Abstract
ZnO, comprising nanosize particles (approximately 40 nm) has been prepared by heating (300°C) ZnCO3, which was obtained as precipitate by mixing ZnSO4 and (NH4)2CO3 solutions. The prepared ZnO was characterized by X-ray diffraction, scanning electron microscopy (SEM), laser-induced breakdown spectroscopy, and adsorption studies. It has been used to catalyze the decolorization of Ponceau S (PS), a model diazo dye, in an aqueous suspension under visible light (I ≈ 1.8 × 10−4 W cm−2). This ZnO was found to be more efficient as a photocatalyst compared to pristine ZnO. ZnO samples with higher temperatures (500°C and 700°C) show less catalytic activity. SEM images show that the particle size of ZnO increases with the increase in calcined temperature of ZnO through agglomeration, resulting in a decrease in surface area. Photodecolorization of PS is affected by its and ZnO concentrations, but unaffected by the initial pH of the solutions in the range of 4 to 7. Illumination for a sufficiently long time completely mineralizes the dye, but no Zn2+ can be detected in the clear solution.
Photodegradation kinetics in the ZnO suspension obeys the Langmuir-Hinshelwood equation, and some activation of the ZnO surface by light is indicated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
A substantial amount of dyestuff is lost during the dyeing process in the textile/dyeing industries. The discharged wastewater is highly colored and is of complex and variable nature [1]. This wastewater has been a long-standing concern, and different methods of removal of the color are still under trial. After the successful demonstration of the photocatalytic ability of titania (TiO2) [2], studies on its use for removal of pollutants, particularly dyes, from wastewater attracted wide attention. The source of light has been mostly ultraviolet (UV). The recent studies on various parameters to make TiO2 as an effective photocatalyst for this purpose, even with solar light, have been reviewed [3–6].
Zinc oxide (ZnO), with comparable bandgap energy as that of TiO2 (rutile), has hardly drawn as much attention as a photocatalyst. The reluctance to use ZnO probably originates from a study of the effect of UV illumination of its aqueous slurry [7]. ZnO dissolves forming Zn2+(aq) and simultaneously produces H2O2 in the presence of air. On the other hand, TiO2 is photochemically stable. However, one of the early studies of ZnO as a photocatalyst shows that it has high reaction and mineralization rates [8] because of its more efficient hydroxyl ion generation efficiency [9]. Subsequent studies discover that ZnO absorbs a large fraction of the solar spectrum and more light quanta than TiO2[10–14]. These studies have shown that ZnO acts as a better photocatalyst than TiO2 under the given conditions. ZnO has been found to satisfactorily degrade an azo dye, brilliant orange [11], and a non-azo dye, Bractive T Blue [12], from aqueous suspensions at the normal pH, approximately 6, of the dyes by visible light with an intensity of approximately 0.18 mW cm−2. Many more examples of the use of ZnO as a photocatalyst are available in the literatures [15–17]. One of the examples is the study showing that ZnO can be used as an alternative photocatalyst to TiO2[17].
The recent trend in the study of ZnO photocatalysis is predominantly based on nanosize ZnO and its amenability of structural modification for improved visible light absorption [18]. ZnO nanocrystals have been investigated in terms of operational parameters and kinetics of photocatalytic degradation [19]. A photocatalytic paper using zinc oxide nanorods has also been developed [20].
Photocatalysis using suspended particles to remove contaminants in aqueous systems is an efficient process, but the subsequent removal of the particles from the systems adds extra process steps. In this regard, use of supports for the catalysts is found to be helpful. However, there is an inverse relationship between the adhesion of a catalyst to a support and its photocatalytic activity [21].
Ponceau S (PS), a diazo dye, is used in the textile dying industry and also in coloring leather and paper [22]. It is used in clinical laboratories as a protein-binding dye for staining blood serum proteins [23]. Its biotransformation products have toxic effects on aquatic organisms, and it is a suspect as a carcinogen for the human body [24]. Some methods of its decolorization/degradation are reported [22, 25–27]. Most of these methods need further development in terms of time and cost.
In the present study, nanosize ZnO powder has been prepared and used as a photocatalyst to decolorize/degrade PS in the presence of visible light from fluorescent lamps. Suspended nanosized ZnO particles in the aqueous medium have been preferred to make the process simple to operate. The photodecolorization pattern of PS in this process is expected to be the same as that which will be obtained by a previously made nanosize ZnO attached to a suitable support.
Methods
Materials
PS, acid red 112 (C.I. 27195), was obtained from local dye suppliers. The structure of the dye is shown in Figure 1. Its absorption maximum, λmax, at 520 nm in the visible range is quite sharp (2.8 × 104 M−1 cm−1 at 30°C).
Pristine zinc oxide (>99.9% purity, MZ-500 with a surface area of 38.4 m2/g) is from Tayca Corporation, Japan. Zinc sulfate, ammonium carbonate, sodium hydroxide, and hydrochloric acid were purchased from BDH (Dubai, United Arab Emirates). All the chemicals were used without further purification. The pH of the solutions was adjusted to values between 4 and 7 using dilute solutions of HCl or NaOH. Deionized water was used throughout the experiment.
Preparation of zinc oxide
ZnCO3 was precipitated from 10% zinc sulfate solution at 60°C by ammonium carbonate solution. The precipitate was filtered and washed with water to remove sulfate ions. The dry (at approximately 105°C) ZnCO3 was heated at 300°C in a muffle furnace (OSK, Isuzu Seisa kusu Co., Ltd., Japan) until being in constant weight. Portions of this ZnO were further thermally treated separately at 500°C and 700°C for 1 h.
Characterization of the prepared ZnO
X-ray diffraction (XRD) was done using RINT 2200 (Rigaku Corporation, Tokyo, Japan). Some ideas about the particle sizes of ZnO samples were obtained from scanning electron microscope (SEM; model S-3400N, Hitachi, Tokyo, Japan) images. For further characterization of ZnO, a Spectra-Physics laser source (model LAB-170, pulsed Nd:YAG, Spectra-Physics Inc., Santa Clara, CA, USA) was used to get laser-induced breakdown (LIB) spectra of the samples. The SpectraPro 2758 spectrometer (Spectra-Physics Inc.) and Princeton Instruments PIXIS 100B CCD detector (Trenton, NJ, USA) were used.
Nanosize ZnO was also characterized by adsorption studies. ZnO portions of 0.20 g were soaked overnight in water in stoppered bottles. The volume was made up to 40 mL by adding water and PS solution so that the desired concentration and an initial pH, approximately pH 6, of the dye solution were maintained. The bottles with their contents were shaken in a thermostat shaker (NTS-4000AL, Tokyo Rika Kikai Co., Ltd, Tokyo, Japan) at 30°C. Samples were withdrawn at definite intervals of time and centrifuged, and their dye concentrations were determined by measuring absorbance of the clear solution at 520 nm (UV-Visible spectrophotometer, UV-160A, Shimadzu Corporation, Kyoto, Japan).
Decolorization experiment
The visible light illumination source comprised two 40-W fluorescent lamps, each with a length of 20 cm, placed side by side. The lamp house was a wooden box with the internal surface covered with aluminum foil. The temperature inside the box was maintained at 30°C by regulated flow of cool air through it. The air entered the box at the bottom and flowed out at the top. The approximate intensity of the visible light at the position of the reactor was approximately 1.8 × 10−4 W cm−2 as measured by a spectroradiometer (model IL-588, International Light Technologies, Peabody, MA, USA).
Fifty-milliliter beakers, with an internal diameter of 4.0 cm, were used as reactors. Each reactor was placed on the magnetic stirrer, and the distance of the solution surface from the lower part of the lamps was kept fixed.
ZnO portions of 0.20 g were taken in each of the several reactors containing 10 mL of water each. They were left overnight, and PS solutions of desired concentrations were added to the reactors to make the total volume in each to 40.0 mL. The contents of the reactors were agitated ultrasonically for 5 min prior to subjecting them to visible light illumination for definite times. The weight of each reactor was recorded. During the illumination, the suspensions were continuously stirred magnetically while the air passed through the lamp house. After illumination, the weights of the reactors were checked, and the weights were made up by the addition of water. Clear solutions, obtained after centrifugation, were analyzed spectrophotometrically at 520 nm to find out the concentration of the remaining PS in each reactor. The clear solutions (0 dye concentrations) were analyzed for free Zn2+ ions by AAS (Shimadzu) with reference to the standard ZnSO4 solution.
Percent decolorization and mineralization
Decolorization or color removal of PS was calculated as (X t /Xo) × 100, where X t is the absorbance at time ‘t’ and Xo is that at t = 0. On the other hand, the percent color remaining was [(Xo − X t ) ∕ Xo] × 100.
In the case of mineralization/degradation, Xo is the total organic carbon (TOC, ppm) of PS at t = 0, and X t (ppm) is its TOC at time ‘t’. The total organic carbon analyzer (CPH/CPN and 638 - 4241C, Shimadzu) was used for this purpose.
The initial rate of decolorization
The equation Ri = k[PS]i was used to determine the initial rate (Ri) by multiplying the apparent first-order rate constant k with the initial concentration of PS, [PS]i. k was determined from the slope of the plot ln([PS]i [PS]t) vs time.
Results and discussion
Characterization of ZnO
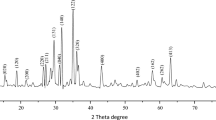
Figure 2 shows the XRD pattern of the prepared ZnO and commercially available ZnO (Tayca Corporation, Osaka, Japan). Results show that the prepared ZnO are crystalline and identical with wurtzite ZnO, and ZnO at 300°C contains a minor amount of Zn(OH)2.
Figure 3 shows the SEM images of thermally treated ZnO powders. ZnO particles made at 300°C are of approximately 40 nm in sizes. Agglomeration of the particles of ZnO made at 300°C to larger sizes results from heating.
Adsorption of PS on the prepared nanosize ZnO particles from aqueous suspension reveals some of their additional characteristics. The Langmuir equation for adsorption in the present system at equilibrium is expressed here as follows:
where KL is the Langmuir constant (L/mg) or adsorption constant. The ZnO-PS system obeys the Langmuir isotherm as the plots of Ce/(x/m) vs Ce are straight lines in all cases. Qm and KL values were computed from the slopes and intercepts, respectively, of the straight lines. The maximum amount of adsorption of PS corresponding to the complete monolayer formation on ZnO particles is represented by Qm (mg/g), and it is also known as the Langmuir constant related to the capacity of adsorption. Ce (mol/L, M) is the equilibrium concentration of PS in the suspension, and x/m (mg/g) is the amount of PS adsorbed per gram ZnO, at equilibrium, for its different initial concentrations.
PS molecules from the aqueous suspension, with [PS]i = 8.50 × 10−5 M, ZnO = 0.20 g/40 mL, pH = 6.02, and temp = 30°C, take about 100 min for their adsorption on ZnO (300°C) particles to reach equilibrium. The equilibrium times, under these conditions, are longer for other ZnO samples. Qm is the highest in the case of the prepared ZnO (300°C; Table 1). This is due to large surface area of the nanosize ZnO at 300°C. Previous studies also show that a larger surface area provides more surface active sites for the adsorption of the dye molecules resulting in efficient photocatalytic activity [28–30].
LIB spectra (Figure 4) of both the commercial and prepared ZnO (300°C) show three peaks with different intensities (in au). The peak positions as well as intensities of the commercial ZnO are very much identical to those of the prepared sample.
On the basis of the above results, the prepared nanosized ZnO (300°C) has been chosen for studying the photocatalytic decolorization of PS.
ZnO-mediated visible light decolorization of PS
No detectable decolorization or degradation of PS was observed under the longest illumination by the light source in the absence of ZnO. About 30% decolorization of the dye from the aqueous suspension took place by adsorption on the ZnO surface under the specified conditions (Figure 5). However, visible light decolorization is much faster as it accompanies degradation. About 35% Yellow direct 12 has also been reported [31] to be decolorized by ZnO in the dark under similar experimental conditions. Visible light generates reactive oxygen species (e.g., ·OH, O2·−) on the surface of ZnO in the aerated aqueous suspension containing the dye, and these species are responsible for the degradation of PS molecules [32, 33]. Of these species, · OH is the most prominent and most active.
Effect of solution pH on decolorization
It is to be noted that the initial pH of the PS solution in the range of 4 to 7 did not affect its photodecolorization. This is because the zero point charge of ZnO (made at 300°C) in the aqueous medium is 8.9. PS is an anionic dye, and the surface of the nanosized ZnO particles carries predominantly a positive charge up to the solution pH of <8.9. The initial pH in most of the present studies has been fixed at a pH of approximately 6. After complete decolorization, the pH of the clear solution has been found to decrease (≥4).
In a previous study, decolorization of brilliant golden yellow in ZnO suspension has also been found to decrease [14].
Decolorization mechanism
The spectrum of the PS solution (Figure 6) has characteristics peaks at 520, 345, 306, and 235 nm corresponding to the chromophore containing two azo linkages, a naphthalene moiety, benzene rings, and benzene and naphthalene rings, respectively. The decrease in absorbance, hence decolorization, of the solution at 520 nm with visible light illumination of PS in the ZnO suspension is due to the breakdown of the chromophore which is responsible for the characteristic solution color.
The -N=N- bonds of the chromospheres of PS molecules are the most susceptible sites for oxidative attack [34–36]. Figure 6 shows that the characteristic peaks gradually disappear with the time of illumination. When the illumination is continued for sufficiently long time, none of the peaks can be detected. The residual peak in the spectrum, observed when water was used as reference, near about 200 nm disappeared when the very dilute solution of Na2SO4 was used as reference in the place of water. This suggests the presence of SO42− ions, one of the products formed during degradation of PS in the ZnO suspension.
PS molecules are adsorbed on ZnO predominantly through their negatively charged −SO3− ends. The molecules of this dye, like some dye molecules containing −SO3Na groups in their structures [37, 38], release SO42− ions as one of the initial products of mineralization during photocatalytic degradation. CO2 is unlikely to be produced until some simple carboxylic acid species are available for the photo-Kolbe reaction [39]. When the azo bonds are ruptured, the naphthalene and benzene moieties are expected to be separated releasing some SO42− ions in the suspension. These species need to be remained adsorbed on the ZnO surface to be further oxidized during photolysis to the precursors of CO2, and the subsequent processes lead to the formation of CO2 from them. Accordingly, mineralization of PS molecules in the ZnO suspension, indicated by the formation of CO2, is much slower than its decolorization.
Figure 7 shows how decolorization and disappearance of TOC progress with time of visible light illumination of PS in the ZnO suspension. The decrease of TOC with time of illumination is the actual measure of mineralization of PS, that is, its conversion into CO2, H2O, N2, SO42−, and Na+ ions.
With 0.20 g ZnO in 40 mL of 5.00 × 10−5 M PS solution, it needs visible light illumination for about 300 min for its complete decolorization (Figure 7). Photodecolorization of PS is faster than its photodegradation. Obviously, the photodecolorization of PS is related to its degradation. Accordingly, studies on photodecolorization gave a measure of the photodegradation of PS.
Generation of active species
The principal agent for degradation of dyes by light in the presence of photocatalysts is the ·OH radical. This radical, along with others, is expected to form by visible light illumination of the ZnO suspension containing PS through sequences of steps, some of which are likely to be as follows:
The first step is the excitation of the dye (PS) adsorbed on the ZnO surface, Dads,
*Dads stands for an excited PS molecule which is adsorbed on ZnO.
The excited molecule on the ZnO surface injects an electron to its conduction band.
The trapped electron, ZnO(e), converts O2 adsorbed on the surface into O2·−,
The excited dye can also create an electron (e−)- hole (h+) pair in ZnO.
This hole can react with a water molecule adsorbed on the ZnO surface to produce the ·OH radical.
Another route of generation of ·OH can be through the formation HO2·, which leads to formation of H2O2.
The H2O2 adsorbed on ZnO can also lead to the formation of ·OH.
Accordingly, the overall degradation of PS molecules in the suspension of ZnO by visible light can be summarized as follows:
The step 11, involving ·OH, is likely to be the most favorable. However, this does not exclude the possibility of the oxidative attack at this stage by other species, such as dissolved oxygen, HO2·, and radicals that are subsequently formed from intermediates. The azo linkages, -N=N-, of PS are, of course, the most active site for oxidative attack by ·OH radicals.
Effect of catalyst concentration
Increasing the amounts of ZnO in the aqueous suspension increases the PS decolorization.
The initial rate of decolorization increases with the increase of concentration of ZnO in the suspension (Figure 8). Figure 8 suggests that the initial rate of decolorization may increase linearly up to about 0.15 g ZnO. Afterwards, deceleration becomes prominent as the concentration of ZnO in the suspension increases. Accordingly, PS molecules in a particular concentration need an optimum amount of ZnO for their decolorization with the visible light. The time for complete decolorization will certainly depend upon the intensity of the visible light.
An important aspect of increasing the concentration of ZnO in the aqueous suspension is to increase its turbidity. This should have affected the intensity of the visible light photons that cause excitation of the dye molecules adsorbed on the ZnO particles in the aqueous suspensions. However, such excitation by visible light photons does not seem to be spectacularly affected. This could be due to the efficient stirring of the suspension, as it caused fresh surfaces to be continuously exposed to the light of the same intensity.
Effect of initial dye concentration
The trend (Figure 9) in the decolorization of PS in the ZnO suspension with its concentrations seems to be similar to that with the increase of concentrations of ZnO, and the situation arises because of a small number of adsorption sites on ZnO in comparison to the increasingly larger number of PS molecules. Moreover, the inner filter effect [40, 41] becomes predominant with increasing PS concentration in the suspension. The decolorization of PS drops from about 93% to 60% when its concentration is increased from 4.00 × 10−5 M to 8.00 × 10−5 M (Table 2). Of the various factors that cause inner filter effect during the dye decolorization process by visible light in the presence of ZnO particles, the concentration seems to be the most prominent [42].
The kinetic data were applied to the Langmuir-Hinshelwood equation in the following form:
Ri is the initial rate of disappearance of the PS color from ZnO suspension; kL-H is a proportionality constant and provides a measure of intrinsic reactivity of PS adsorbed on ZnO [43]. KL-H is the apparent adsorption equilibrium constant (so-called Langmuir-Hinshelwood constant).
Ri−1 vs [PS]i−1 is a nicely fitted straight line (Figure 10). KL-H and kL-H values are 10.50 × 103 L mol−1 and 8.67 × 10−6 mol min−1, respectively. The higher KL-H value compared to that of KL (8.23 × 103 L mol−1) suggests some surface activation of the ZnO surface by visible light.
Conclusion
In the presence of visible light, ZnO can efficiently catalyze the decolorization of PS in the aqueous suspension. ZnO prepared at 300°C shows superior photocatalytic activity compared to the ZnO samples treated at 500°C and 700°C and also to the pristine ZnO. Photodecolorization of PS is unaffected by the initial pH of its solution in the range of 4 to 7. The prepared ZnO has been found to be quite stable under visible light illumination at a pH of approximately 6, and there is some activation of the surfaces of ZnO particles by this light.
References
Grau P: Textile industry wastewaters treatment. Water Sci. Technol. 1991, 24: 97.
Fujishima A, Honda K: Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238: 37. 10.1038/238037a0
Fox MA, Dulay MT: Heterogeneous photocatalysis. Chem. Rev.. 1993, 93: 341.
Fujishima A, Rao TN, Tryk DA: Titanium dioxide photocatalysis. J. Photochem. Photobiol. C: Photochem. Rev. 2000, 1: 1. 10.1016/S1389-5567(00)00002-2
Gaya UI, Abdullah AH: Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: a review of fundamentals, progress and problems. J. Photochem. Photobiol. C: Photochem Rev 2008, 9: 1. 10.1016/j.jphotochemrev.2007.12.003
Mohamed AR, Teh CM: Roles of titanium dioxide and ion-doped titanium dioxide on photocatalytic degradation of organic pollutants (phenolic compounds and dyes) in aqueous solutions: a review. J. Alloys. Compd. 2011, 509: 1648. 10.1016/j.jallcom.2010.10.181
Domenech J, Prieto A: Stability of ZnO particles in aqueous suspensions under UV illumination. J. Phys. Chem. 1986, 90: 1123. 10.1021/j100278a031
Poulios I, Makri D, Prohaska X: Photocatalytic treatment of olive milling waste water: oxidation of protocatechuic acid. Global Nest: the Int. J. 1999, 1: 55.
Carraway ER, Hoffman AJ, Hoffmann MR: Photocatalytic oxidation of organic acids on quantum-sized semiconductor colloids. Environ. Sci. Technol. 1994, 28: 786. 10.1021/es00054a007
Shakthivel S, Neppolian B, Shankar MV, Arabindo B, Palanichamy M, Murugesan V: Solar photocatalytic degradation of azo dye: comparison of photocatalytic efficiency of ZnO and TiO 2 . Solar Energy Materials & Solar Cells 2003, 77: 65. 10.1016/S0927-0248(02)00255-6
Mahmood AJ, Islam MS, Hossain MM: ZnO mediated degradation of brilliant orange by visible light. J. Bang. Chem. Soc. 2003, 16: 35.
Mahmood AJ, Islam MS, Hossain MM: Removal of Bractive T Blue from aqueous solution by ZnO. Dhaka Univ. J. Sci. 2003, 52: 13.
Mahmood AJ, Jabbar MA, Akhtar S: Influence of light on the degradation of a dye in homogeneous and heterogeneous media. J. Bang. Chem. Soc. 2003, 16: 57.
Habib MA, Ismail IMI, Mahmood AJ, Ullah MR: Photocatalytic decolorization of brilliant golden yellow over TiO2 and ZnO suspensions. J. Saudi Chem. Soc. 2012, 5: 34.
Yu J, Yu X: Hydrothermal synthesis and photocatalytic activity of zinc oxide hollow spheres. Environ. Sci. Technol. 2008, 42: 4902. 10.1021/es800036n
Liu S, Li C, Yu J, Xiang Q: Improved visible-light photocatalytic activity of porous carbon self-doped ZnO nanosheet-assembled flowers. Cryst. Eng. Comm. 2011, 13: 2533.
Salari ND, Khataee AR: Photocatalytic degradation of azo dye acid red 14 in water on ZnO as an alternative catalyst to TiO2. J. Photochem. Photobio. A: Chem. 2004, 162: 317. 10.1016/S1010-6030(03)00378-2
Baruah S, Rafique RF, Dutta J: Visible light photocatalysts by tailoring crystal defects in zinc oxide nanostructures. Nano: Brief Reports Rev. 2008, 3: 399.
Daneshvar N, Aber S, Seyed DMS, Khataee AR, Rasoulifard MH: Preparation and investigation of photocatalytic properties of ZnO nanocrystals: effect of operational parameters and kinetic study. Inter. J. Chem. Biol. Eng. 2008, 1: 24.
Baruah S, Jaisai M, Imani R, Nazhad MM, Dutta J: Photocatalytic paper using zinc oxide nanorods. Sci. Technol. Adv. Mater. 2010, 11: 055002. 10.1088/1468-6996/11/5/055002
Pozzo RL, Baltanas MA, Cassano AE: Supported titanium oxide as photocatalysts in water decontamination: state of the art. Catal. Today 1997, 39: 219. 10.1016/S0920-5861(97)00103-X
Meena RC, Pachwarya RB, Meena VK, Arya S: Degradation of textile dyes Ponceau-S and Sudan IV using recently developed photocatalyst, immobilized resin Dowex-11. Am. J. Environ. Sci. 2009, 5: 444.
Bannur VS, Kulgod VS, Metkar SS, Mahajan KS, Sainis KJ: Protein determination by Ponceau S using digital color image analysis of protein spots on nitrocellulose membranes. Anal. Biochem. 1999, 267: 382. 10.1006/abio.1998.3020
Chung KT, Stevens SEJ, Cerniglia CE: The reduction of azo dyes by the intestinal microflora. Crit. Rev. Microbiol. 1992, 18: 175. 10.3109/10408419209114557
Vijaykumar MH, Vaishamapayan PA, Shouche YS, Karegoudar TB: Decolourization of naphthalene-containing sulfonated azo-dyes by Kerstersia sp. strain VKY1. Enzyme Microb. Technol. 2007, 40: 204. 10.1016/j.enzmictec.2006.04.001
Chen H, Wang RF, Cerniglia CE: Molecular cloning, over expression, purification, and characterization of an aerobic FMN-dependent azo-reductase from Enterococcus faecalis. Protein Expression Purif. 2004, 34: 302. 10.1016/j.pep.2003.12.016
El-Desoky HS, Ghoneim MM, Zidan NM: Decolorization and degradation of Ponceau S azo-dye in aqueous solutions by the electrochemical advanced Fenton oxidation. Desalination 2010, 264: 143. 10.1016/j.desal.2010.07.018
Yu J, Liu S, Yu H: Microstructures and photoactivity of mesoporous anatase hollow microspheres fabricated by fluoride-mediated self-transformation. J. Catal. 2007, 249: 59. 10.1016/j.jcat.2007.03.032
Yu JG, Su YR, Cheng B: Template-free fabrication and enhanced photocatalytic activity of hierarchical macro-/mesoporous titania. Adv. Funct. Mater. 2007, 17: 1984. 10.1002/adfm.200600933
Yu J, Yu JC, Leung MK-P, Ho W, Cheng B, Zhao X, Zhao J: Effects of acidic and basic hydrolysis catalysts on the photocatalytic activity and microstructures of bimodal mesoporous titania. J. Catal. 2003, 217: 69.
Rao AN, Sivasankar B, Sadasivam V: Kinetic studies on the photocatalytic degradation of Direct Yellow 12 in the presence of ZnO catalyst. J. Mol. Catal. A: Chem 2009, 306: 77. 10.1016/j.molcata.2009.02.028
Turchi CS, Ollis DF: Photocatalytic degradation of organic water contaminants: mechanisms involving hydroxyl radical attack. J. Catal. 1990, 122: 178. 10.1016/0021-9517(90)90269-P
Xiang Q, Yu J, Wong PK: Quantitative characterization of hydroxyl radicals produced by various photocatalysts. J. Colloid Interface Sci. 2011, 357: 163. 10.1016/j.jcis.2011.01.093
Li J, Chen C, Zho J, Zhu H, Orthman J: Photodegradation of 5 dye pollutants on TiO2 nanoparticles dispersed in silicate UV-Visible irradiation. J. Appl. Catal. 2002, 37: 331. 10.1016/S0926-3373(02)00011-5
Sun Z, Chen Y, Ke Q, Yang Y, Yuan J: Photocatalytic degradation of cationic azo dye by TiO2/bentonite nanocomposites. J. Photochem. Photobiol. A: Chem 2002, 149: 169. 10.1016/S1010-6030(01)00649-9
Xie YB, Li XZ: Interactive oxidation of photoelectrocatalysis and electro-Fenton for azo dye degradation using TiO 2 -Ti mesh and reticulated vitreous carbon electrodes. Mater. Chem. Phys. 2006, 95: 39. 10.1016/j.matchemphys.2005.05.048
Lachheb H, Puzenat E, Houas A, Ksibi M, Elaloui E, Guillard C, Herrmann JM: Photocatalytic degradation of various types of dyes (Alizarins, Crocein Orange G, Methyl red, Congo red, Methylene blue) in water by UV-irradiated titania. Appl. Catal. B Environ. 2002, 39: 75. 10.1016/S0926-3373(02)00078-4
Saquib M, Muneer M: Semiconductor mediated photocatalyzed degradation of an anthraquinone dye, Remazol Brilliant Blue R under sunlight and artificial light source. Dyes Pigments 2002, 53: 237. 10.1016/S0143-7208(02)00024-4
Krautler B, Bard AJ: Heterogeneous photocatalytic synthesis of methane from acetic acid - new Kolbe reaction pathway. J. Am. Chem. Soc. 1978, 100: 2239. 10.1021/ja00475a049
Chen D, Roy AK: Photodegradation kinetics of 4-nitrophenols in TiO 2 suspension. Water Res. 1998, 32: 3223. 10.1016/S0043-1354(98)00118-3
Neppollian B, Sankar MV, Murugesan V: Semiconductor assisted photodegradation of textile dye. J. Sci. Ind. Res. 2002, 6: 224.
Muslim M: Photoelectrochemical studies of some dyes. PhD thesis. University of Dhaka, Bangladesh; 2009.
Lagrasta C, Bellobono IR, Bonardi M: Photobleaching and photomineralization of azobenzene and substituted azobenzenes in aqueous solution by photocatalytic membranes immobilizing titanium dioxide. Photochem. Photobiol. A 1997, 110: 201. 10.1016/S1010-6030(97)00179-2
Acknowledgment
Muhammad Muslim gratefully acknowledges the grant of study leave and deputation by the Ministry of Education, and his UGC PhD Fellowship by Bangladesh University Grants Commission (UGC).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
MM is a PhD student and his work is the basis of the paper. AJM and TSAI are the supervisor and co-supervisor, respectively. MAH and IMII have helped with the instrumental analysis and final preparation of the paper. All the authors read and approved the final version of the manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Muslim, M., Habib, M.A., Mahmood, A.J. et al. Zinc oxide-mediated photocatalytic decolorization of Ponceau S in aqueous suspension by visible light. Int Nano Lett 2, 30 (2012). https://doi.org/10.1186/2228-5326-2-30
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2228-5326-2-30