Abstract
Ni0.8-xCu0.2Zn x Fe2O4 (x = 0.0 ≤ 0.6 with steps of 0.2) ferrite nanophase was achieved by sol–gel auto-combustion technique. The as-prepared samples were thermally characterized by thermogravimetry/differential thermal analysis to obtain firing temperature of the materials. The X-ray diffraction pattern indicates the formation of a single-phase cubic spinel structure and shows strong influence of the incorporation of Zn2+ metal ions on the spinel structure. The annealing treatment does not alter the crystal structure but increases the crystallinity of the samples. The morphological investigations and nanometric sizes of the samples were studied by scanning electron microscopy and transmission electron microscopy. The crystallographic texture due to annealing and Zn2+ ion doping was systematically investigated by Fourier transform infrared spectroscopy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
The development of civilization has been intimately linked with the ability of human beings to work with advanced magnetic nanomaterials. Nanocrystalline magnetic particles with specific properties can be synthesized by different chemical techniques [1–4]. These properties are strongly dependent on their shape, size, crystallinity, and distribution of the cations among the tetrahedral (A) and octahedral (B) sites of the spinel structure. The use of nanoparticles for desired applications has attracted considerable attention in recent years because nanoparticles provide high surface area-to-volume ratios [5, 6]. Magnetic particles are gaining attraction due to a variety of technological applications such as high-density information storage nanodevices, ferrofluids, magnetic refrigeration, residential cooling, and biomedical applications like magnetic separations, biosensors, resonance imaging, hyperthermia, and targeted and controlled drug delivery. In this regard, spinel ferrites are particularly important because of their excellent magnetic properties that can be tuned to suit the requirement using chemical manipulations [7–11]. However, the development of novel techniques for improvements of the magnetic and the structural properties of soft magnetic spinel ferrites has been the objective of numerous studies in past decades.
Recently, Ni-Cu-Zn spinel ferrites with nanometric dimensions were extensively studied in order to develop multilayer chip inductors [12–15]. Many methods such as sol–gel auto-combustion technique [16], chemical coprecipitation [17], citrate precursor method [18], oxalate precursor technique [19], and ceramic method [20] are used to prepare Ni-Cu-Zn spinel ferrite. Among them, the sol–gel auto-combustion method has unique advantages such as excellent composition control, low temperature process, low production cost, and better results.
In the present investigation, we report the sol–gel auto-combustion synthesis of Ni-Cu-Zn ferrite. The Ni-Cu spinel ferrite material allows the introduction of Zn2+ cation in the spinel matrix, which causes the change in the structure and surface morphology considerably. Optimization of Zn2+ concentration with respect to Ni2+ will be investigated. Correlation between compositions of Ni-Cu-Zn spinel ferrites and sintering temperatures on structural, infrared, and morphological behaviors has been carried out.
Methods
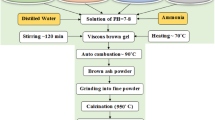
A spinel ferrite compositions of Ni0.8-xZn x Cu0.2Fe2O4 (with x = 0.0, 0.2, 0.4, and 0.6) nanoparticles were prepared using the sol–gel auto-combustion technique from the high-purity analytical grade solutions of Fe(NO3)3·9H2O, Cu(NO3)2·3H2O, Zn(NO3)2·6H2O, and Ni(NO3)2·6H2O. Citric acid was used as fuel. The weighed amounts of these salts were completely dissolved in distilled water, and the solution was stirred for half an hour. This solution was then added to citric acid in such a way that in the final sample, the molar ratio of these nitrates and citric acid becomes 1:3. A small amount of ammonia was simultaneously added drop-wise to maintain the pH of 7 with continuous stirring of the solution. The as-prepared spinel ferrite materials were annealed at 400°C and 700°C for 2 h after confirmation by thermogravimetry (TG)-differential thermal analyses (DTA). The schematic of the chemical reaction involved in the present investigated ferrite system is depicted in Figure 1. The general nitrate-citrate combustion reaction may be written as follows:
The thermal behavior analysis of the as-prepared sample was carried out on a PerkinElmer system (model Diamond TG/DTA, PerkinElmer, Waltham, MA, USA) under air atmosphere in the temperature range from ambient to 1,000°C. The phase identification and structure analysis of the thermally treated powders were carried out by X-ray diffraction (XRD) analysis using a Philips X-ray diffractometer (model PW 3710, Philips, Amsterdam, The Netherlands). The morphological studies were carried out by field-emission gun scanning electron microscopy using JSM-7600F with an accelerating voltage of 0.1 to 30 kV and transmission electron microscopy (TEM) using Philips (model CM200) operating at 20 to 200 kV with a resolution of 2.4 Å. The Fourier transform infrared (FTIR) spectroscopy (model MAGNA 550, Nicolet Instruments Corporation, Madison, WI, USA)was taken in the range of 400 to 4,000 cm-1.
Results and discussion
In order to investigate the thermal growth process of Ni0.8-xZn x Cu0.2Fe2O4 nanoparticles, TG-DTA of the as-prepared powder samples were carried out from 30°C to 1,000°C. The typical TG-DTA curve for the sample x = 0.4 is shown in Figure 2. From TGA, a total weight loss of approximately 35% was observed when the sample was heated between ambient to 1,000°C in air. A weight loss of approximately 10% occurred between 30°C to 300°C that could be assigned to the elimination of surface water and solvents that remained in the material even after drying. The major weight loss at 300°C to 400°C attributed to the loss of structural water and the decomposition of organic compounds and nitrates. As expected, the decomposition reaction is strongly exothermic. In the DTA curve, we can observe that an exothermic peakat 370°C associated withthe decomposition of organic compounds in the structure due to the crystallization of the spinel ferrite material is relatively sharp and intense. The weight loss that is about 3% at 400°C to 600°C is due to the dehydroxylation of the ferrite material, which occurs up to high temperatures, followed by the collapse of the pores and the densification of the nanoparticles.
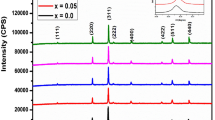
Figure 3 represents the typical room-temperature powder X-ray diffraction patterns for the annealed samples (at 400°C and 700°C) which confirm the formation of a single-phase cubic spinel ferrite. The annealing treatment does not alter the crystal structure but increases the crystallinity of the samples. The lower peak intensity and broadening in XRD patterns show the nanocrystalline nature of the materials. The sintered samples show a sharp XRD peak indicating the increase in the crystalline nature of the samples. The average crystalline size of each composition is estimated from the XRD line width of the (311) diffraction peak using the Scherrer formulae (D = 0.9λ/β cosθ, where D is the crystallite diameter, λ is the radiation wavelength, and θ is the incidence angle) [21], and values are presented in Table 1. It is evident from Table 1 that an average homogeneous crystalline spinel phase enhances as the doping of Zn2+ ions and sintering temperature increases. The variation of the lattice constant with the composition (x) calculated using inter planner spacing (d) and Miller indices (hkl) is presented in Table 1.
Microstructural and chemical changes occurring in the system during heat treatment and Zn2+ substitutions are visible in Figures 4, 5, 6, 7, 8. The scanning electron microscope images of the samples obtained after heat treatment at 400°C and 700°C and presented in Figures 4 and 5 show the nanocrystalline nature, and crystallinity increases as the annealing temperature increases. This may be attributed to the fact that the growth of the crystal takes place in different orientations. The crystallographic images clearly indicate that the particles are stacked on top of each other because of their mutual magnetic interactions.
The TEM images together with the SAED pattern (x = 0.2) for representative compound results of Ni0.8-xZn x Cu0.2Fe2O4 (x = 0.2, 0.4, and 0.6) nanoparticles are predicted in Figures 6, 7, 8. It has been observed that particles are aggregated during annealing and metal ion incorporation. The parallel lattice fringe is observed as uniformly extended over the primary building blocks, grain boundaries, and pores in the samples at x = 0.6 for all compositions, as shown in the insets of the said figures. Thus, it can be concluded that the nanoparticles are organized into an iso-oriented attached structure by sharing identical lattice planes.
The Fourier transform infrared spectra of the powder (as pellets in KBr) samples in the range 4,000 to 400 cm-1 were represented in Figure 9. The FTIR spectroscopy is a very useful technique to deduce not only the structural investigation and redistribution of cations between octahedral and tetrahedral sites of spinel ferrite nanoparticles, but also the lattice vibrational modes [22]. The FTIR spectra of all the samples exhibit two main absorption bands at wave numbers 600 and 400 cm-1 corresponding to intrinsic lattice vibrations of the ferrite skeleton for tetrahedral (A) and octahedral (B) sites, respectively [23]. The impurity peak is observed around 1,380 cm-1 in all the samples. The observed peak is attributed to the carboxyl group of C-O bonding. This may be due to the unreacted citric acid compound, which is used as fuel. The band around 1,600 cm-1 corresponds to the bending mode of H2O molecules [24], and one more is observed at 3,100 to 3,450 cm-1 corresponding to hydrogen-bonded O-H groups [1]. During the combustion process, high temperature was generated. All carboxyl and hydroxyl groups appear with less intensity and disappear as the annealing temperature increases, confirming the single-phase nature of the sample as evident from the XRD results. The increase in the bandwidth and area of 564 cm-1 is attributed to the increase in crystallinity after the heat treatment; reverse observations are observed in SHI-irradiated nickel ferrite thin films by Dixit et al. [25]. The careful observation of FTIR data shows that the peak intensity at the octahedral site decreases as annealing temperature and Zn2+ contents increase showing that cations migrate from the octahedral (B) site to tetrahedral (A) site. This observation is also supporting the cation distribution obtained from the XRD results and exothermic peak observed at 370°C in DTA analysis.
Conclusions
The influence of Zn2+ doping in nanocrystalline Ni-Cu spinel ferrite was successfully studied by synthesizing the materials by the sol–gel auto-combustion technique and characterizing them using different tools. Thermal behavior of the as-prepared samples was confirmed by TG-DTA. The X-ray diffraction pattern reveals the formation of single-phase cubic spinel structure for all the compositions and temperatures. The crystallite size of the product was found in nanometric dimensions using a line profile fitting of the XRD pattern. The novel TEM morphological results with variations in the composition and sintering conditions are promising candidates for MLC technological applications. The chemical analysis and infrared spectral analysis of the prepared material confirm the formation of the spinel ferrite phase and the effect of preparation aids.
References
Toksha BG, Sagar E, Shirsath ML, Mane SM, Patange SS, Jadhav SS, Jadhav KM: Auto-combustion high-temperature synthesis, structural and magnetic properties of CoCr x Fe 2-x O 4 (0 ≤ x ≤ 1.0). J. Phys. Chem. C 2011, 115: 20905. 10.1021/jp205572m
Cannas C, Ardu A, Musinu A, Peddis D, Piccaluga G: Spherical nanoporous assemblies of iso-oriented cobalt ferrite nanoparticles: synthesis, microstructure and magnetic properties. Chem. Mater. 2008, 20: 6364. 10.1021/cm801839s
Nandapure AI, Kondawar SB, Sawadh PS, Nandapure BI: Effect of zinc substitution on magnetic and electrical properties of nanocrystalline nickel ferrite synthesized by refluxing method. Physica. B. 2012, 407: 1104. 10.1016/j.physb.2012.01.081
Praveena K, Sadhana K, Murthy SR: Elastic behaviour of microwave hydrothermally synthesized nanocrystalline Mn 1- x –Zn x ferrites. Mater. Research Bulletin 2012, 47: 1096. 10.1016/j.materresbull.2011.11.054
Astruc D, Lu F, Aranzaes JR: Nanoparticles as recyclable catalysts: the frontier between homogeneous and heterogeneous catalysis. Angew. Chem. Int. Ed. 2005, 44: 7852. 10.1002/anie.200500766
Reetz MT, Maase M: Redox-controlled size-selective fabrication of nanostructured transition metal colloids. Adv. Mater. 1999, 11: 773. 10.1002/(SICI)1521-4095(199906)11:9<773::AID-ADMA773>3.0.CO;2-1
Rety F, Clement O, Siauve N, Cuenod CA, Carnot F, Sich M, Buisine A, Frija G: MR lymphography using iron oxide nanoparticles in rats: pharmacokinetics in the lymphatic system after intravenous injection. Magn. Reson. Imaging 2000, 12: 734. 10.1002/1522-2586(200011)12:5<734::AID-JMRI10>3.0.CO;2-R
Rosensweig R: Ferrohydrodynamics. Cambridge, U.K.: Cambridge University Press; 1985.
Philip J, Jaykumar T, Sundaram PK, Raj B: A tunable optical filter. Meas. Sci. Technol. 2003, 14: 1289. 10.1088/0957-0233/14/8/314
Pankhurst QA, Connolly J, Jones SK, Dobson J: Applications of magnetic nanoparticles in biomedicine. J. Phys. D: Appl. Phys. 2003, 36: R167. 10.1088/0022-3727/36/13/201
Yavuz CT, Mayo JT, Yu WW, Prakash A, Falkner JC, Yean S, Cong LL, Shipley HJ, Kan A, Tomson M, Natelson D, Colvin VL: Low-Field Magnetic Separation of Monodisperse Fe3O4 Nanocrystals. Science 2006, 314: 964. 10.1126/science.1131475
Shirsath SE, Kadam RH, Patange SM, Mane ML, Ali G, Akimitsu M: Enhanced magnetic properties of Dy3+ substituted Ni-Cu-Zn ferrite nanoparticles. Appl. Phys. Lett. 2012, 100: 042407. 10.1063/1.3679688
Murbe J, Topfer J: High permeability Ni–Cu–Zn ferrites through additive-free low-temperature sintering of nanocrystalline powders. J. Europ. Ceramic Soc. 2012, 32: 1091. 10.1016/j.jeurceramsoc.2011.11.021
Li Y, Zhao J, Han J, He X: Combustion synthesis and characterization of NiCuZn ferrite powders. Mater. Research Bulletin 2005, 40: 981. 10.1016/j.materresbull.2005.02.018
Penchal Reddy M, Balakrishnaiah G, Madhuri W, Venkata Ramana M, Ramamanohar Reddy N, Siva Kumar KV, Murthy VRK, Ramakrishna Reddy R: Structural, magnetic and electrical properties of NiCuZn ferrites prepared by microwave sintering method suitable for MLCI applications. J. Phys. Chem. Solids 2010, 71: 1373. 10.1016/j.jpcs.2010.06.007
Raghavender AT, Biliškov N, Skoko Ž: XRD and IR analysis of nanocrystalline Ni–Zn ferrite synthesized by the sol–gel method. Mater. Lett. 2011, 65: 677. 10.1016/j.matlet.2010.11.071
Han QJ, Ji DH, Tang GD, Li ZZ, Hou X, Qi WH, Liu SR, Bian RR: Estimating the cation distributions in the spinel ferrites Cu0.5–xNi0.5ZnxFe2O4 (0.0≤x≤0.5). J. Magn. Magn. Mater 2012, 324: 1975. 10.1016/j.jmmm.2012.01.039
Jadhav PA, Devan RS, Kolekar YD, Chougule BK: Structural, electrical and magnetic characterizations of Ni–Cu–Zn ferrite synthesized by citrate precursor method. J. Phys. Chem. Solids 2009, 70: 396. 10.1016/j.jpcs.2008.11.019
Ghodake SA, Ghodake UR, Sawant SR, Suryavanshi SS, Bakare PP: Magnetic properties of NiCuZn ferrites synthesized by oxalate precursor method. J. Magn. Magn. Mater. 2006, 305: 110. 10.1016/j.jmmm.2005.11.041
Murbe J, Topfer J: Low temperature sintering of sub-stoichiometric Ni–Cu–Zn ferrites: shrinkage, microstructure and permeability. J. Magn. Magn. Mater. 2012, 324: 578. 10.1016/j.jmmm.2011.08.040
Mane ML, Dhage VN, Sundar R, Ranganathan K, Oak SM, Shengule DR, Jadhav KM: Effect of Nd: YAG laser irradiation on structural, morphological, cation distribution and magnetic properties of nanocrystalline CoFe 2 O 4 . Appl. Surf. Sci. 2011, 257: 8511. 10.1016/j.apsusc.2011.05.004
Prakash I, Nallamuthu N, Muralidharan P, Venkateswarlu M, Manjusri M, Amar M, Satyanarayana N: Preparation and characterization of nanocrystalline CoFe2O4 deposited on SiO2: in situ sol–gel process. J. Sol–gel, Sci. Technol. 2011, 58: 24. 10.1007/s10971-010-2350-2
Waldron RD: Infrared spectra of ferrites. Phys. Rev. 1955, 99: 1727. 10.1103/PhysRev.99.1727
Huang Z, Zhu Y, Wang S, Yin G: Controlled growth of aligned arrays of Cu-Ferrite nanorods. Cryst. Growth Des. 2006, 6: 1931. 10.1021/cg0505517
Dixit G, Jitendra Pal S, Srivastava RC, Agrawal HM: Study of 200 MeV Ag15+ ion induced amorphisation in nickel ferrite thin films. Nucl. Instrum. Methods B 2011, 269: 133. 10.1016/j.nimb.2010.10.011
Acknowledgments
VVA acknowledges financial support from BCUD, University of Pune and SAIF, IIT Mumbai for SEM, TEM facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
VA, SR, MM, and KM have equal contributions. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Awati, V.V., Rathod, S.M., Mane, M.L. et al. Influence of Zn2+ doping on the structural and surface morphological properties of nanocrystalline Ni-Cu spinel ferrite. Int Nano Lett 3, 29 (2013). https://doi.org/10.1186/2228-5326-3-29
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2228-5326-3-29