Abstract
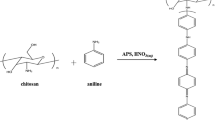
Polyaniline (PAni) as a conductive polymer and chitosan (CS) as a natural polymer have been reacted with formaldehyde as a grafting agent and potassium persulfate as an initiator. The effect of using specific primer, different ratios of monomers, and the solubility of synthesized copolymer has been studied and analyzed here. This new copolymer was characterized by Fourier transform infrared spectroscopy (FT-IR), UV-visible, scanning electron microscopy (SEM), and differential scanning calorimetry (DSC) techniques.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
Conducting polymers such as polyaniline (PAni) have drawn considerable interest for their wide applications. PAni is one of the most attractive conducting polymers due to its high conductivity and good stability. PAni as a conductive polymer has attracted the most of the attentions due to its simple preparation and doping procedure, good environmental stability, relatively high conductivity, and low cost and also due to its wide spectrum of applications.
The nanofibrillar morphology significantly improves the performance of polyaniline in many conventional applications involving polymer interactions with its environment. The highly conjugated polymeric structure of polyaniline produces new nanoscale phenomena that are not accessible with current inorganic systems. Most studies in the field were devoted to the preparation of PAni/noble metal composites. Conducting polymers find applications in the fields like sensors, electrocatalysts, microelectronics, electromagnetic shielding, rechargeable batteries, and controlling systems [1–4].
Because of these problems, many methods are reported. Grafting with other polymers having good mechanical and physical properties is one of them. Many potential applications of PAni are demonstrated recently [5–8]. The polymers should be insoluble in the whole range of pH to gain some of the applications. One way to overcome these problems is chemical grafting of PAni onto the radiation cross-linked chitosan (CS). PAni is of great interest because of its good conductivity and ease of formation, which is a good candidate for this research. CS is a polysaccharide derived from the chitin of crustaceans. The properties of cationic polysaccharides are the low toxicity and good biocompatibility that make them interesting for study as a drug excipient [9]. The nitrogen electrons in the amino and N-acetyl amino groups can establish dative bonds with transition metal ions, and some hydroxyl groups in CS may function as donors [10]. CS can chelate metal ions by itself, especially those listed transition metals. It can apply as a matrix for immobilization of enzymes too [11]. Due to its physical and chemical properties, CS is used in different products such as pharmaceutical and cosmetic products to water treatment and plant protection. In different applications, different properties of CS are required [12]. Therefore, the aim of this study is to improve the physico-chemical properties of PAni by varying the grafting ratio of CS. Because of these reasons, the results were investigated by Fourier transform infrared spectroscopy (FT-IR), UV-visible (UV-vis), scanning electron microscopy (SEM), and differential scanning calorimetry (DSC) techniques.
Methods
The CS, acetic acid, hydrochloric acid (37% purification), N-methyl pyrrolidine (NMP), and ammonium persulfate (APS) with 98% purification were prepared from Merck Company (Whitehouse Station, NJ, USA) and used without further purification.
All infrared spectra were obtained from samples in KBr pellets using a Bruker Tensor27 Ger FT-IR spectrophotometer (Ettlingen, Germany). 1H NMR spectrum was taken using a Bruker WP 200 SY spectrometer operating at 250 MHz in DMSO. To review the samples, UV-vis has been used (model PerkinElmer Lamer Lambda25, Waltham, MA, USA) too. SEM instrument model was XL30, Philips, Holland, The Netherlands. Also, the thermal behavior of the polymers was obtained from the STA S-1500 Scinco model that is made in Korea. Finally, the samples were mixed together with Alfa magnetite stirrer (model: Hs-860, Karlsruhe, Germany).
Preparation of polyaniline
One milliliter of freshly distilled aniline was dissolved in 30 ml of hydrochloric acid (1 M) and was brought to temperature of 4°C to 0°C by ice bath. Then, a certain amount of KPS was added to 20 ml of hydrochloric acid (1 M) and stirred. The prepared solution was added to the first solution drop by drop. A few minutes after injection of the oxidant, the solution turned bluish green color. The color intensity of the reaction was greater by the time. After 5 h of stirring, the solution was filtered through a filter paper. Greenish black deposits remaining on the filter paper were washed with water and dried in an electric oven at 60°C for 12 h finally. Quantitative details of this process are given in Table 1.
Preparation of copolymer
A certain amount of CS was solved in 20-ml acetic acid solution (2% w/w) and stirred for 1 h. Then, 10 ml of KPS solution (as described previously) was added to the first solution drop by drop at 5°C. Again, a solution of certain amount of freshly distilled aniline in 10 ml of distilled water was prepared and was added to the final solution drop by drop over 20 min. Stirring continued for 12 h. Then, the solution was filtered and dried. Dark green to black powder is the expected copolymer.
Grafting of copolymer
A certain amount of chitaline (as described previously) was dissolved in 20 ml of 2 M ammonia, stirred at room temperature for 1 h and then was filtered. The precipitate was dried in an oven at 60°C for 3 h. Then, the obtained solid was stirred with 2 ml of 1 M acetic acid until fully dissolved. Thus, by adding excess amounts of acetic acid, the pH raised to 4. Meanwhile, by adding drop by drop a certain amount of formaldehyde, the grafting process is started. Stirring was continued for 12 h at room temperature. The solid was filtered, washed with distilled water, and dried at 60°C for 12 h. A dark green powder was obtained including the chitosan-graft-polyaniline (chit-g-c-PAni). Different ratios of aniline and formaldehyde were examined.
Results and discussion
1H NMR analysis of CS-graft-PAni copolymer
The 1H NMR spectrum confirmed the grafting of PAni chain onto CS. In Figure 1, the 1H NMR spectrum of the final CS-graft-PAni copolymers, marked peak at 1.71 ppm, belongs to the CH2 protons along the chain. The wide peak at 2.5 ppm indicates the CH protons of CS that have overlaps with protons in the DMSO. The wide peak at 3.7 ppm is the especial peak of hydroxyl groups along the chain that are overlapping with H2O. There is also a broad peak in the region between 6.97 and 7.69 ppm belonging to the protons of aromatic ring and benzene groups in PAni. All these evidences affirmed the structure of CS-graft-PAni copolymers.
Fourier transform infrared analysis of PAni and CS-graft-PAni copolymer
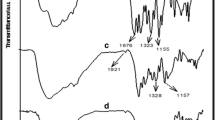
The FTIR spectrum of PAni is shown in Figure 2. The peak at 3,452 cm−1 is related to N-H stretching vibrations, the area of 1,560 to 1,650 cm−1 is related to NH flexural vibrations, and the peak at 1,560 cm−1 is attributed to quinoid (Q) ring vibrations of PAni. The peak at 1,481 cm−1 is due to benzoid vibrations. Also, the vibrations of N = Q = N have characteristic absorption at 1,132 cm−1. Aromatic C-N stretching vibrations appear at 1,298 cm−1, and the peak at 504 to 879 cm−1 is due to CH bending vibrations of substituted benzene ring.
Various vibrations of final grafted product (Figure 3) are analyzed too. The wide peak which is observed in the area of 3,200 to 3,500 cm−1 is due to the overlapping between O-H stretching vibrations in CS and NH stretching vibrations in PAni. Also, there are peaks at the areas of 2,858 and 2,925 cm−1 which are due to CH stretching vibrations, and the peak at 1,636 cm−1 is due to C = O stretching vibrations of CS. The area at 11,459 cm−1 belongs to the C = C and C = N stretching vibrations in benzoid rings of PAni. Stretching vibrations of the aromatics is approximately at 1,301 cm−1.
As can be seen, stretching vibrations of N = Q = N, after linking CS with PAni, have shifted from 1,132 to 1,040 cm−1. NH vibration of CS was seen in the region around the peak at 1,655 cm−1 too.
TGA-DSC and SEM characterization of CS-graft-PAni copolymer
Figure 4 shows the STA spectrum of the graft copolymer. As can be seen, the polymer begins to soften from the temperature about 50°C, and it continues until the temperature of 210°C. This issue is caused by the loosening of connections between PAni and CS (Figure 5). At this point, the player loses almost 10% of its weight. This mass loss can be due to loss of moisture and solvent of polymer. Degradation begins at 210°C and continues to 500°C. The reason is PAni and CS chain degradation that caused to 50% mass reduction. Complete degradation occurs above 500°C temperatures that continue to 600°C. Instead of burning, the polymer remains less than 10% ash.
Conclusion
The information on 1H NMR, FT-IR, STA, and SEM spectra were derived from the structure of the synthesized copolymer. Solubility test results showed that the synthesized copolymer link is sparingly soluble in solvents tested; also, the electrical conductivity of the grafted copolymer is less than the Ani with unclear reason. Besides the chemical structure, many factors including the morphology and shape of the polymer chain may affect the electrical conductivity; also, the molar ratio of Ani and APS affects the electrical conductivity of the synthesized copolymer as it is shown in Table 1. The electrical conductivity increases when the initiator amount is greater than the Ani. In general, high value of initiator or high temperature has a positive effect on the polymerization rate, but it reduces the degree of polymerization. Also, half of the CS amount led to a slight increase in electrical conductivity, but the quality of the synthesized copolymer would not be good. It is known that the electrical properties of the conducting polymer are dependent on the size and shape of the particles.
References
Sajjad Sedaghat and Fariba Golbaz: In situ oxidative polymerization of aniline in the presence of manganese dioxide and preparation of polyaniline/MnO 2 nanocomposite. J. Nanostruct. Chem 2013, 3: 65–68. http://www.jnanochem.com/content//
Chen CH: Thermal and mechanical properties of PVDF/PANI blends. J. Appl. Polym. Sci 2003, 89: 2142–2148. 10.1002/app.12361
Yin W, Li J, Li Y, Wu Y, Gu T: Conducting IPN based on polyaniline and crosslinked cellulose. Polym. Int 1997, 42: 276–280. 10.1002/(SICI)1097-0126(199703)42:3<276::AID-PI718>3.0.CO;2-F
Lee YM, Kim JH, Kang JS, Ha SY: Grafting of polyaniline onto the radiation crosslinked chitosan. Macromolecules 2000, 33: 7341.
Shibaev PV, Schaumburg K, Bjornholm T, Norgaard K: Conformation of polythiophene derivatives in solution. Synth. Met 1998, 97: 97–104. http://www.sciencedirect.com/science/article/pii/S0379677998000939 10.1016/S0379-6779(98)00093-9
Liu L, Li Y, Liu H, Fang Y: Synthesis and characterization of chitosan-graft-polycaprolactone copolymers. Eur. Polym. J 2004, 40: 2739–2744. 10.1016/j.eurpolymj.2004.07.016
Lee YM, Nam SY, Ha SY: Application of the Pervaporation Process to Separate Azeotropic Mixtures. J. Membr. Sci 1999, 159: 41–46. 10.1016/S0376-7388(99)00051-4
Agbor NE, Petty MC, Monkman AP: Pervaporation of water/isopropanol mixtures through polyaniline membranes doped with poly acrylic. Sens. Actuators B 1995, 28: 173. 10.1016/0925-4005(95)01725-9
Yang S, Tirmizi SA, Burns A, Barney AA, W. Chitaline materials: Soluble Chitosan-polyaniline copolymers and their conductive doped forms. Synth. Met 1989, 32: 191–200. 10.1016/0379-6779(89)90841-2
Ismail YA, Shin SR, Shin KM, Yoon SG, Shon K, Kim SI: S, Electrochemical actuation in chitosan/polyaniline microfibers for artificial muscles fabricated using an in situ polymerization. J. Kim, Sens. Actuators B: Chem 2008, 129: 834–840. 10.1016/j.snb.2007.09.083
Shi Q-H, Tian Y, Dong X-Y, Bai S, Sun Y: Chitosan coated silica as immobilized metal affinity support for protein adsorption. Biochem. Eng. J 2003,16(3):317–322. 10.1016/S1369-703X(03)00095-0
Sedaghat S: Synthesis and evaluation of chitaline nanocomposite. J. Nanostruct. Chem 2011,2(1):53–57.
Acknowledgements
We warmly thank islamic azad university for any supports.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
As we can see in the results the synthesized copolymer has improved properties and can be develpoed in future for some applications.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Sedaghat, S. Synthesis and characterization of new biocompatible copolymer: chitosan-graft-polyaniline. Int Nano Lett 4, 2 (2014). https://doi.org/10.1186/2228-5326-4-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2228-5326-4-2