Abstract
Herein, the interaction of hydrogen sulfide with inside and outside single-wall carbon nanotube of (5,0) and (5,5) is investigated using density functional theory at B3LYP/6-31G* level of theory in the gaseous phase by Gaussian 09. The adsorption energies, thermodynamic properties, highest occupied molecular orbital, lowest unoccupied molecular orbital, energy gaps, and partial charges of the interacting atoms are also studied during two kinds of rotation of hydrogen sulfide (H2S) molecules as vertical and horizontal to the main axes of the nanotube. For these systems, the binding energy of H2S-single-wall carbon nanotubes is low and the process is thermodynamically near-simultaneous.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
The discovery of carbon nanotubes (CNTs) has caused considerable interest in many different scientific areas to investigate their physical and mechanical properties toward the development of potential technological applications. It has been demonstrated that theoretical methods and experimental procedures remarkably improve nanotube properties such as stiffness and strength [1]. Carbon nanotubes also have exceptionally high mechanical [2–4], electrical [5–8], and thermal conductivities [9–11] as well as high aspect ratio (length/diameter) and low density [12].
Single-walled carbon nanotube (SWCNT) properties are highly structure-/size-dependent and are influenced by atomic arrangement (chirality), nanotube diameter and length, and morphology or nanostructure. Depending on chirality, carbon nanotubes can either be conducting or semiconducting [13]. Wei et al. [14] demonstrated that multi-walled carbon nanotubes have extraordinarily high-current carrying capacity, sustaining current densities greater than 109 A/cm2. These novel electrical properties have generated substantial interest in utilizing carbon nanotubes in nanoelectronics [15].
Sensing gas molecules is crucial for many process control technologies, and advances in chemical and physical sensors continue to get improved sensitivity, lower power consumption, and faster response. There is a strong demand for improved sensitivity, selectivity, stability, and low-power consumption beyond what is offered by commercially available sensors. In order to satisfy this demand, nanotechnologies are employed, providing new materials, devices, and systems with structures and components that can exhibit novel and significantly improved physical and chemical properties because of their nanoscale size [16]. The operation at the room temperature with sensitivity as high as 103, besides their small size, is the main advantage of semiconducting-SWCNT sensors. The fast response of a nanotube sensor can be attributed to the full exposure of the nanotube surface area to chemical environments [17].
Seo et al. [18] reported the density-functional calculations of NO2 adsorption on SWCNT. They found that NO2 adsorption is both energetic- and kinetics-limited and strongly electronic structure and strain dependent. The NO2 adsorption on metallic nanotubes was energetically more favorable than that on semiconducting nanotubes and, furthermore, the adsorption became less stable with increasing diameters of nanotubes. The diameter dependence of the adsorption energetics and kinetics in the chiral nanotubes are other important parameters in the adsorption process.
The effect of the shape and defect edge of SWCNT on the adsorption of O2 and N2 gases on the graphite has been studied theoretically by means of density functional theory coupled with cluster models [19, 20]. The calculated adsorption energy 0.26 and 1.04 kcal/mol for O2 and N2, respectively, physisorbed on the clean basal surface, is in good agreement with the experimental value, suggesting that the one-layer simple cluster model is an effective way to investigate the adsorption of molecular gases on the graphite surface.
The doping effects of B and N on atomic and molecular adsorption of hydrogen in SWCNTs were investigated through density functional theory (DFT) calculations [21]. The results show that the B doping increases the hydrogen atomic adsorption energies both in zigzag and armchair nanotubes and a coordination-like B-H bond forms. The N doping decreases the hydrogen atomic adsorption energies.
Humidity-assisted desorption of SO2 and NO2 has been experimentally investigated on carbon nanotubes [22]. The results showed that the resisted desorption of SO2 on carbon nanotubes in dichloroethane solution increases at high humidity level (92%) and no change was observed in low humidity level, while there was also no change in desorption of NO2 in the different levels of humidity.
There are many literatures about the carbon nanotube sensors as a detector for many much gases; for example, O2[23], NH3[17, 24], NO2[25, 26], and SO2[27]; and the change in electrical conductivity occurs after adsorption of molecules. These sensors have fast response time and high sensitivity to special gas molecules which is very favorable for certain applications [23].
There has been only few gas nanosensors developed for hydrogen sulfide (H2S) detection including a catalytic chemiluminescence sensor made of R-Fe2O3 nanotubes [28], suppressed electron hopping occurred in gold nanoparticles [29], and networks made of hybrid polyaniline nanowires and gold nanoparticles [30]. There is little attention to the SWCNTs for this purpose because there is no chemical interaction with H2S or other gases that may interfere [31].
In the previous work, the effective parameters of (5,0) and (5,5) SWCNTs during the interaction with CO2 as sensors were determined [32]. The interaction of CO2 molecules and its rotation around tube axles vertically and parallel to internal and external walls of the nanotubes were studied using Gaussian 03 coding by density functional theory (DFT) at the B3LYP/6-311G level of theory. The carbon dioxide molecule was predicted to bind only weakly to nanotubes, and the tube-molecule interactions can be identified as physisorption. CO2 adsorption is stronger on the external walls than on internal walls, and adsorption on the external wall of (5,0) is stronger than on the external wall of (5,5); the adsorption energies are exothermic. In the present study, the interaction of hydrogen sulfide with the inside and outside of (5,0) and (5,5) SWCNT is investigated.
Method
Computational method
Single-wall zigzag (5,0) and armchair (5,5) carbon nanotubes are considered. The diameters of the nanotubes are 4 and 6.85 Å, the lengths of the nanotubes are 8.5 and 9.8 Å, respectively, and the average bond length is 1.42 Å. The (5,0) SWCNT containing 50 carbon atoms and 10 hydrogen atoms and the (5,5) SWCNT containing 100 carbon atoms and 20 hydrogen atoms were selected for this purpose. Hydrogen sulfide in the vicinity of internal and external walls of the nanotubes is placed, and these structures are optimized by Gaussian 09 software [33].
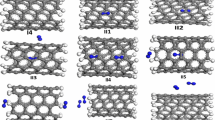
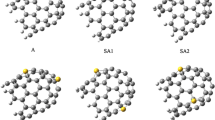
DFT is used to study the structural and electric properties of the tube-molecule systems during adsorption of H2S molecule on the SWCNTs. In these cases the calculations are carried out with the B3LYP/6-31G* level of theory [34]. The geometry of all molecules under investigation is determined by optimizing all geometrical variables without any symmetry constraints. All optimization processes were carried out by supercomputer up to 32 processors via 20GB shared memory. The harmonic frequencies are computed from analytical derivatives for all species in order to define the minimum energy structures. Figure 1 shows the optimal structures.
The calculated parameters are the energy interaction of H2S with inside and outside wall of SWCNT, Eads, through the following formula:
where Enanotube-H2S is the total energy of the optimized nanotube-H2S system, Enanotube is the total energy of the optimized nanotube and EH2S is the total energy of the isolated H2S molecule. By this explanation, E ads < 0 corresponds to exothermic adsorption, which leads to a stable structure.
Total zero point energy, total internal energy, and total enthalpy are calculated to the same formula.
Results and discussion
Thermodynamic parameters
The results of the Gaussian output files, after tedious efforts for structure optimization with high-time-consuming thermodynamic parameters related to the electronic structure of (5,0) and (5,5) nanotubes in the presence of H2S molecule, in the vicinity of internal and external walls have been listed in Table 1. Total Gibbs free energy of H2S-SWCNT system was calculated for inside and outside of (5,0) are 347.0703, 4.8947, and of (5,5) are 41.7915 and 4.5180 kcal/mol, respectively. Calculated Gibbs free energy shows that H2S adsorption process is not thermodynamically favorable. The lowest total zero-point energy, internal energy, and enthalpy relate to the process of H2S adsorption on the external wall of (5,0) nanotubes. The lowest Gibbs free energy is achieved during the interaction of H2S on the external wall of (5,5) nanotubes. Despite low and positive values of the Gibbs free energies of H2S adsorption on the external wall of (5,0) and (5,5) nanotubes, the adsorption energies are negative. It confirms no interaction between H2S gas and nanotube [15]. For obtaining more information, the optical properties under rotation of H2S molecule and natural bond orbital (NBO) analysis of H2S fluid-nanotube systems are studied.
Optical parameters
The effect of both adsorption of H2S molecule on the optical parameters of the systems has been listed and compared in Table 2. The results show that the adsorption on the external is more effective than the internal walls. The adsorption on the internal wall of (5,0) nanotube is the chemical adsorption and on the external wall of (5,0) and internal and external walls of (5,5) nanotubes are the physical adsorption. For these systems, the binding energy of the target gas analytes is low so that the analyte only fast desorbs from the CNTs after the sensor is exposed to an analyte-free atmosphere.
The lowest of energy gap is achieved in the process of H2S adsorption on the external wall of (5,5) nanotube. The energy gap is increased after the interaction of H2S on the internal and is decreased for external wall of (5,5) and (5,0) nanotubes. The lowest of HOMO and LUMO energies is obtained in the process of the adsorption on the external wall of (5,0) nanotube.
H2S rotation on the nanotubes
H2S molecule rotation in two kinds of the horizontal and the vertical rotation relative to the axes of nanotubes have been shown in Figure 2. The effect of the internal and external adsorption of SWCNTs on the adsorption energy has been studied during these rotations at 30°, 60°, 90°, 120°, 150°, and 180° angles. The H2S molecule rotations are classified as follows:
-
1.
Rotations 1a and 2a denote the rotations on the inside wall of (5,0) nanotube in the vertical and horizontal directions to the nanotube, respectively.
-
2.
Rotations 1b and 2b indicate the rotations on the outside wall of (5,0) nanotube in the vertical and horizontal direction to the nanotube, respectively.
-
3.
Rotations 1c and 2c represent the rotations on the inside wall of (5,5) nanotube in the vertical and horizontal direction to the nanotube, respectively.
-
4.
Rotations 1d and 2d denote the rotations on the outside wall of (5,5) nanotube in the vertical and horizontal direction to the nanotube, respectively.
The origin of rotation angles for 1a, 2a, 1c, 2c, 2b, and 2d rotations is at 90°, and for 1b and 1d rotations is at 0°. The basis set superposition errors (BSSE) have been estimated for the counterpoise correction [35] and their effects on the energy changes during the rotation of H2S molecule inside and outside nanotubes have been calculated. This effect is much low for any considerations. It confirms the convenience of the chosen bases set. Based on the calculations, the most adsorption situations of H2S is at 90° for 1a, 2a, 2b, 1c, 2c, and 2d rotations and is at 0° for 1b and 1d rotations. The most favorable adsorption distance of H2S molecule on the external surface of (5,0) and (5,5) nanotubes is shown in Figure 3, where the distances from the external wall are 2.6 and 2.8 Å, respectively.
NBO calculations
Gases absorption often changes the electronic properties of nanotubes. The environmental sensitivity can be useful in the detection of gas and making of gas sensors. Therefore, NBO calculations for all the rotations in order to calculate the charge difference before and after adsorption of H2S molecule on nanotubes have been performed.
The partial charge differences on the interaction of atoms including sulfur atom, hydrogen atoms and nearest carbone atom of interaction zoon of the (5,0) and (5,5) nanotubes in the presence of H2S molecule in the vicinity of internal and external walls for all rotations have been shown in Tables 3 and 4. They represent the change in the electronic structure of nanotube. They can be used as a response of the adsorption in the sensor equipments.
Conclusions
H2S molecule adsorption on the internal and external wall of (5,0) nanotube is both chemical and physical adsorption. The absorption on both internal and external walls of (5,5) nanotube is physical absorption. Based on the calculations, the most adsorption situations of H2S occur at 90° during the parallel and vertical rotations of the H2S molecule to the main axes inside of the nanotube. This situation is the same as the parallel rotation outside of the nanotube. The vertical rotation outside of the nanotube has the most adsorption energy at 0°. The most favorable adsorption distances from the molecule to external of (5,0) and (5,5) SWCNTs are 2.6 and 2.8 Å, respectively. The results of the thermodynamic quantities certifies no simultaneous H2S adsorption process, while the NBO analysis shows the change in the electronic structure of nanotubes which may be suitable fabricating sensors in providing an immediate feedback on the environment by SWCNTs. In these sensors, the electrical properties of nanostructures are dramatically changed when exposed to the target gas analytes and the analyte desorbs from the SWCNTs after the sensor is exposed to an analyte-free atmosphere.
References
Thostenson ET, Ren Z, Chou TW: Advances in the science and technology of carbon nanotubes and their composites: a review. Compos. Sci. Technol. 2001, 61: 1899–1912. 10.1016/S0266-3538(01)00094-X
Thostenson ET, Li WZ, Wang DZ, Ren ZF, Chou TW: Carbon nanotube/carbon fiber hybrid multiscale composites. J. Appl. Phys. 2002, 91: 6034–6037. 10.1063/1.1466880
Suhr J, Koratkar N, Keblinski P, Ajayan P: Viscoelasticity in carbon nanotube composites. Nat. Mater. 2005, 4: 134–137. 10.1038/nmat1293
Gojny FH, Wichmann MH, Köpke U, Fiedler B, Schulte K: Carbonnanotube-reinforced epoxy-composites: enhanced stiffness and fracture toughness at low nanotube content. Compos. Sci. Technol. 2004, 64: 2363–2371. 10.1016/j.compscitech.2004.04.002
Du F, Fischer JE, Winey KI: Effect of nanotube alignment on percolation conductivity in carbon nanotube/polymer composites. Phys. Rev. B 2005, 72: 121404–12144.
Vigolo B, Coulon C, Maugey M, Zakri C, Poulin P: An experimental approach to the percolation of sticky nanotubes. Science 2005, 309: 920–923. 10.1126/science.1112835
Wang L, Dang ZM: Carbon nanotube composites with high dielectric constant at low percolation threshold. Appl. Phys. Lett. 2005, 87: 042903–042903–3.
Sandler JKW, Kirk JE, Kinloch IA, Shaffer MSP, Windle AH: Ultra-low electrical percolation threshold in carbon-nanotube-epoxy composites. Polymer 2003, 44: 5893–5899. 10.1016/S0032-3861(03)00539-1
Che J, Cagin T, Goddard WA: Thermal conductivity of carbon nanotubes. Nanotechnology 2000, 11: 65–69. 10.1088/0957-4484/11/2/305
Huang H, Liu CH, Wu Y, Fan S: Aligned carbon nanotube composite films for thermal management. Adv. Mater. 2005, 17: 1652–1656. 10.1002/adma.200500467
Biercuk MJ, Llaguno MC, Radosavljevic M, Hyun JK, Johnson AT, Fischer TE: Carbon nanotube composites for thermal management. Appl. Phys. Lett. 2002, 80: 2767–2769. 10.1063/1.1469696
Thostenson ET, Li C, Chou TW: Nanocomposites in context. Compos. Sci. Technol.. 2005, 65: 491–516. 10.1016/j.compscitech.2004.11.003
Rao CNR, Satishkumar BC, Govindaraj A, Nath M: Nanotubes. Chem. Phys. Chem.. 2001, 2: 78–105. 10.1002/1439-7641(20010216)2:2<78::AID-CPHC78>3.0.CO;2-7
Wei BQ, Vajtai R, Ajayan PM: Reliability and current carrying capacity of carbon nanotubes. Appl. Phys. Lett. 2001, 79: 1172–1174. 10.1063/1.1396632
Mubeen S, Zhang T, Chartuprayoon N, Rheem Y, Mulchandani A, Myung NV, Deshusses MA: Sensitive detection of H2S using gold nanoparticle decorated single-walled carbon nanotubes. Anal. Chem. 2010, 82: 250–257. 10.1021/ac901871d
Maruccio G, Cingolani R, Rinaldi R: Projecting the nanoworld: concepts, results and perspectives of molecular electronics. J. Mater. Chem. 2004, 14: 542–554. 10.1039/b311929g
Kong J, Franklin NR, Zhou C, Chapline MG, Peng S, Cho K, Dai H: Nanotube molecular wires as chemical sensors. Science 2000, 287: 622–625. 10.1126/science.287.5453.622
Seo K, Park KA, Kim C, Han S, Kim B, Lee YH: Chirality- and diameter-dependent reactivity of NO2 on carbon nanotube walls. J. Am. Chem. Soc. 2005, 127: 15724–15729. 10.1021/ja052556y
Xu YJ, Li JQ: The interaction of molecular oxygen with active sites of graphite: a theoretical study. Chem. Phys. Lett. 2004, 400: 406–412. 10.1016/j.cplett.2004.11.010
Xu YJ, Li JG: The interaction of N2 with active sites of graphite: a theoretical study. Chem. Phys. Lett. 2005, 406: 249–253. 10.1016/j.cplett.2005.02.119
Zhou Z, Gao X, Yan J, Song D: Doping effects of B and N on hydrogen adsorption in single-walled carbon nanotubes through density functional calculations. Carbon 2006, 44: 939–947. 10.1016/j.carbon.2005.10.016
Yao F, Duong DL, Lim SC, Yang SB, Hwang HR, Yu WJ, Lee IH, Günes F, Lee YH: Humidity-assisted selective reactivity between NO2 and SO2 gas on carbon nanotubes. J. Matt. Chem. 2011, 21: 4502–4508. 10.1039/c0jm03227a
Collins PG, Bradley K, Ishighami M, Zettl A: Extreme oxygen sensitivity of electronic properties of carbon nanotubes. Science 2000, 287: 1801–1804. 10.1126/science.287.5459.1801
Feng X, Irle S, Witek H, Morokuma K, Vidic R, Borguet E: Sensitivity of ammonia interaction with single-walled carbon nanotube bundles to the presence of defect sites and functionalities. J. Am. Chem. Soc. 2005, 127: 10533–10538. 10.1021/ja042998u
Li J, Lu Y, Ye Q, Cinke M, Han J, Meyyappan M: Carbon nanotube sensors for gas and organic vapor detection. Nano Lett. 2003, 3: 929–933. 10.1021/nl034220x
Goldoni A, Larciprete R, Petaccia L, Lizzit S: Single-wall carbon nanotube interaction with gases: sample contaminants and environmental monitoring. J. Am. Chem. Soc. 2003, 125: 11329–11333. 10.1021/ja034898e
Ciraci S, Dag S, Yildirim T, Gülseren O, Senger RT: Functionalized carbon nanotubes and device applications. J. Phys. Condens. Matter 2004, 16: R901. 10.1088/0953-8984/16/29/R01
Sun Z, Yuan H, Liu Z, Han B, Zhang X: A highly efficient chemical sensor material for H2S: α-Fe2O3 nanotubes fabricated using carbon nanotube templates. Adv. Mater. 2005, 17: 2993–2997. 10.1002/adma.200501562
Geng J, Thomas MDR, Shephard DS, Johnson BFG: Suppressed electron hopping in a Au nanoparticle/H2S system: development towards a H2S nanosensor. Chem. Commun. 2005, 14: 1895–1897.
Shirsat MD, Bangar MA, Deshusses MA, Myung NV, Mulchandani A: Polyaniline nanowires-gold nanoparticles hybrid network based chemiresistive hydrogen sulfide sensor. Appl. Phys. Lett. 2009, 94: 083502–083503. 10.1063/1.3070237
Zhang T, Mubeen S, Myung NV, Deshusses MA: Recent progress in carbon nanotube-based gas sensors. Nanotechnology 2008, 19: 332001–332014. 10.1088/0957-4484/19/33/332001
Oftadeh M, Gholamalian B, Hamadanian M: Investigation of the interaction of carbon dioxide fluid with internal and external single-wall carbon nanotubes by DFTJ. Nanostruct. 2012, 1: 213–223.
Frisch MJ, Trucks GW, Schlegel GB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, et al.: Gaussian 09 software. 2009.
Becke AD: Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98: 5648–5652. 10.1063/1.464913
Lendvay G, Mayer I: Some difficulties in computing BSSE-corrected potential surfaces of chemical reactions. Chem. Phys. Lett. 1998, 297: 365–373. 10.1016/S0009-2614(98)01191-9
Acknowledgments
We thank the Universiti Teknologi Malaysia (UTM). This research was in part supported by a grant from Payame Noor University (PNU) (1388/0/8/125).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
MO conceived of the study, participated in the design of the study and in the sequence alignment and drafted the manuscript. MG participated in drafting the manuscript and its design and coordination. HHA participated for his assistance and efforts for optimizing the 3D geometry of the structures studied. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Oftadeh, M., Gholamian, M. & Abdallah, H.H. Investigation of interaction hydrogen sulfide with (5,0) and (5,5) single-wall carbon nanotubes by density functional theory method. Int Nano Lett 3, 7 (2013). https://doi.org/10.1186/2228-5326-3-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2228-5326-3-7