Retraction
This article was mistakenly published twice. For this reason this duplicate article has now been retracted. For citation purposes please cite the original:http://www.inljournal.com/?_action=articleInfo&article=16
Abstract
Nanosensing properties of hydrogenated edge armchair graphene nanoribbons (HAGNR) are investigated. Using non-equilibrium Green's function method in the tight-binding approach, the effects of hydrogen and oxygen adsorption on current–voltage (I-V) characteristics and also the electrical conductivity of these systems are calculated. We found that the I-V curves of these systems change by the adsorption of hydrogen or oxygen molecules. Also, we found that conductivity of these systems at low adsorption concentrations increases, while at high adsorption, concentrations decrease. This could be explained in terms of semiconducting or metallic properties of the adsorbed system which was obtained from electronic properties of our clean HAGNR system. On the other hand, the local density of states of some sites has a metallic behavior, and that of other sites has a semiconducting behavior.
Note that our results are investigated at a fixed temperature T = 300 K, i.e., room temperature. By calibrating conductivity in terms of adsorbed gas molecules, one can make a gas nanosensor
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
In recent years, much attention has been focused on nanoelectronic devices[1–3] and molecular gas adsorption on single-walled carbon nanotubes and graphene system surfaces[4–13]. Since gas adsorption by these systems could change their electronic properties, these properties could be used as a gas sensing property. Electronic and transport properties of these systems such as local density of states (LDOS), transmission coefficient, and current–voltage (I V) characteristics are investigated by the Landaure formula and non-equilibrium Green's function (NEGF) formalism[14–19].
One of the various forms of these devices that have attracted a great deal of attention is graphene nanoribbons. The electronic properties of nanoribbons could be determined by the atomic geometry along their edges, namely, zigzag graphene nanoribbons (ZGNR) or armchair graphene nanoribbons (AGNR)[14, 20–23]. Usually, ZGNRs have a metallic feature, while AGNRs are semiconducting[14].
Here, we extend our previous work[14] to investigate the gas sensing properties of these systems. We focus on the AGNRs which were hydrogenated from their edges, called hydrogenated AGNR (HAGNR). Note that, in the previous work, we considered a clean AGNR and ZGNR without adsorption of hydrogen or oxygen molecules, but in this paper, we considered a HAGNR where hydrogen or oxygen molecules were adsorbed on its surface. Our results show that at low adsorption concentrations, more adsorption of hydrogen or oxygen molecules leads to the increase of conductivity of the system, while at high adsorption concentrations, more adsorption leads to the reduction of conductivity. Therefore, conductivity could identify the percentage of adsorbed gas molecules.
This paper is organized as follows: In the ‘Methods’ section, we describe the model and theoretical tools that we used to calculate the local density of states, transmission, and current–voltage curves of the system. In the ‘Results and discussion’ section, we applied this method to our system and analyzed the obtained results. The last section is the conclusion part.
Method
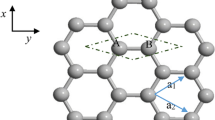
Consider a HAGNR which is attached to two leads from the left to the right. The HAGNR is called the device. To convert this system to a one-dimensional system, we divided it into similar cells along the transport direction where each cell included N0 atoms (each lattice site included N0 sublattice atoms). This is illustrated in Figure 1.
In the NEGF formalism, in the absence of applied voltage, the total transmission is given by[14, 16],
where Imx stands for the imaginary part of x, and is the retarded (advance) Green function matrix from i unit cell to i′. The unit cell number i = 0 refers to the right edge of the left lead, and i = N + 1 refers to the left edge of right lead. Note that we have N unit cells in the device region (i = 1,…,N) and that in our system, N is equal to 5. The retarded and advance Green functions, Gr and Ga, are defined by
and
respectively, where S and I are the overlap matrix and unitary matrix, respectively. H is the Hamiltonian function of the system, and η is a positive infinitesimal energy.
∑L/R is the left/right lead self-energies. By applying the mode-matching method and using the boundary conditions (the source is put in the left lead), these self-energies are given by
where they contain all information about the coupling between the leads and scattering device region (HAGNR) as well as the information about the scattering boundary condition. HLD/RD stands for coupling matrix between neighbor cells in the left/right lead and the device. Note that self-energy functions are energy-dependent and expressed in terms of the Bloch matrices FL/R where the Bloch matrix depends on the lead modes.
In the real space, H is a Hamiltonian matrix where its elements are matrices with a dimension of N0 × N0. For definition of H matrix elements, we used the tight-binding representation in the nearest neighbor's approximation.
We consider the hopping between the nearest neighbor atoms in the device equal to 2.75 eV and calculate other hopping and on-site energies with respect to this value.
Using the Landaur approach, we apply a weak bias voltage between the left and right regions in the transport direction. In addition, we ignored phonons effects, and we fixed temperature at room temperature, T = 300 K.
The total current using scattering formalism is given as follows:[15]
where is the Fermi function, and μ is the chemical potential. Note that the chemical potential of the two leads varies by the applied bias voltage, V, as (source is at the left lead). We assumed that before applying bias voltage, the chemical potential of the whole system is −6β where β is the hopping energy in the device. After applying bias voltage, all atoms in the left lead cells have the same chemical potential μL, while the atoms in the right lead have the same chemical potential μR. However, in the device region, the chemical potential varies linearly with respect to the distance from the left edge of the device, i.e., along the transport direction (as shown in Figure 1).
Our clean system is a HAGNR which included 90 atoms (70 carbon and 20 hydrogen atoms). Now, we consider hydrogen and oxygen molecule adsorption on the top of this system. We assumed that the leads are two-dimensional semi-infinite periodic wires of copper with body centered square lattices where the widths of all regions are the same. In the next section, we present our results.
Result and discussion
To see effects of hydrogen/oxygen molecule adsorption on the electronic properties of HAGNRs, first, we investigate electronic properties of our clean HAGNR system. Figure 2a,b,c,d illustrates the comparison of LDOS of sites 39, 41, 43, and 45 along the width of the clean HAGNR, respectively. It is obvious that LDOS of sites 39 and 45 have a metallic behavior, while those of sites 41 and 43 show a semiconducting feature. Therefore, the upper (lower) edge sites along the length of the HAGNR construct metallic regions separated by semiconducting regions.
Figure 3a shows the metallic and semiconducting regions for the lower edge nanowire. The middle nanowire is constructed from metallic and semiconducting regions of double-site atoms along the length of the HAGNR. Figure 3b shows this nanowire. Hence, at low adsorptions, adsorption of a hydrogen/oxygen molecule by a metallic or semiconducting carbon atom of the HAGNR could change the electronic properties of the HAGNR in different ways. Adsorptions of H2/O2 by metallic sites lead to a reduction of current, while those by semiconducting sites lead to an increase in current. Figure 4a,b shows the current–voltage characteristics of our system when one hydrogen/oxygen molecule is adsorbed by sites 39, 41, 43, and 45 along the width of the HAGNR. To explain this result, we should look at the LDOS of these sites for the adsorbed system. Figures 5a,b,c,d and6a,b,c,d show LDOS of sites 39, 41, 43, and 45 when one H2/O2 is adsorbed by sites 39, 41, 43, or 45. These figures show that adsorption of H2/O2 by semiconducting sites converted these sites to metallic sites. Hence, this adsorption increases the current, while adsorption of H2/O2 by metallic sites reduces the current. To clarify this result, we plotted LDOS of the neighbor sites of adsorption site 43. Figures 7a,b,c and8a,b,c show LDOS of sites 26, 41, and 45, respectively, when one H2/O2 is adsorbed by site 43. The semiconducting sites 26 and 41 are converted to metallic sites. In the next stage, we investigate adsorption concentration effects on the I-V curve of the HAGNR. Figure 9a,b shows I-V curves of the HAGNR when three H2 or three O2 molecules are adsorbed by (a) sites 21, 39, and 57; (b) sites 23, 41, and 59; (c) sites 25, 43, and 61; and (d) sites 27, 45, and 63 along its length, respectively. We found that at high adsorption concentrations, adsorption by semiconducting sites with bigger energy gap increases current more than smaller energy gap semiconductor sites, and adsorption by more metallic sites decreases current with respect to those sites with low metallic features. Figure 10 illustrates the comparison of I-V curves in which metallic sites 27, 45, and 63 adsorbed three H2/O2 molecules (Figure 10a); sites 9, 27, 45, 63, and 81 adsorbed five H2/O2 molecules (Figure 10c); semiconducting sites 23, 41, and 59 adsorbed three H2/O2 molecules (Figure 10b); and sites 5, 23, 41, 59, and 77 adsorbed five H2/O2 molecules (Figure 10d). We found that by increasing adsorption concentration, current decreases. To complete our results, we calculated the conductivity of the system in terms of H2/O2 adsorption concentration. Figure 11a,b illustrates conductivity in terms of H2/O2 concentration, respectively. Since at high adsorptions these systems have metallic features, so for high concentrations, we expect such reductions.
Comparison of I - V curves. Comparison of I-V curves when metallic sites 27, 45, and 63 and sites 9, 27, 45, 63, and 81 adsorbed (a) three and (c) five H2/O2 molecules, respectively. Semiconducting sites 23, 41, and 59 and sites 5, 23, 41, 59, and 77 adsorbed (b) three and (d) five H2/O2 molecules, respectively
Conductivity of HAGNR in Figure 1 in terms of (a) H 2 and (b) O 2 adsorption concentration
Conclusions
Using the NEGF (non equilibrium Green's function) method in the tight-binding approach, effects of gas adsorption on the electronic properties of hydrogenated armchair graphene nanoribbons are investigated. We found that at a low adsorption concentration, adsorption by metallic HAGNR sites decreases current, while adsorption by semiconductor HAGNR sites increases current in the current–voltage curve of these systems. At high adsorption concentrations, the whole system becomes metallic, so by increasing adsorption concentrations, current always decreases. Also, we found that at low adsorption, the conductivity of the system increases by increasing adsorption, while at high adsorptions, the conductivity of the system decreases by increasing adsorption. These results could be used to make a gas nanosensor.
References
Anantram MP, Leonard F: Physics of carbon nanotube electronic devices. Rep. Prog. Phys. 2006, 69: 507. 10.1088/0034-4885/69/3/R01
Lu W, Lieber CM: Semiconductor nanowires. J. Phys. D: Appl. Phys. 2006, 39: R387-R406. 10.1088/0022-3727/39/21/R01
Wang H, Wang X, Li X, Dai H: Chemical self-assembly of graphene sheets. Nano. Res. 2009, 2: 336–342. 10.1007/s12274-009-9031-x
Moradian R, Mohammadi Y: Finite-concentration gas molecule adsorption on carbon nanotubes investigated by a tight-binding approach. Phys. Rev. B. 2007, 76: 155432.
Moradian R, Mohammadi Y, Ghobadi N: Investigation of gas sensing properties of armchair graphene nanoribbons. J. Phys. Condens. Matter 2008, 20: 425211. 10.1088/0953-8984/20/42/425211
Panella B, Hirscher M, Roth S: Hydrogen adsorption in different carbon nanostructures. Carbon 2005, 43: 2209–2214. 10.1016/j.carbon.2005.03.037
Sevinçli H, Topsaka M, Durgun E, Ciraci S: Electronic and magnetic properties of 3d transition-metal atom adsorbed graphene and graphene nanoribbons. Phys. Rev. B. 2008, 77: 195434.
Giannozzi P, Car R, Scoles G: Oxygen adsorption on graphite and nanotubes. J. Chem. Phys. 2003, 118: 1003. 10.1063/1.1536636
Schimmel HG, Kearley GJ, Nijkamp MG, Visser CT, de Jong KP, Mulder FM: Hydrogen adsorption in carbon nanostructures: comparison of nanotubes, fibers, and coals. Chem. Eur. J. 2003, 9: 4764–4770. 10.1002/chem.200304845
Schimmel HG, Nijkamp MG, Kearley GJ, Rivera A, de Jong KP, Mulder FM: Hydrogen adsorption in carbon nanostructures compared. Materials Science and Engineering B 2004, 108: 124. 10.1016/j.mseb.2003.10.091
Zhu Z, Lu GQ, Finnerty J, Yang RT: Electronic structure methods applied to gas-carbon reactions. Carbon 2003, 41: 635. 10.1016/S0008-6223(02)00380-9
Ataca C, Aktürk E, Ciraci S: Hydrogen storage of calcium atoms adsorbed on graphene: first-principles plane wave calculations. Phys. Review B. 2009, 79: 041406.
Jagiello J, Thommes M: Comparison of DFT characterization methods based on N2, Ar, CO2, and H2 adsorption applied to carbons with various pore size distributions. Carbon 2004, 42: 1227–1232. 10.1016/j.carbon.2004.01.022
Moradian R, Zereshki P, Haseli S, Hayati M: Investigation of electronic transport through graphene nanoribbon quantum dots. Physica E 2009, 41: 801. 10.1016/j.physe.2008.12.014
Ferry DK, Goodnick SM: Transport in Nanostructures. Cambridge: Cambridge University Press; 1997.
Datta S: Electronic Transport in Mesoscopic Systems. Cambridge: Cambridge University Press; 1997.
Castro Neto AH, Guinea F, Peres NMR, Novoselov KS, Geim AK: The electronic properties of grapheme. Rev. Mod. Phys. 2009, 81: 109. 10.1103/RevModPhys.81.109
Koshelkin AV: Two-particle Green's functions in non-equilibrium matter. Physics Letters B. 1999, 471: 202–207. 10.1016/S0370-2693(99)01373-8
Xue Y, Datta S, Ratner MA: First-principles based matrix Green's function approach to molecular electronic devices: general formalism. Chem. Phys. 2002, 281: 151–170. 10.1016/S0301-0104(02)00446-9
Son YW, Cohen ML, Louie SG: Energy gaps in graphene nanoribbons. Phys. Rev. Lett. 2006, 97: 216803.
White CT, Li J, Gunlycke D, Mintmire JW: Hidden one-electron interactions in carbon nanotubes revealed in graphene nanostrips. Nano Lett. 2007, 7: 825–830. 10.1021/nl0627745
Brey L, Fertig HA: Electronic states of graphene nanoribbons studied with the Dirac equation. Phys. Rev. B. 2006, 73: 235411.
Ezawa M: Peculiar width dependence of the electronic properties of carbon nanoribbons. Phys. Rev. B. 2006, 73: 045432.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
The authors' contributions in this article are the same. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Moradian, R., Nazeri, S. Retracted: Electrical conductivity of hydrogenated armchair nanoribbon as a gas sensor using non-equilibrium Green's function method. Int Nano Lett 2, 3 (2012). https://doi.org/10.1186/2228-5326-2-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2228-5326-2-3