°C until their analysis.
Apparatus
Analysis was performed using an HP 6890 gas chromatograph (Agilent Technologies, Palo Alto, CA, USA) with FID and equipped with the HP Chemstation software (version A.09.01) used for data analysis. The GC column used for analysis was ZB-WAX, 30 m × 0.32 mm × 0.50 μm (Phenomenex, Torrance, CA, USA). Ultrapure-grade helium (99.9999%, Sabalan Co., Iran) was utilized as the carrier gas at a flow rate of 34 mL/min. Hydrogen for FID was UHP-grade, and air was purified using a Domnick Hunter ZA3500 (EST Analytical, Fairfield, OH, USA) with an additional moisture trap with 13X molecular sieve. The oven program increased from 60°C to 75°C at 15°C/min to 90°C at 3°C/min, then held for 3 min, and extended to 190°C at 25°C/min. Inlet and detector temperatures were 225°C and 300°C, respectively. FID conditions consisted of a hydrogen flow of 40 mL/min, airflow of 450 mL/min, and a helium makeup flow of 40 mL/min. Injection volumes were 2.0 μL with a split of 20:1, and injector temperature was set at 210°C. The solution was stirred with a magnetic heater-stirrer (Heidolph MR 3001 K, Heidolph Instruments, Schwabach, Germany). A simple water bath placed on the heater-stirrer was used for controlling the temperature of the sample solutions.
Methods
An aqueous solution (10 mL) containing a mixture of the BTEX compounds (2.0 μg L-1) was introduced into a vial containing a magnetic stirrer. Then, 15.0 μL of an immiscible extracting solvent (1-undecanol) was dropped onto the surface, and then, the vial was sealed with an aluminum seal with a PTFE septum and stirred for 15 min at 45°C. Subsequently, the sealed vial was placed in an ice bath. After a short time, the organic extractant solidified and was transferred to a clean conical vial where it melted quickly. Finally, 2.0 μL of the extractant was removed for analysis by GC-FID.
References
Alberici RM, Zampronio GC, Poppi RJ, Eberlin MN: Analyst. 2002, 127: 230–234.
Alegretti AP, Thiesen FV, Maciel GP: J Chromatogr B. 2004, 809: 183–187.
Irwin RJ, Van Mouwerik M, Stevens L, Seese MD, Basham W: Environmental contaminants encyclopedia. National Park Service, Fort Collins; 1997.
Bianchi F, Careri M, Marengo E, Musci M: J Chromatogr A. 2002, 975: 113–121.
Jochmann MA, Kmiecik MP, Schmidt TC: J Chromatogr A. 2006, 1115: 208–216.
Nakamura S, Daishima S: Anal Chim Acta. 2005, 548: 79–85.
Rosell M, Lacorte S, Ginebreda A, Barcel’o D: J Chromatogr A. 2003, 995: 171–184.
Tanabe A, Tsuchida Y, Ibaraki T, Kawata K, Yasuhara A, Shibamoto T: J Chromatogr A. 2005, 1066: 159–164.
Lin Z, Wilson JT, Fine DD: Environ Sci Technol. 2003, 37: 4994–5000.
Zwank L, Schmidt TC, Haderlein SB, Berg M: Environ Sci Technol. 2002, 36: 2054–2059.
Bahramifar N, Yamini Y, Shariati-Feizabadi S, Shamsipur M: J Chromatogr A. 2004, 1042: 211–217.
Robert S, Dungan S: Anal Lett. 2005, 38: 2393–2405.
Dron J, Garcia R, Millan E: J Chromatogr A. 2002, 963: 259–264.
Ji J, Deng C, Shen W, Zhang X: Talanta. 2006, 69: 894–899.
Kubinec R, Adamuscin J, Jurdakova H, Foltin M, Ostrovsky I, Kraus A, Sojak L: J Chromatogr A. 2005, 1084: 90–94.
Beketov VI, Parchinski VZ, Zorov NB: J Chromatogr A. 1996, 731: 65–73.
Stan HJ, Kirsch NH: Int J Environ Anal Chem. 1995, 60: 33–40.
Perez Pavon JL, del Nogal Sanchez MD, Garcia Pinto C, Fernandez Laespada ME, Moreno Cordero B: Anal Chem. 2006, 78: 4901–4908.
Liu H, Dasgupta PK: Anal Chem. 1996, 68: 1817–1821.
Jeannot MA, Cantwell F: Anal Chem. 1996, 68: 2236–2240.
Ye C, Zhou Q, Wang X: J Chromatogr A. 2007, 1139: 7–13.
Psillakis E, Kalogerakis N: Trends Anal Chem. 2002, 21: 54–64.
Sarafraz-Yazdi A, Amiri AH, Es’haghi Z: Chemosphere. 2008, 71: 671–676.
Shen G, Lee HK: Anal Chem. 2002, 74: 648–654.
Sarafraz-Yazdi A, Amiri AH, Es'hagi Z: Talanta. 2009, 78: 936–941.
Herrador EA, Lucena R, Cardenas S, Valcarce M: J Chromatogr A. 2008, 1201: 106–111.
Assadi Y, Ahmadi F, Milani Hossieni MR: Chromatographia. 2010, 71: 1137–1141.
Khalili Zanjani MR, Yamini Y, Shariati S, Jonsson JA: Anal Chim Acta. 2007, 585: 286–293.
Faraji H, Saber Tehrani M, Husain SW: J Chromatogr A. 2009, 1216: 8569–8574.
Khalili Zanjani MR, Yamini Y, Yazdanfar N, Shariati S: Anal Chim Acta. 2008, 606: 202–208.
Farahani H, Norouzi P, Dinarvand R, Ganjali MR: J Sep Sci. 2009, 32: 314–320.
Dai L, Cheng J, Matsadiq G, Liu L, Li JK: Anal Chim Acta. 2010, 674: 201–205.
Chen X, Zhang T, Liang P, Li Y: Microchim Acta. 2006, 155: 415–420.
Leong MI, Huang SD: J Chromatogr A. 2008, 1211: 8–12.
Zhao L, Lee HK: J Chromatogr A. 2001, 919: 381–388.
Bagheri H, Khalilian F: Anal Chim Acta. 2005, 537: 81–87.
Psillakis E, Kalogerakis N: J Chromatogr A. 2001, 907: 211–219.
Helena P, Locija Zupan IK: Trends Anal Chem. 1999, 18: 272–282.
Aguilera-Herrador E, Lucena R, Cardenas S, Valcarcel M: J Chromatogr A. 2008, 1201: 106–111.
Mottola HA: Analyst. 1993, 118: 675–679.
Kramer KE, Andrews ARJ: J Chromatogr B. 2001, 760: 27–36.
Ugland HG, Krogh M, Rasmussen KE: J Chromatogr B. 2000, 749: 85–92.
Psillakis E, Kalogerakis N: J Chromatogr A. 2003, 999: 145–153.
Bagheri H, Saber A, Mousavi SR: J Chromatogr A. 2004, 1046: 27–33.
Lambropoulou AD, Albanis TA: J Chromatogr A. 2005, 1072: 55–61.
U.S. Environmental Protection Agency: Primary drinking-water regulations, maximum contaminant levels. In: Appendix B to part 136, National primary drinking-water regulations. U.S. Code of Federal Regulations, Washington, D.C; 1990. Title 40, parts 100–149, revised as of July 1
Meng L, Wang B, Luo F, Shen G, Wang Z, Guo M: Forensic Sci Int. 2011, 209: 42–47.
Sarafraz-Yazdi A, Piri moghadam H, Es'haghi Z, Sepehr S: Anal Methods. 2010, 2: 746–752.
Sarafraz-Yazdi A, Amiri AH, Es'hagi Z: Intern J Environ Anal Chem. 2010, 90: 1036–1047.
Vickackaite V, Pusvaskiene E: J Sep Sci. 2009, 32: 3512–3520.
Acknowledgements
The authors gratefully acknowledge the financial support provided by the Research Council of the Varamin Branch, Islamic Azad University to carry out this project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
HF initiated the study, prepared the design of the study and supervised the acquisition of the data carried out the data analysis, prepared the graphics and prepared the manuscript for submission, review and final acceptance. RMAT carried out the experimental studies and prepared the first draft of the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Faraji, H., Tehrani, R.M.A. Liquid-phase microextraction based on the solidification of a floating organic microdrop for trace analysis of BTEX in the aqueous sample. Int J Ind Chem 3, 12 (2012). https://doi.org/10.1186/2228-5547-3-12
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2228-5547-3-12