Abstract
During the past decade, much attention has been paid to the replacement of homogeneous catalysts by solid acid catalysts. Friedel-Crafts benzylation of toluene with benzyl chloride (BC) in liquid phase was carried out over highly active, nano-crystalline sulfated titania systems. These catalysts were prepared using the sol gel method. Modification was done by loading 3% of transition metal oxides over sulfated titania. Reaction parameters such as catalyst mass, molar ratio, temperature, and time have been studied. More than 80% conversion of benzyl chloride and 100% selectivity are shown by all the catalysts under optimum conditions. Catalytic activity is correlated with Lewis acidity obtained from perylene adsorption studies. The reaction appears to proceed by an electrophile, which involves the reaction of BC with the acidic titania catalyst. The catalyst was regenerated and reused up to four reaction cycles with equal efficiency as in the first run. The prepared systems are environmentally friendly and are easy to handle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Friedel and Crafts first reported the alkylation of benzene with alkyl chlorides in the presence of aluminum chloride in 1877 [1]. Their groundbreaking discovery initiated an unimaginable flow of new synthetic processes. Over the past century, overwhelming numbers of compounds have been synthesized through the modification of the original approach, a field now commonly referred to as the Friedel-Crafts chemistry. Friedel-Crafts alkylation is an important means of attaching alkyl chains to aromatic rings and hence is a key reaction in organic chemistry. The liquid-phase benzylation of benzene and other aromatic compounds by benzyl chloride and benzyl alcohol is an important process for the production of diphenyl methane and substituted diphenyl methane which are industrially important compounds used as pharmaceutical intermediates, heat-transfer fluids, aromatic solvents, fragrances, monomers for polycarbonate resins, and fine chemicals [2]. Despite more than 120 years of history, the Friedel-Crafts alkylation and acylation reactions are still in the forefront of organic synthesis research. The catalysts used in these reactions are often homogeneous, such as HF, H2SO4, AlCl3, and BF3. Though readily available and inexpensive, these catalysts have numerous drawbacks, which include violent decomposition with water, liberating HCl, its corrosive nature, and poor selectivity, leading to undesired polysubstituted and isomerized by-products. During the reaction work-up, the catalyst is destroyed which not only generates large volumes of gaseous effluent (HCl), but also Al-rich acidic effluents, which are difficult to deal with. Thus, these traditional catalysts have difficulty in separation and recovery, disposal of spent catalyst, corrosion, high toxicity, etc. Moreover, these catalysts are highly moisture-sensitive and hence require moisture-free solvent and reactants and anhydrous conditions, and also dry atmosphere for their handling [3]. Unfortunately, they do not fulfill the current requirements of environmental protection and safety standards. These drawbacks become a major disadvantage in times of environmental concern, emphasizing the need to develop an alternative to the conventional homogeneous Lewis acid catalyst. Replacing homogeneous catalytic systems with heterogeneous ones is advantageous mainly because of easy catalyst recovery, reusability, and work-up of reaction products [4–6]. During the past decade, much attention has been paid to the replacement of homogeneous catalysts by solid acid catalysts such as zeolites [7, 8], sulfated ZrO2 or Fe2O3, sulfated Al2O3-ZrO2 or TiO2[9, 10], clays [11, 12], metal oxides [13], and modified silica [14].
Benzylation of toluene with either benzyl alcohol or benzyl chloride (BC) has extensively been studied previously. For example, alkylation of toluene with BC in the presence of AlSBA-15 as catalyst has been carried out with 31% conversion and 88% para-selectivity [15]. A similar reaction catalyzed by Fe-SBA-15 afforded the corresponding para-alkylated product with 66% conversion and 100% selectivity [16]. Utilization of benzyl alcohol in the presence of Ga-Al-MCM-41 has converted 78% of toluene to a mixture of ortho- and para-selectivity as well as ether products at 383 K for 6 h [14]. Catalytic activities of niobium phosphate and Sc(OTf)3 in the benzylation of toluene with benzyl alcohol have also been reported [17, 18]. In most cases, reactions either need long reaction times or are carried out at relatively high temperatures. More importantly, they mostly end up with a mixture of ortho- and para-alkylated products [8, 18–20]. The fundamental concept is to identify new, stable, and recyclable catalysts as replacements for the conventional liquid acids and ultimately develop environmentally safe industrial processes. Herein, we report a recyclable, easily separable, eco-friendly, and highly effective catalytic system TiO2/SO42- and its modified forms for the benzylation of toluene with BC, a typical example of Friedel-Crafts alkylation.
Methods
Materials and apparatus
All reagents used were of analytical grade purity and were procured from Sigma-Aldrich (St. Louis, MO, USA).
The absorbance measurements were done using a Shimadzu UV-160A spectrophotometer (Kyoto, Japan). Analysis of the products was done in a Chemito 8610 gas chromatograph (Mumbai, India) equipped with a flame ionization detector and an SE-30 column.
Catalyst preparation and characterization
Cr-, Mn-, Fe-, Co-, Ni-, Cu-, and Zn-loaded (3%) sulfated titania nano-powders were prepared using the sol-gel technique. Titanium isopropoxide was used as the precursor of titania. Ti(OC3H7)4 (25 mL) was hydrolyzed in 300 mL of water containing 2.5 mL of nitric acid. Precipitates formed were stirred continuously at room temperature to form a highly dispersed sol. To this, Cr, Mn, Fe, Co, Ni, Cu, and Zn nitrate solutions (3 wt.%) were added separately and stirred again for about 4 h. After keeping the sol for aging, it was concentrated and dried at 333 K. Sulfation was done using a 0.5-M sulfuric acid solution (2 mL g-1 of the hydroxide). The samples, after overnight drying at 383 K, were calcined for 5 h at 773 K. The general sample notation STX3 stands for sulfated titania with 3 wt.% of X metal oxide, whereas T and ST denote titania and sulfated titania, respectively. A detailed procedure for the preparation of the catalysts and their characterization by X-ray diffraction (XRD), FT-IR, BET surface area, TGA, EDX, and UV-vis DRS had been explained in our earlier paper [21].
Acidity measurements by temperature-programmed desorption of ammonia (NH3-TPD) and 2,6-dimethylpyridine (2,6-DMP) adsorption-desorption studies of these catalyst were also explained in the literature [21]. Perylene adsorption experiments were carried out in a 50-mL stoppered U-tube by stirring 5 mL of freshly prepared perylene in benzene solutions of varying perylene concentrations (0.01 to 0.02 mol L-1 in benzene) and accurate amounts of catalyst for 4 h. The contents were filtered after 24 h, and absorbance is measured. In all cases, absorbance measurements were performed in the adsorbate concentration range, where the Beer-Lambert law is valid. From Langmuir plots, the limiting amount of perylene adsorbed on the catalyst surface, a measure of the Lewis acidity, is obtained. The absorbance measurements were done at λmax = 439 nm using a 10-mm quartz cell.
Benzylation of toluene with benzyl chloride
The liquid-phase benzylation was carried out in a closed 50-mL round-bottom glass flask equipped with a reflux condenser, magnetic stirrer, and provision for withdrawing product samples. In a typical run, appropriate amounts of toluene, BC, and a catalyst were allowed to react at specified temperatures under magnetic stirring. Reaction mixture was withdrawn at specific intervals, filtered, and analyzed using a gas chromatograph (N2 carrier gas; injection port temperature, 503 K; column temperature, 353 K; at a heating rate of 3 K; detector temperature, 523 K). The reaction always yielded a single product under the present reaction conditions and is named monoalkylated product (MAP). The percentage conversion (wt.%) of BC is the total percentage of BC transformed into the product.
The present work also attempts a closer look into the metal leaching and deactivation of the systems under the reaction conditions to obtain a better understanding of the nature and course of the reaction. To study the reusability of the catalyst, the catalyst used in the particular reaction was removed from the reaction mixture by filtration, washed, dried, and activated before reusing in the next reaction. In order to test the effect of moisture on catalyst performance, the catalyst and substrate were saturated with water vapor by keeping them over deionized water in a desiccator for 48 h at room temperature. The reaction was carried out as before. To test the metal leaching, the reaction mixture was separated after a particular time from the reaction mixture at the reaction temperature, and the filtrate was again subjected for reaction for 30 min more, and the products were monitored as before. The filtrate was further subjected to qualitative analysis for testing the presence of leached metal ions.
Results and discussion
Textural parameters of the prepared catalysts were listed in Table 1 from our earlier study [21]. NH3-TPD is now widely used for evaluating the surface acidity of the solid catalysts [22, 23]. The distribution of acid sites and total acidity values of the different systems are shown in Table 2. The ammonia thermodesorption results give clear evidence for the presence of surface acid sites of different strengths going from weak to strong acidities. Titania shows only low acidity, and sulfation increases its acidity. Incorporation of metal ions also changes the acidity considerably. The nature of the acid sites is greatly altered by the nature of the ions incorporated into the lattice. The distribution change may be a coupled effect of the crystalline and structural changes. The change in the acid strength distribution for the different systems may be related to the interaction of the added metal cations with the TiO2. A thermodesorption study of 2,6-DMP was carried out with an intention of obtaining a comparative evaluation of the Brönsted acidity in the samples. Satsuma et al. [24] reported a complete elimination of the coordinatively adsorbed 2,6-DMP after purging at an appropriate temperature (above 573 K). Thus, we presume that the amount of 2,6-DMP desorbed at temperatures above 573 K is due to the desorption from Brönsted acid sites. The results are represented in Table 2. Brönsted acidity increases in the case of metal-loaded systems, compared to titania. Among the different metal-incorporated systems, manganese, iron, and zinc show low Brönsted acid sites, while other metal ions do not show much change.
Perylene adsorption studies give information regarding Lewis acid sites in the presence of Brönsted sites. This study is based on the ability of the catalyst surface site to accept a single electron from an electron donor like perylene to form charge-transfer complexes. The adsorption of perylene from a solution in benzene was done at room temperature. The pale yellow or white color of the samples changed into green after adsorption. After electron donation, perylene gets adsorbed on the Lewis sites as radical cation [25, 26]. As the concentration of perylene in the solution increases, the amount adsorbed also increases up to a certain limiting value after which it remains constant. A clear picture of the adsorption and limiting value can be obtained by plotting the amount of perylene adsorbed against the equilibrium concentration. These graphs convince the Langmuir type of adsorption over solid surfaces. However, perylene being large, along with its sole ability for binding with electron acceptor centers, has access only to exposed Lewis acid sites. The limiting amount adsorbed is a measure of the Lewis acidity or the electron-accepting capacity. The results of perylene adsorption studies for various catalytic systems are presented in Table 2. TiO2 adsorbs a very low amount of perylene. Substantial amount of perylene gets adsorbed on TiO2/SO42-. Introduction of transition metals, even in small amounts, increases significantly the limiting value of the one-electron donor adsorbed on the catalyst surface. This gives a clear picture about the Lewis acidity of the catalysts.
Toluene benzylation
To optimize the reaction parameters, the benzylation of toluene using BC was carried out by STCr3 as a representative catalyst by varying the reaction conditions such as catalyst mass, molar ratio, temperature.
The effect of the catalyst
We made the reaction run using benzyl chloride and toluene in the absence of a catalyst (blank run) and in the presence of the catalyst (0.1 g STCr3) at the refluxing temperature of the mixture. We observed a percentage conversion of 2.1 and 80.9 after 60 min of reaction in the absence and presence of the catalyst, respectively. Low yield for the reaction in the absence of the catalyst is due to the higher activation energy of the uncatalyzed reaction. Addition of the catalyst significantly reduced the activation energy, and the reaction proceeded through a different path with lower activation energy, resulting in a higher percentage conversion.
The effect of catalyst mass
To determine the optimum mass of the catalyst, reactions were carried out by varying the mass of the catalyst from 0.05 to 0.2 g. The addition of 0.05 g catalyst changes the percentage conversion from 1.8% to 61.7% (Figure 1). An initial steep increase in the conversion is observed when 0.1 g catalyst is used and reaches 100% when the amount is 0.2 g. These results indicate that only a small amount of catalyst is needed for the reaction to take place. At high catalyst concentration, a decrease in selectivity to MAP is observed. As the amount of catalyst is increased, there is a steady increase in the conversion, because of the increase in the total number of acid sites available for the reaction [27]. The product yield is found to be proportional to the amount of the catalyst taken, establishing that the reaction proceeds through a pure heterogeneous mechanism. Singh et al. [28] studied the influence of catalyst concentration in the benzylation of o-xylene using zeolite catalysts and found similar results.
Influence of toluene/BC molar ratio
The influence of toluene/BC molar ratio on the conversion and product distribution over STCr3 at 373 K is shown in Table 3. As the molar ratio increased from 5:1 to 20:1, the BC conversion also increased. At lower toluene to BC ratios, the amount of polyalkylation is negligible, while it considerably increases at higher molar ratios. The results show that benzylation is favored with a lower concentration of benzylating agent. Rohan et al. [29] reported that when the concentration of the benzylating agent is high, alkylated products may enhance the poisoning effect, which is strongly adsorbed on the catalyst surface. This restricts further adsorption of the reactant molecules and thus reduces the conversion of BC. At high arene-to-BC molar ratio, this inhibition would be less significant which helps to desorb the products formed from the catalyst surface easily. A similar observation is also reported for benzylation of toluene over H-Y [30] and o-xylene over H-β zeolites [28].
The effect of reaction temperature
In order to study the effect of temperature, the benzylation was carried out from 343 to 383 K with a 10:1 molar ratio (toluene/BC). The result (Figure 2) shows that an increase in the temperature results in enhanced activity. This is due to the speedy desorption of the alkylated product from the catalyst surface as the temperature increases which facilitates the further adsorption of reactant molecules, resulting in the increased conversion of BC. Maximum conversion is found to be at the refluxing temperature with 100% selectivity to the monoalkylated product.
The effect of substrate
Catalytic activity largely depends on the substrate used. Variation of reactivity with substrate was studied by carrying out the reaction using benzene, toluene, and o-xylene over the same catalyst under the same reaction conditions. Table 4 gives the details of the results for reactions conducted with different substrates. The reaction is in the order o-xylene > toluene > benzene. Sebti et al. [31] reported similar results over different Lewis acids supported on hydroxyapatite for benzylation reaction. The observed order of reactivity is exactly the same as the order of electron-releasing effect of the substituting group in the benzene ring. The inductive effect of methyl group makes the reaction more facile with toluene and still higher for xylene due to the cumulative effect of two methyl groups. Similar results have been reported by Jun and Ryoo [30] and Manju and Suganan [26].
Stability, reusability, and leaching
To confirm the involvement of Lewis acid sites in catalyzing the reaction, the influence of moisture on catalytic activity was tested. The activated catalyst and substrate were saturated with water vapor, by keeping them over deionized water in a desiccator for 72 h at room temperature. The reactions were carried out at 383 K for 90 min by taking toluene-to-BC molar ratio 10:1. For comparison, parallel runs were conducted using activated fresh catalysts. The percentage conversion suddenly rose from 15.7% to 59.3% in the time range of 45 to 60 min (Table 5). This may be correlated with the reversible transformation of the Lewis acid sites into Brönsted acid sites upon exposure to moisture. Thus, there was a time period for which the catalyst was inactive towards reaction, when it is adsorbed with moisture. However, after the induction period, reaction proceeds with almost the same rate. The moisture gets adsorbed on the active sites on the catalyst surface and prevents the interaction of BC molecules with these sites [26]. Once the sites are freed from moisture, they are active towards the desired reaction. These catalysts do not demand stringent moisture-free conditions to be highly active in the benzylation process.
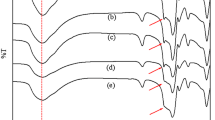
One of the major objectives guiding the development of solid acid catalysts includes the easy separation of final products from the reaction mixture and efficient catalyst recovery. The reusability of the catalyst systems was also subject to investigation. The catalyst was removed by filtration from the reaction solution, washed thoroughly with acetone, then dried, and activated. No pronounced change was observed in the XRD pattern (Figure 3), except for a slight lowering of intensity, symptomatic of the retention of the crystalline nature. It was tested for catalytic activity in four cycles, and no noticeable change in conversion was detected (Table 6), indicating the absence of metal leaching and the reusability of the catalyst. This suggests the resistance to rapid deactivation.
To prove the heterogeneous character of the reactions, the reactants were carefully withdrawn and filtered while hot and charged into an empty round-bottom flask equipped with a condenser maintained at the same temperature. The system was allowed to react further without the catalyst. The filtrate was further subjected to qualitative analysis for testing the presence of leached metal ions. There was no metal leaching in all the systems under study. We conclude that metal chloride, if at all formed by the probable reaction of BC or HCl evolved in the reaction, exists on the catalyst surface. Thus, the reaction can be assumed to be mainly heterogeneous in nature. The low metal content may be the reason for the heterogeneous character of the reaction. The metal seems well dispersed on the catalyst surface as a part of the complex structure, which explains its stability. The same observation was seen in the XRD results. No peaks corresponding to the transition metal oxide is detected in the XRD, suggesting that it exists as the amorphous phase without getting incorporated into the TiO2 phase, i.e., they are in a highly dispersed form on the surface.
The effect of metal loading
Catalytic activities of all the systems were evaluated at a molar ratio of 10:1, using a 0.1-g catalyst and at a reaction temperature of 373 K. Table 7 presents the results of benzylation of toluene using BC over different metal-loaded TiO2/SO42- systems. The sulfated system gives comparatively higher conversion than titania. The modified TiO2-SO42- shows a good activity to BC conversion. Cent percent selectivity to the monobenzylated product remains the same in all metal-loaded systems. Among the various metal-loaded systems, a notable deviation was observed in the case of iron-incorporated systems. Abnormally high conversions (within 20 min), which do not commensurate with the Lewis acidity values, may be attributed to the reducible character of the iron. The conversion becomes 100% within 15 min in the case of iron-loaded systems. This may be an indication of the fact that the iron content or the Lewis acidity is not the only factor favoring the reaction. It is reported that catalysts containing reducible cations like Fe3+, Sn4+, Cu2+, Sb3+ regardless of their low Lewis acidities exhibit high alkylation activity [32–34].
The acid-base properties of metal oxide carriers can significantly affect the final selectivity of heterogeneous catalysts [30]. Friedel-Crafts alkylation is an aromatic electrophilic substitution reaction in which the carbocation is formed by the complexation of alkyl halide with the catalyst. The carbocation attacks the aromatic species for alkylation, and hence, formation of carbocation is an important step in the reaction mechanism. Lewis acidic centers on the catalyst surface facilitate the carbocation formation [35]. Thus, an attempt is made to correlate the catalytic activity with the acidic characteristics determined by perylene adsorption studies. Conversion of BC for different systems is in agreement with the amount of Lewis acid sites measured from perylene adsorption studies (Figure 4). Metal loading and sulfate modification provoke considerable synergistic effect leading to an enhanced activity. The metal oxide surface contains both Brönsted and Lewis acid sites and the above observations clearly indicate the dominating impact of Lewis acid sites for the benzylation reaction over the sulfated titania systems. Introduction of sulfate ions increases the surface acidity due to the electron-withdrawing nature of the sulfate groups, which increases the number of Lewis sites.
Mechanism of benzylation reaction
The reaction appears to proceed by an electrophile, which involves the reaction of BC with the acidic titania catalyst. The acidic catalyst polarizes the benzylating agent and, in turn, produces an electrophile (C6H5CH2+). Thus, the generated electrophilic species attack the benzene ring, resulting in the formation of the corresponding dimethyl diphenyl derivative [36]. Nonpolar nature of the substrate molecules also supports the formation of the electrophilic species by adsorption of BC molecule on the catalyst surface. A plausible mechanism for the reaction can be represented schematically, as shown in Figure 5. The percentage conversions obtained in the case of iron-loaded systems were much higher than those expected from Lewis acidity. The high activity of iron systems, which does not commensurate with the acidity values, can be attributed to the redox or free radical mechanism (Figure 6). Choudary et al. [34] suggested the possibility of a redox mechanism for reducible cations when BC was the alkylating agent. Considering all the aspects, we propose the existence of a redox or a free radical mechanism in the case of Fe-loaded samples side by side with the involvement of Lewis acid sites. Radicals are powerful reductants, which can readily be oxidized to cations in the presence of reducible metal cations. Thus, the high activity associated with these reducible cations involves the initiation of the reaction by the homolytic cleavage of the carbon-chlorine bond followed by the oxidation of the radical to the corresponding ion.
Conclusions
Transition metal-loaded TiO2/SO42- resulted in materials with good acidic and textural properties. Studies on elemental composition and surface area indicate the presence of loaded metals. Benzylation of toluene occurs efficiently over these catalysts. Cent percent monoalkylated product selectivity is obtained with these catalysts. A good relationship between acid strength of the catalyst and its catalytic activity could be observed. The prepared systems are environmentally friendly and are easy to handle. The catalyst can be reused. Mostly, the inexpensive and environmentally friendly nature of these catalysts led to their broad use in research laboratories and even to their application in the industry for Friedel-Crafts alkylation reactions. The trend is clear: the strengthening environmental regulations and safety concerns will generate even further interest in the application of these catalysts.
Authors' information
The authors did not provide this information.
References
Friedel C, Craft JM: Compt. Rend. Acad. Sci. Compt. Rend. Acad. Sci, Paris 1877.
Olah GA: Friedel-Crafts Chemistry. Wiley, New York; 1973.
Ratnaswamy P, Singh AP, Sharma S: Appl. Catal. A. 1996, 135: 25. 10.1016/0926-860X(95)00210-3
Sartori G, Maggi R: Chem. Rev.. 2006, 106: 1077. 10.1021/cr040695c
Trong OD, Desplantier-Giscard D, Danumah C, Kaliaguine S: Appl. Catal. A. 2003, 253: 545. 10.1016/S0926-860X(03)00195-9
Selvaraj M, Lee TG: Microporous Mesoporous Mater.. 2005, 85: 59. 10.1016/j.micromeso.2005.05.042
Cao Y, Kessas R, Naccache C, Ben Taarit Y: Appl. Catal. A. 1999, 184: 231. 10.1016/S0926-860X(99)00089-7
Narender N, Krishna Mohan KVV, Kulkarni SJ, Ajit Kumar Reddy I: Catal. Commun. 2006, 7: 583. 10.1016/j.catcom.2006.01.013
Song X, Sayari A: Catal. Rev. Sci. Eng.. 1996, 38: 299. 10.1080/01614949608006461
Quaschning V, Deutsch J, Druska P, Niclas HJ, Kemnitz E: J. Catal.. 1998, 177: 164. 10.1006/jcat.1998.2098
Smith K, Pollaud GM, Matthews I: Green Chem.. 1999, 1: 75. 10.1039/a901394f
Nehate M, Bokade VV: Appl. Clay Sci.. 2009, 44: 255. 10.1016/j.clay.2009.02.011
Willock DJ: Metal Oxides. In Encyclopedia of Catalysis. Edited by: Horvath IT. Wiley, New York; 2003.
Gracia MJ, Losada E, Luque R, Capelo JM, Luna D, Marinas JM, Romero AA: Appl. Catal. A. 2008, 349: 148. 10.1016/j.apcata.2008.07.023
Vinu A, Sawanat DP, Ariga K, Hartmann M, Halligudi SB: Microporous Mesoporous Mater.. 2005, 80: 195. 10.1016/j.micromeso.2004.12.012
Anand C, Srinivasu P, Alam S, Balasubramanian VV, Sawant DP, Palanichamy M, Murugesan V, Vinu A: Microporous Mesoporous Mater.. 2008, 111: 72. 10.1016/j.micromeso.2007.07.011
Henrique M, de la Cruz C, Francisco J, da Silva C, Lachter ER: Appl. Catal. A.. 2003, 245: 377. 10.1016/S0926-860X(02)00639-7
Mantri K, Komura K, Kubota Y, Sugi Y: J. Mol. Catal. A.. 2005, 236: 168. 10.1016/j.molcata.2005.04.020
Narayanan S, Deshpande K: Appl. Catal. A.. 2000, 193: 17. 10.1016/S0926-860X(99)00402-0
Ghiaci M, Abbaspur A, Kia R, Belver C, Trujillano R, Rives V, Vicente MA: Catal. Commun.. 2007, 8: 49. 10.1016/j.catcom.2006.05.003
Sunajadevi KR, Sugunan S: Mater. Lett.. 2004, 58: 3290. 10.1016/j.matlet.2004.06.019
Lin S, Hsu R: J. Chem. Soc. Chem. Commun.. 1992, 1469.
Arene F, Dario R, Parmaliana A: Appl. Catal. 1998, A 170: 127.
Satsuma A, Kamiya Y, Westi Y, Hattori T: Appl. Catal. A. 2000, 194: 253.
Suja H, Deepa CS, Sreeja Rani K, Sugunan S: Appl. Catal. A. 2002, 230: 33.
Manju K, Suganan S: Bullt. Catal. Soc. Ind.. 2050, 4: 88.
Jacob B, Sugunan S, Singh AP: J. Mol. Catal. A: Chem.. 1999, 139: 43. 10.1016/S1381-1169(98)00187-3
Singh AP, Jacob B, Sugunan S: Appl. Catal. A. 1998, 174: 51. 10.1016/S0926-860X(98)00194-X
Rohan D, Canaf C, Romeafin E, Guismet M: J. Catal.. 1998, 177: 2743.
Jun S, Ryoo R: J. Catal.. 2000, 195: 237. 10.1006/jcat.2000.2999
Sebti S, Tahir R, Nazih R, Boulaajaj S: Appl. Catal. A. 2001, 218: 25. 10.1016/S0926-860X(01)00599-3
Despande AB, Bajpai AR, Samant SD: Appl. Catal. A. 2001, 209: 229. 10.1016/S0926-860X(00)00777-8
Cseri T, Bekassy S, Figueras F, Rizner S: J. Mol. Catal. A: Chem.. 1995, 98: 101. 10.1016/1381-1169(95)00016-X
Choudary BM, Kantam ML, Sateesh M, Rao KK, Santhi PL: Appl. Catal. A. 1997, 149: 257. 10.1016/S0926-860X(96)00310-9
Ghorpade SP, Dharsane VS, Dixit SG: Appl. Catal. A.. 1998, 166: 135. 10.1016/S0926-860X(97)00266-4
Coq B, Gourves V, Figueras F: Appl. Catal. A.. 1993, 100: 69. 10.1016/0926-860X(93)80116-8
Acknowledgments
The authors did not provide this information.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors did not provide this information.
Authors' contributions
The authors did not provide this information.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Devi, K.R., Sreeja, P.B. & Sugunan, S. Environmentally benign Friedel-Crafts benzylation over nano-TiO2/SO42-. Int Nano Lett 3, 40 (2013). https://doi.org/10.1186/2228-5326-3-40
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2228-5326-3-40