Abstract
The present study is aimed to investigate the formulation and in vitro anticancer activities of solid lipid nanoparticles (SLNs) of 5-fluorouracil (5-FU) prepared using glyceryl monostearate (GMS) and cetyl palmitate (CP) by hot homogenization method. The lipids were selected based on the partition coefficient of 5-FU in lipids. The lipid nanoparticles were optimized for process and formulation parameters. The optimized nanoparticles were characterized for their zeta potential, morphology, release kinetics, and anticancer activity. Higher entrapments were achieved using a combination of emulsifiers. The zeta potential of the optimized CP and GMS SLN formulation were −8.26 and −9.35 mV, respectively. Both the optimized formulations were spherical. The in vitro release studies of SLNs of both the lipid carriers followed Peppas-Korsenmeyer equation when carried out at pH 3.5 and 7.4. The chemosensitivity assay carried out in B16F10 cell lines revealed that CP SLNs had better cytotoxicity than 5-FU solution and GMS SLNs at 48 h of incubation. Subtoxic concentration of 5-FU-loaded CP SLNs (0.12 μg/mL) possessed comparable antimigrational activity, colony inhibition activity, and cytopathic as that of 5-FU solution effects. The results indicated that encapsulating 5-FU in CP would be a promising delivery system for delivering 5-FU.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
5-Fluorouracil (5-FU) is one of the oldest chemotherapeutic drugs, and it has been used commonly against colon, stomach, breast, and pancreatic cancers [1, 2]. It is a fluorinated analog of pyrimidine base uracil, which is metabolized intracellularly to its active form, fluorodeoxyuridine monophophate. The active form inhibits DNA synthesis by inhibiting the normal production of thymidine. 5-FU is an S-phase-active anticancer agent, and it has no activity when cells are in G0 or G1[3]. It is sparingly soluble in water [4]. On intravenous administration, it causes severe toxic effects of gastrointestinal, hematological, neural, cardiac, and dermatological origin [5]. The bioavailability of 5-FU is greatly limited by rapid catabolism in the blood, liver, and other organs. After IV injection in humans, the drug has a half-life in blood of only 8 to 20 min [6]. Therefore, 5-FU requires an effective delivery system for appropriate therapy. Solid lipid nanoparticles (SLNs) have emerged as a promising delivery system for delivery of anticancer drugs. SLNs combine the advantages of polymeric nanoparticles, fat emulsions, and liposomes. In addition, they also avoid some of their disadvantages. Solid matrices in SLNs provide controlled release of drugs, thereby avoiding the burst release generally associated with fat emulsions. Hot homogenization method is one of the methods for the preparation of SLN that is found appropriate for production scale [7].
Encapsulating 5-FU in SLN formulations has been attempted by a few researchers previously. Mao et al. investigated formulation factors influencing the properties of 5-FU-loaded SLNs prepared by hot homogenization method [8]. 5-FU SLNs prepared using phospholipids were found to effectively reduce MDA-MB-468 tumor growth [9]. As an inhalation therapy, 5-FU SLNs have been found effective in lung cancer [10, 11]. Multiple emulsion-ultrasonication method has also been employed to prepare 5-FU, which was found to be a promising alternative for passive targeting therapeutic agents for curing primary lung carcinoma [12]. In a recent report, 5-FU SLNs were formulated using Dynasan as lipid matrix to treat colon cancer [13].
In the present study, SLNs of 5-FU were prepared by hot homogenization method. Cetyl palmitostearate (CP) and glyceryl monostearate (GMS) were selected as lipid carriers based on the partitioning of 5-FU in various lipids. The process variables such as speed of stirrer (ULTRA-TURRAX, IKA, Rawang, Selangor, Malaysia), homogenization pressure, and homogenization cycles were optimized. The stability of the optimized SLNs was assessed by electroflocculation method. The morphology of SLNs was studied using a transmission electron microscope (TEM). The in vitro studies carried out on the prepared SLNs included the release studies in pH 3.5 and 7.4. The SLNs were also assessed for its anticancer activity using metastatic B16F10 melanoma cell lines through functional assays such as chemosensitive assay, wound assay, colony formation assay, and Leighton tube assay.
Methods
Materials and apparatus
5-FU was a gift sample received from M.S. Otto Kemi (Mumbai, India), and glyceryl monostearate, cetyl palmitate, sodium taurocholate (STC), and poloxamer 407 (P407) were gifts provided by Colorcon India Ltd. (Mumbai, India). All other chemicals and reagents used were of laboratory grade and used as such.
The apparatus that were used in this study are the following: spectrophotometer (Shimadzu UV 1601, Shimadzu Corporation, Kyoto, Japan), high-shear apparatus (ULTRA-TURRAX T18, IKA, Rawang, Selangor, Malaysia), high-pressure homogenizer (Emulsiflex E5, AVESTIN, Inc., Ottawa, Ontario, Canada),particle size analyzer (Malvern Hydro 2000SM particle size analyzer, Malvern Instruments, Worcestershire, UK), zeta potential analyzer (Malvern Zetasizer, Nano ZS90, Malvern Instruments, Worcestershire, UK); TEM apparatus (Zeiss TEM 109, Carl Zeiss, Inc., Oberkochen, Germany), TEM size-measuring device (ultrastructuresize calculator (Pello scale), Van Loenen Instruments, Zaandam, The Netherlands), MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] test apparatus (Spectramax 190 microplate spectrometer, Sunnyvale, CA, USA),wound width-measuring apparatus (laser capture microdissection microscope with PalmRobo software, Carl Zeiss, Inc., Oberkochen, Germany, and Leighton tube apparatus (inverted-light microscope, Shamboo Scientific Glass Works, Haryana, India).
Cell and culture conditions
B16F10, a highly metastatic lung selected subline derived from C57/BL6 murine melanoma, was purchased from National Center for Cell Science (Pune, India). The cell line was maintained as a continuous culture in Iscove’s minimum Dulbecco’s medium (IMDM;Gibco-BRL, Gaithersburgh, MD, USA), supplemented with 10% fetal bovine serum (FBS; Himedia, Mumbai, India), 100 U/mL penicillin, and 100 μg/mL streptomycin. Cells were grown in a humidified atmosphere of 5% CO2 and 95% air at 37°C. Media were replenished every three days.
Partitioning behavior of 5-FU in various lipids
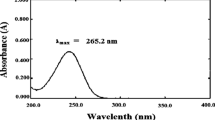
Ten milligrams of 5-FU was dispersed in a mixture of melted lipid (1 g) and 1 mL of hot distilled water and shaken for 30 min in a hot water bath. Aqueous phase was separated after cooling and analyzed spectrophotometrically at λmax 267.
Preparation of solid lipid nanoparticles
SLNs were prepared by modified hot homogenization method. Briefly, CP and GMS were dissolved in ethanol and added to a hot P407/P407-to-STC solution (5°C above the melting point of the lipid) using the high-shear apparatus. The resultant dispersion was then passed through the high-pressure homogenizer at 65°C for 3 cycles.
Particle size analysis
The particle size was measured in a particle size analyzer. The SLN dispersion was added to the sample dispersion unit containing stirrer and stirred in order to minimize the interparticle interactions. The obscuration range was maintained between 5% and 20%. The instrument was set to measure the sample three times at a rate of 3,000 snaps (or counts) per second, and the average volume mean diameter was obtained.
Percent entrapment efficiency
The prepared SLNs were passed through a column of sephadex G25. The in-house sephadex G25 column had a dimension of 1.5 cm × 4 cm. Initially, a blank check was carried out on a 1-mL column and was saturated. Lipid nanoparticle suspensions of 0.2, 0.3, 0.5, and 1 mL were loaded in column with water elution. The flow rate was found to be 1.0 mL/min−1. It was possible to separate encapsulated and free drug from 0.2 mL of sample (column recovery, 99.26; RSD, 1.54%). An aliquot of 5 mL of this formulation was used for analysis. The SLNs were shaken with 5 mL of chloroform in a separating funnel. The chloroform portion was separated and analyzed for 5-FU content spectrometrically at λmax 267 nm against chloroform as reference.
Zeta potential
Zeta potential of nanoparticles was measured using the zeta potential analyzer. The nanoparticles were dispersed in water, and the zeta potential was determined.
Transmission electron microscopy
TEM was performed to measure the morphology and size distribution of nanoparticles. The apparatus was operated at an acceleration voltage of 60 kV. To measure the morphology and size distribution of nanoparticles, a drop of sample solution was placed onto a 300-mesh copper grid coated with carbon. Approximately 2 min after deposition, the grid was tapped with filter paper to remove surface water and was air-dried. Negative staining was performed using a droplet of 2 wt.% aqueous uranyl acetate. The size of the nanoparticles was measured using the ultrastructure size calculator (Pello scale).
In vitro release studies
The in vitro release of 5-FU-loaded CP/GMS SLNs was evaluated by diffusion technique. Dialysis bags with a molecular weight cutoff of 12,000 (Himedia, Mumbai, India) were used in the study. Before performing the in vitro release, the dialysis bag was tested for permeation study of the drug. One milliliter of SLN dispersion was loaded in the dialysis tube, and the dialysis tube tied firmly at both ends was immersed in 50 mL phosphate-buffered saline (PBS) (pH 3.5 and 7.4). Aliquots of 5-mL samples were withdrawn from the medium and replaced with the same volume of fresh dissolution medium every time. The samples were estimated spectrophotometrically at λmax 268.
In vitro anticancer studies
Chemosensitivity assay
The cytotoxicity of 5-FU and its CP/GMS SLNs were evaluated by MTT method. The experiment was carried out as follows: 100 μL of cell culture medium (IMDM supplemented with 10% FBS and antibiotics) containing 4 × 104 cells was added in each well in a 96-well plate and incubated for 48 h. The confluent wells were treated with 5-FU drug solution and 5-FU-loaded CP/GMS SLN concentrations prepared in culture media between 10° and 5 × 101 μg/mL. The empty nanoparticles of similar dilutions were added to the control wells.
After 48 h of incubation (in moisture atmosphere, enriched by 5% CO2), the plates were washed with PBS. An aliquot of 100 μL of culture medium containing 20 μL MTT was added to the plates and incubated for further 4 h at 37°C. After this, the contents of the plates were replaced with 50 μL DMSO, and optical density at 540 nm was measured after background correction at 690 nm using the Spectramax 190 microplate spectrometer.
Wound assay
B16F10 cells were plated in 35-mm petriplates and were allowed to grow to 60% confluency. The plates were treated with subtoxic doses of 5-FU solution, 5-FU-loaded CP/GMS SLNs, and their corresponding blanks (for 24 and 48 h). At the end of incubation, the cells were washed with PBS, and the wound was prepared on the monolayer. A zero time point wound was kept as reference plate. Remaining plates were incubated for 24 and 48 h in the presence of serum-free IMDM. The plates were fixed with methanol and stained with crystal violet (Sigma-Aldrich, St. Louis, MO, USA). The wound widths were measured using the laser capture microdissection microscope using PalmRobo software after 24 and 48 h of incubation. The width of the wound was calculated for each dose on the pictures, and percentage migration was expressed as follows:
Twenty-five wound readings of each formulation were taken. The experiment was performed in duplicate. Percent relative wound widths were calculated, and the statistical significance in the case of relative wound widths were calculated using SPSS package.
Colony formation
B16F10 melanoma cells were incubated for 24 h (4 × 103cells per plate) and 48 h (2 × 103 cells per plate). The plates were then treated with subtoxic concentrations of 5-FU solution, 5-FU-loaded CP/GMS SLNs, and their corresponding blanks. After incubation, the plates were washed with PBS and then incubated with complete medium (10% FBS and IMDM) for another 48 h. The cells were then fixed using 70% alcohol and stained using the crystal violet. Colonies having more than 50 cells were counted, and the percent colony inhibition (PCI) capacity of the formulations was calculated as follows:
Leighton tube studies
B16F10 melanoma cells were grown on cover slips in 30-mm petriplates in the presence of complete medium (10% FBS with IMDM). The plates were treated with sub-toxic concentrations of 5-FU solution, 5-FU-loaded CP/GMS SLNs, and their corresponding blanks and incubated for 24 and 48 h. After incubation, the cells are fixed using 70% alcohol and then stained using hematoxylin and counter stained with eosine. The cover slips were washed in xylene and finally mounted on the slides. The changes in morphology of B16F10 cells after the treatments of 5-FU solution, 5-FU-loaded CP/GMS SLNs, and their corresponding blanks were assessed under the inverted-light microscope.
Results and discussion
Preparation and optimization of SLNs
Drug partitioning between various lipids and water has been used by several researchers to select appropriate lipids in the preparation of lipid nanoparticles [14, 15]. The lipids used in the study and the partition coefficients of 5-FU in these lipids are provided in Table 1. The partition coefficient of 5-FU in CP and GMS was high, and therefore, these lipids were selected for further studies.
Influence of process parameters
Initially, the influence of process parameters such as speed of stirrer (ULTRA-TURRAX), homogenization pressure, and homogenization cycles were investigated. The investigation was carried out using blank SLNs using 4% lipid (CP/GMS) and 1% P407 as emulsifier. The influence of stirring speedsof ULTRA-TURRAX on SLNs was studied and found to be 6,500 and 9,500 rpm at time durations of 5, 10, and 15 min. The stirring speed of 9,500 rpm for 15 min provided the maximum mean particle size of SLNs (5 μm). Since no decrease in mean particle size was observed on further increase of the stirring speed and stirring time, 9,500 rpm for 15 min was used for preparing SLNs. Similarly, the effects of homogenization cycles and homogenizer pressure were evaluated by measuring the mean particle sizes at 5,000, 10,000, and 15,000 psi at 1, 2, and 3 cycles. Homogenization speed of 5,000 psi pressure for 3 cycles gave a mean particle size of 526 nm. At 15,000 psi for 3 cycles, maximum reduction in mean particle size was observed, which was found to be 198 nm.
Effect of lipid and emulsifier concentrations
The lipid concentration and the emulsifier content are known to influence the particle size and the drug loading of the SLNs [14]. Lipid concentrations between 2%and 4% were used in the optimization of SLNs. In the optimization of both the lipid matrices (CP and GMS), the percent entrapment efficiencies (EE%) increased with the increase in lipid concentration from 2% to 4% and at P407 concentration of 1%. In the case of CP SLNs, the EE% increased from 49.65% to 69.46% (Table 2). While in the GMS SLNs, the increase was from 41.82% to 60.27%. Increase in lipid content beyond 4% resulted in agglomeration of SLNs.
Formulations with varying concentrations of 5-FU from 40 to 120 mg were used in optimizing 5-FU concentration. Increase in drug concentration brought about an increase in EE% in both the SLN formulations. The increase was observed till 120 mg beyond which no increase in EE% was observed. The increases in CP and GMS SLNs were 84.26 and 72.68, respectively.
STC and P407 were used in the optimization which were incorporated individually or in combination. The EE% was less when STC was used in comparison with P407. When used in combination, the increase in STC composition resulted in the decrease in EE%. In the CP SLNs, the EE% increased from 72.62% to 86.94% when the STC/P407 ratio was increased from 1:1 to 1:2. However, further increase in STC content did not increase the EE% of 5-FU. A similar phenomenon was observed with GMS SLNs; however, higher EE% was achieved with STC/P407 (1:3). The percent drug loading of 5-FU in CP and GMS SLNs were found to be 2.608% and 2.358%. A previous study using phospholipids has also observed a loading of 3% when prepared by hot homogenization method [8]. The lipid and emulsifier concentrations had little effects on the mean particle size of the SLNs.
The formulation parameters did not affect the particle size of both the SLN formulations. It ranged from 153 to 208 nm in the case of GMS SLNs and 168 to 193 nm in the case of CP SLNs. The zeta potential of the optimized CP and GMS SLN formulation was found to be −8.26 and −9.35 mV, respectively.
Transmission electron microscopy
The 5-FU-loaded SLNs prepared from CP and GMS were found to be of spherical shape. The mean particle sizes were 150 and 200 nm (Figure 1a,b). Previously, SLN preparations with CP and GMS have shown spherical shape nanoparticles [15, 16].
In vitro release studies
The release kinetics of 5-FU from the SLNs was assessed at pH 3.5 and 7.4. The lipid carriers effectively retarded release of 5-FU in both media, although it was observed that the release of 5-FU was faster in acidic medium (Figure 2). The release pattern in both formulations showed burst release. The burst release was similar to what is observed with SLNs commonly [13]. In pH 7.4, the release was complete in 24 h, while it took only 16 h in pH 3.5. Burst release of a drug generally takes place in lipid nanoparticles that are obtained as a ‘drug-enriched shell with core shell model.’ In these nanoparticles, the drug partitions to water phase during production. The lipid precipitates on cooling, and as a result of phase separation, the lipid forms a core that is drugfree. Meanwhile, drug re-partitions into the liquid lipid phase and concentrates in the outer shell. The drug partitioning into the aqueous phase increases with increase in solubility in aqueous phase.
The release kinetics of drug from the carrier was assessed using theoretical dissolution equations of zero-order, first-order, Higuchi, and Peppas-Korsenmeyer kinetic models (Table 3). The release of 5-FU from both lipid carriers in both media fitted into the Peppas-Korsenmeyer model. The n in the Peppas-Korsenmeyer equation is indicative of the mechanism of action. If the value of n is below 0.45, it denotes that the release follows Fickian class I controlled drug release. If n has a value of 1, it indicates a non-Fickian drug release. In the present study, the values of n of both the nanoparticles CP and GMS were below 0.45 which means that the release from the nanoparticles followed the Fickian class I release.
In vitro anticancer studies
Chemosensitivity assay
The free drug and SLN formulations demonstrated a concentration-dependent antiproliferative activity (Figure 3). At the end of 48 h, the free drug and SLN formulations completely inhibited the growth of B16F10 melanoma cells. The SLN formulations showed better proliferative activity compared to the drug solution (p < 0.05). Among the SLN formulation, CP SLNs performed better than GMS SLNs (p < 0.05). Previously, lipid nanoparticles of 5-FU has been reported to effectively inhibit MDA-MB-468 breast cancer cells [9]. The IC50 values of 5-FU solution, 5-FU-loaded CP, and 5-FU-loaded GMS SLNs were found to be 0.24, 0.16, and 0.52 μg/mL at 24 h. At 48 h, the IC50 values were 0.02, 0.02, and 0.04 μg/mL for 5-FU drug solution, 5-FU-loaded CP SLN, and 5-FU-loaded GMS SLN, respectively. The subtoxic concentrations for further studies were selected based on these IC50 values which were 0.12, 0.08, and 0.26 μg/mL for 5-FU, 5-FU-loaded CP SLNs, and 5-FU-loaded GMS SLNs, respectively at 24 h. At 48 h, the subtoxic concentrations selected were 0.01, 0.01, and 0.02 μg/mL, respectively, for 5-FU, 5-FU-loaded CP SLNs, and 5-FU-loaded GMS SLNs.
Wound assay
The in vitro analysis of migration of cells can be easily carried out using wound assay [17]. Cell motility is an important component of cell invasion and spread of cancer cells through the body. The ability to exploit factors that enable cell motility may endow a tumor cell with a greater ability to metastasize. 5-FU is known to inhibit cell migration. In the study, 5-FU and its SLN formulations were found to significantly exert antimigrational effect on B16F10 cell lines at subtoxic concentrations compared to the control (p > 0.5) (Figure 4a). At the end of 48 h, 5-FU solution (0.02 μg/mL) had better inhibitions compared to the SLN formulations. Among the SLN formulations, 5-FU-loaded CP SLN (0.12 μg/mL) was found to have comparable inhibition of cell motility of B16F10 with that of 5-FU solution. As anticipated, the blank formulations did not have any antimigrational activity. Photographic representation of wound widths of each formulation studied is given in Figure 4b.
Percent relative wound widths and photo samples of 5-FU-loaded CP and GMS SLNs. (a) Percent relative wound widths of 5-FU-loaded CP and GMS SLNs compared to 5-FU solution at 48 h; (b) photo samples of wound widths of 5-FU-loaded CP and GMS SLNs, 5-FU solution as well as their respective blank nanoparticles taken using the laser capture microdissection microscope.
Evaluation of cell migration using scratch test carried out on nanoparticulate formulation has been reported in several studies. The method established titanium oxide nanoparticles to be effective against tumor cells [18]. Lipid nanoparticles of opioids enhanced inhibition capacities of opioids by twofold in comparison with CMS nanotransporters when evaluated in HaCaT cells [19].
Colony formation assay
In the study, the subtoxic concentrations of all 5-FU formulations exerted concentration-dependent colony-inhibiting activity compared to control (p < 0.05) (Figure 5). The 5-FU solution (0.02 μg/mL) had a better colony-inhibiting activity compared to the SLN formulations. Among the SLNs, only 5-FU CP SLN (0.12 μg/mL at 24 h and 0.01 μg/mL at 48 h) had comparable colony inhibition with that of 5-FU solution. As anticipated, the blank formulations did not possess any antimigrational activity. Podophyllotoxin encapsulated in its lipid nanoparticulate form provided a long-term cancer growth suppression of 293 T and HeLa cells as determined from colony formation assay [20].
Leighton tube
Leighton tube study was carried to assess the extent of cellular damage such as destruction of the cell wall and release of cellular content by the 5-FU SLN formulations in comparison with the 5-FU solution. The study showed that the extent of cellular damage caused by 5-FU SLN formulations were similar to that of 5-FU solution (Figure 6). At the end of 48 h, drastic morphological damage was observed with 5-FU-loaded CP SLN (0.01 μg/mL) compared to other formulations and drug solution. No morphological changes were observed with blank formulations. Similar studies carried out on chitosan nanoparticles showed necrotic morphology when it inhibited cell proliferation of MGC803 cells [21].
Conclusions
SLNs prepared using CP and GMS by hot homogenization method provided high entrapment (86.94% and 78.56%, respectively). The method employed showed consistent and reproducible mean particle sizes (172 to 253 nm). In vitro release studies revealed that the lipid matrix could retard the release irrespective of the pH, although it was found to release it faster in acidic conditions. In vitro antiproliferative studies on B16F10 revealed that the activity of 5-FU is retained despite being encapsulated in CP/GMS. The 5-FU encapsulated in CP showed comparable antimigrational activity, colony inhibition activity, and cytopathic as that of 5-FU solution, and the effects of 5-FU CP SLNs were better than the effects of 5-FU GMS SLNs. On the basis of these results, it could be concluded that 5-FU-loaded CP SLNs can be considered a promising system for in vivo 5-FU delivery. Further studies in murine metastatic B16F10 melanoma model can provide vital information on the potency of CP as a potential carrier for the delivery of 5-FU.
Authors' information
RSRM was a professor at the Department Pharmacy, The M S University of Baroda, Fatehgunj, Vadodara, Gujarat 390 002, India. Presently, he is the Director Academics, I S F College of Pharmacy, Moga, Punjab. RPG is a scientific officer at the Department of Chemotherapy, Tata Memorial Center, Cancer Research Institute, Kharghar, Navi Mumbai 410208, India. VSS was a research scholar at the Department Pharmacy, The M S University of Baroda, Fatehgunj, Vadodara, Gujarat 390 002, India.
References
Grem JL: Cancer Chemotherapy and biotherapy: Principles and Practice. Edited by: Chabner BA, Longo DL. Philadelphia: Lippincott-Raven; 1996.
Grem JL, Nguyen D, Monahan BP, Kao V, Geoffroy FJ: Biochem Pharmacol. 1999, 58: 477. 10.1016/S0006-2952(99)00099-4
McEnvoy GK, Litvak K, Welsh OH (Eds): AHFS Drug Information. Bethesda, MD: American Society of Health-System Pharmacists, Inc; 1998.
van Kuilenburg ABP, Haasjes J, Richel DJ, Zoetekouw L, Van Lenthe H, De Abreu RA, Maring JG, Vreken P, van Gennip AH: Clin. Cancer Res. 2000, 6: 4705.
Di Paolo A, Danesi R, Falcone A, Cionini L, Vannozzi F, Masi G, Allegrini G, Mini E, Bocci G, Conte PF, Del Tacca M: Ann. Oncol. 2001, 12: 1301. 10.1023/A:1012294617392
Heggie GD, Sommadossi J, Cross DS, Huster WJ, Diasio RB: Cancer Res. 1987, 47: 2203.
Jenning V, Lippacher A, Gohla SH: J. Microencapsulation. 2002, 19: 1. 10.1080/713817583
Mao S, Wang P, Bi D: Pharmazie. 2005, 60: 273.
Jain SK, Chaurasiya A, Gupta Y, Jain A, Dagur P, Joshi B, Katoch VM: J. Microencapsulation. 2008, 25: 289. 10.1080/02652040701799598
Hitzman CJ, Elmquist WF, Wattenberg LW, Wiedmann TS: J. Pharm. Sci. 2006, 95: 1114. 10.1002/jps.20591
Hitzman CJ, Elmquist WF, Wiedmann TJ: J. Pharm. Sci. 2006, 95: 1127. 10.1002/jps.20590
Du B, Yan Y, Li Y, Wang SY, Zhang ZZ: Pharm. Dev. Technol. 2010, 15: 346. 10.3109/10837450903246390
Yassin AEB, Anwer MK, Mowafy HA, El-Bagory IM, Bayomi MA, Alsarra IA: Int. J. Med. Sci. 2010, 7: 398.
Muller H, Mader K, Gohla S: Eur. J. Pharm. Biopharm. 2000, 50: 161. 10.1016/S0939-6411(00)00087-4
zur Muhlen A, Schwarz C, Mehenert W: Eur. J. Pharm. Biopharm. 1998, 45: 149. 10.1016/S0939-6411(97)00150-1
Venkateswarlu V, Manjunath K: J. Controlled Release. 2004, 95: 627. 10.1016/j.jconrel.2004.01.005
Griffioen AW, Molema G: Pharmacol. Rev. 2000, 52: 237.
Sungkaworn T, Triampo W, Nalakarn P, Triampo D, Tang IM, Lenbury Y, Picha P: Int. J. Biol. Life Sci. 2007, 2: 67.
Wolf NB, Küchler S, Radowski MR, Blaschke T, Kramer KD, Weindl G, Kleuser B, Haag R, Schäfer-Korting M: Eur. J. Pharm. Biopharm. 2009, 73: 34. 10.1016/j.ejpb.2009.03.009
Zhu RR, Qin LL, Wang M, Wu SM, Wang SL, Zhang R, Liu ZX, Sun XY, Yao SD: Nanotechnology. 2009, 20: 055702. 10.1088/0957-4484/20/5/055702
Qi LF, Xu ZR, Li Y, Jiang X, Han XY, World J: Gastroenterol. 2005, 11: 5136.
Acknowledgments
The authors would like to acknowledge the assistance of Mr. Jishnu PS, senior executive, BioQuest Solutions, Bangalore, India, for his contributions in making the illustrations.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
RSRM and VSS designed and interpreted the results of preparation and characterization of nanoparticles. RPG designed the cell line studies and the interpretation of the results. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Shenoy, V.S., Gude, R.P. & Murthy, R.S.R. In vitro anticancer evaluation of 5-fluorouracil lipid nanoparticles using B16F10 melanoma cell lines. Int Nano Lett 3, 36 (2013). https://doi.org/10.1186/2228-5326-3-36
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2228-5326-3-36