Abstract
A green and efficient procedure for the preparation of bis(indolyl)methanes via the condensation of indoles with various carbonyl compounds in the presence of catalytic amount of nanosilica gel under ultrasonic irradiation in good to excellent yields (83% to 94% ) in solvent-free condition is described. Short reaction time, easy and quick isolation of the products, environmentally friendly procedure, excellent chemoselectivity, and excellent yields are the main advantages of this procedure. Native nanosilica gel was used as an inexpensive and readily available catalyst without any support of reagent or modification on it. The reaction is carried out in solvent-free condition in the absence of ultrasonic irradiation at 80°C, too. This methodology offers significant improvements for the synthesis of bis(indolyl)methanes with regard to the yield of products, simplicity in operation, and green aspects by avoiding toxic catalysts and solvents. Recovery and recycling of the silica nanoparticle catalyst were also described.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Recently, the applications of nanoparticles (NPs) in catalysis have attracted considerable attention because of their improved efficiency and facile reaction conditions [1, 2]. These materials have enormously large and highly reactive surface area, so these materials exhibit some unique properties in comparison to bulk materials. Because of individual property of silica-based NPs compared to others, it has been well studied. Silica NPs are easily prepared in room temperature, and their sizes can be easily tuned. On the other hand, silica NPs are stable in organic solvent and are environmentally friendly materials [3–12].
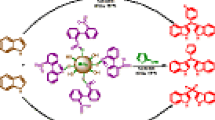
The ultrasonic irradiation is applicable to a broad range of practical syntheses. Some advantages of ultrasound procedure are short reaction times, mild reaction conditions, formation of purer products, and waste minimization. Ultrasonic irradiation can also be used to influence selectivity and yields of reactions [13–15]. Despite the vast advantages of this technique, the use of ultrasound in the synthesis of organic compounds is not fully developed. To develop the application of ultrasound in organic reaction, herein, we wish to describe the reaction of indoles with aldehydes in the presence of a catalytic amount of native silica gel nanoparticles under ultrasonic irradiation (Scheme 1).
Bis(indolyl)methanes, indole, and their derivatives are known as important intermediates in organic synthesis and pharmaceutical chemistry, and exhibit various physiological properties [16]. Bis(indolyl)methanes are found in cruciferous plants and are known to promote beneficial estrogen metabolism [17] and induce apoptosis in human cancer cells. Therefore, there is great interest in the synthesis of these compounds [18–21]. Synthetically, the reaction of indole with aldehydes or ketones produces azafulvenium salts that react further with a second indole molecule to form bis(indol-3-yl)methanes [22]. In recent years, syntheses of this class of molecules under mild conditions have been reported, with promoters such as montmorillonite clay K-10 [23, 24], trichloro-1,3,5-triazine [25], AlPW12O40[26], sodium dodecyl sulfate [27], ZrCl4[28], I2[29], In(OTf)3/ionic liquid [30], CuBr2[31], MW/Lewis acids (FeCl3, BiCl3, InCl3, ZnCl2, CoCl2) [32], NaHSO4 and Amberlyst-15 [33], silica sulfuric acid [34], metal hydrogen sulfates [35], NaHSO4/ionic liquid [36], CAN [37], NBS [38], and Ph3CCl [39]. However, most of the existing methods involve toxic metal ions and solvents, have high costs, use corrosive reagents, and have cumbersome work-up procedures. Consequently, new procedures that address these drawbacks are desirable.
Methods
Materials and apparatus
All chemicals were purchased from Nanostructured & Amorphous Materials, Inc. (nano SiO2 15 nm; TX, USA), Sigma-Aldrich Co. (MO, USA), or Merck & Co., Inc (NJ, USA) chemical companies and used without further purification. Products were characterized by comparing their spectral (IR, 1HNMR, and TLC) and physical data (m.p. and b.p.) with those of authentic samples. Infrared spectra were recorded on a WQF-510 FTIR spectrometer (Rayleigh Instruments Ltd., Essex, England). 1H NMR spectra were recorded with a DRX-500 AVANCE spectrometer (Bruker Corporation, MA, USA) at 500 MHz (1H) in CDCl3 or DMSO-d6 as the solvent and tetramethyl silaneas internal reference. Melting points were measured with Electrothermal 9300 (Electrothermal, Essex, UK). Soltec Sonica Mod 3200 ETH ultrasonic cleaner bath (Soltec S.r.l., Milan, Italy) was used for ultrasonic irradiation. The reaction vessel placed inside the ultrasonic bath contained water.
General procedure
A mixture of indole (1 mmol), aldehyde (2 mmol), and silica NPs (20 mol%) was mixed and added some drop of diethyl ether to it. Further, the reaction mass was irradiated under ultrasonic irradiation at 80°C for appropriate time (Table 1). The progress of reaction was monitored by TLC. After the completion of reaction, the colored product obtained was recrystallized from ethanol and filtered to get the pure product.
Results and discussion
The methodology developed is simple with high to excellent yields. We first compared the catalyst effect on different solvents for synthesis of bis(indol-3-yl)methanes using native silica NPs, and the results are summarized in Table 2.
In a typical experiment, the reaction of indole and benzaldehyde in solvent or solvent-free condition was carried in the presence of silica NPs to afford the corresponding bis(indol-3-yl)methanes. We kept the catalyst concentration constant and used different solvents like dichloromethane, chloroform, toluene acetonitrile, THF, ethanol, and acetonitrile which afforded lower yields. We have also studied the sonochemical effect on model reaction using different solvents. In all cases, the experimental results show that the yields of the products are very low under sonication. Using lower amount of catalyst resulted in lower yields, while higher amount of catalyst did not affect the reaction times and yields, and in the absence of catalyst, the yield of the product was not found. The best results were obtained using 20 mol% of catalyst (Table 2, entry 7). Based on the results of this study, it seems that the reaction times and yields will improve in the solvent-free condition under ultrasound irradiation. The obtained results are summarized in Table 2, entries 1 to 7. These results suggest that the absence of solvent is the best condition for synthesis of bis(indol-3-yl)methanes. Furthermore, the effect of catalyst load on reaction time and yield is explored in Table 3.
The best result is obtained with 20 mol% of the catalyst. After optimizing the conditions, the generality of this method was examined by the reaction of several substituted aryl aldehydes with indole. The results are shown in Table 1. The products were isolated and identified by melting point, NMR, and IR (Scheme 1).
Conclusion
Silica NPs in solvent-free condition were found to be selective and effective catalysts in green synthesis of bis(indolyl)methanes under ultrasonic irradiation. This catalyst provides clean conversion; greater selectivity and easy work-up make this protocol practical and economically attractive.
References
Beletskaya IP, Cheprakov AV: Chem. Rev.. 2000, 100: 3009. 10.1021/cr9903048
Astruc D, Lu F, Aranzaes JR: Angew. Chem. Int. Ed.. 2005, 44: 7852. 10.1002/anie.200500766
Ramazani A, Mahyari A, Farshadi A, Rouhani M: Helv. Chim. Acta.. 2011, 94: 1831–1837. 10.1002/hlca.201100076
Ramazani A, Mahyari A: Helv. Chim. Acta.. 2010, 93: 2203–2209. 10.1002/hlca.201000124
Ramazani A, Mahyar A, Lashgari H, Slepokura K, Lis T: Helv. Chim. Acta.. 2011, 94: 611–622. 10.1002/hlca.201000280
Banerjee S, Horn A, Khatri H, Sereda G: Tetrahedron Let.. 2011, 52: 1878–1881. 10.1016/j.tetlet.2011.02.031
Banerjee S, Das J, Santra S: Tetrahedron Let.. 2009, 50: 124–127. 10.1016/j.tetlet.2008.10.110
Banerjee S, Santra S: Tetrahedron Let. 2009, 50: 2037–2040. 10.1016/j.tetlet.2009.01.154
Badr Y, Abd El-Wahed MG, Mahmoud MA: J. Hazard. Mater.. 2008, 154: 245–253. 10.1016/j.jhazmat.2007.10.020
Banerjee S, Sereda G: Tetrahedron Let.. 2009, 50: 6959–6962. 10.1016/j.tetlet.2009.09.137
Sreedhar B, Radhika P, Neelima B, Neha H: J. Mol. Catal. A: Chem.. 2007, 272: 159–163. 10.1016/j.molcata.2007.03.040
Haghnazari N, Karami C, Ghodrati K, Maleki F: Int. Nano. Lett.. 2011, 1: 30–33.
Sinha AK, Joshi BP, Sharma A, Kumar V, Acharya R: Aust. J. Chem.. 2007, 60: 124. 10.1071/CH06380
Sinha AK, Sharma A, Joshi BP: Tetrahedron. 2007, 63: 960. 10.1016/j.tet.2006.11.023
Kumar V, Sharma A, Sharma M, Sharma UK, Sinha AK: Tetrahedron. 2007, 63: 9718. 10.1016/j.tet.2007.07.018
Sundberg RJ: The Chemistry of Indoles. New York: Academic; 1996.
Zeligs MA: J. Med. Food.. 1998, 1: 67. 10.1089/jmf.1998.1.67
Chakrabarty M, Ghosh N, Basak R, Harigaya Y: Tetrahedron Let.. 2002, 43: 4075. 10.1016/S0040-4039(02)00682-2
Bandgar BP, Shaikh KA: Tetrahedron Let.. 2003, 44: 195.
Wang YY, Chen C: J. Chin. Chem. Soc.. 2007, 54: 1363.
Reddy AV, Ravinder K, Reddy VLN, Venkateshwer Goud T, Ravikanth V, Venkateswarlu Y: Synth. Commun.. 2003, 33: 3687. 10.1081/SCC-120025177
Remers WA: Heterocyclic Compounds, pp. 1–226. Edited by: Houlihan WJ. New York: Interscience Publishers; 1972.
Maiti AK, Bhattacharyya P: J. Chem. Res.. 1997., 424:
Chakrabarty M, Ghosh N, Basak R, Harigaya Y: Synth. Commun.. 2004, 34: 421. 10.1081/SCC-120027281
Sharma GVM, Reddy JJ, Lakshmi PS, Krishna PR: Tetrahedron Let.. 2004, 45: 7729. 10.1016/j.tetlet.2004.08.084
Firouzabadi H, Iranpoor N, Jafari AA: J. Mol. Catal. A: Chem.. 2005, 244: 168.
Deb ML, Bhuyan PJ: Tetrahedron Let.. 2006, 47: 1441. 10.1016/j.tetlet.2005.12.093
Zhang ZH, Yin L, Wang YM: Synthesis. 2005., 1949:
Ji SJ, Wang SY, Zhang Y, Loh TP: Tetrahedron. 2004, 60: 2051. 10.1016/j.tet.2003.12.060
Ji SJ, Zhou MF, Gu DG, Wang SY, Loh TP: Syn. Lett.. 2003, 13: 2077.
Mo LP, Ma ZC, Zhang ZH: Synth. Commun.. 2005, 35: 1997. 10.1081/SCC-200066653
Xia M, Wang SH, Yuan WB: Synth. Commun.. 2004, 34: 3175. 10.1081/SCC-200028611
Ramesh C, Banerjee J, Pal R, Das B: Adv. Synth. Catal.. 2003, 345: 557. 10.1002/adsc.200303022
Pore DM, Desai UV, Thopate TS, Wadgaonkar PP: Arkivoc.. 2006, 12: 75.
Niknam K, Zolfigol MA, Sadabadi T, Nejati A: J. Iran Chem. Soc.. 2006, 3: 318. 10.1007/BF03245953
Zhang LP, Li YQ, Zhou MY: Chin. Chem. Lett.. 2006, 17: 723.
Ramesh C, Ravindranath N, Das B: J. Chem. Res.. 2003., 72:
Koshima H, Matsuaka W: J. Heterocycl. Chem.. 2002., 1089:
Khalafi-Nezhad A, Parhami A, Zare A, Moosavi Zare AR, Hasaninejad A, Panahi F: Synthesis.. 2008., 617:
Nagawade RR, Shide DB: Bull. Korean Chem. Soc.. 2005, 26: 12.
Sadaphal SA, Shelke KF, Sonar SS, Shingare MS: Cent. Eur. J. Chem.. 2008, 6: 622. 10.2478/s11532-008-0069-5
Sadaphal SA, Sonar SS, Ware MN, Shingare MS: Green. Chem. Lett. Rev.. 2008, 1: 191. 10.1080/17518250802637819
Acknowledgment
Financial support from the Islamic Azad University of Kermanshah is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The author(s) did not provide this information.
Authors’ contributions
The author(s) did not provide this information.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Ghodrati, K., Hosseini, S.H., Mosaedi, R. et al. Convenient, efficient, and green method for synthesis of bis(indolyl)methanes with nano SIO2 under ultrasonic irradiation. Int Nano Lett 3, 13 (2013). https://doi.org/10.1186/2228-5326-3-13
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2228-5326-3-13