Abstract
Nanobiotechnology is a rapidly growing scientific field of producing and constructing devices utilizing nanosized particles of about nanometer scale level (1 to 100 nm). Nowadays, nanoparticles have potential effects in life sciences and human health care applications. Among the nanoparticles, silver nanoparticles are playing a major role in the field of biomedical nanotechnology and nanomedicine. Silver is a naturally occurring precious metal, most often as a mineral ore in association with other elements. Silver nanoparticle has a natural antimicrobial effect against many pathogens such as bacteria, fungus, viruses, and yeast. In this present study, the silver nanoparticle is produced using Vitis vinifera fruit extract, and the purified nanoparticles will be used as an antibacterial agent against Bacillus subtilis and Klebsiella planticola. This green chemistry for the biosynthesis of silver nanoparticles has several advantages such as cost-effectiveness and compatibility for biomedical and pharmaceutical applications as well as for large-scale commercial production. Apart from this, it is an eco-friendly process, and compared with microorganisms, plant extract biorecovery unit has an added benefit of ease handling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Nanotechnology is a developing interdisciplinary field of research interspersing material science, bionanoscience, and technology. Remarkable advances are made in the field of biotechnology and nanotechnology to harness the benefit of life sciences, health care and industrial biotechnology [1–3]. A reliable and eco-friendly process for synthesis of metallic nanoparticles is an important step in the field of nanotechnology. In recent years, noble metal nanoparticles have been the subject of focused research due to their unique optical, electronic, mechanical, magnetic, and chemical properties that are significantly different from those of bulk materials [4]. There is particular interest in nanoparticulate Ag, due to its ability to act as both an electron sink as well as a redox catalyst [5]. Numerous microorganisms and plant extracts have been applied to synthesize inorganic nanostructures either intracellularly or extracellularly. It can be realized by electrostatic interaction between Ag+ and negatively charged carboxylate groups on the cell surface. In a microorganism the reduction of metal ions occurs on its cell surface by enzymes present in the cell wall [6].The development of green processes for the synthesis of nanoparticles is evolving into an important branch of nanotechnology [7, 8]. Several plant biomass or plant extracts have been successfully used for extracellular biosynthesis of silver nanoparticles. The metal ions reduction occurs very rapidly, and the reduction of Ag ions will be completed within hours. Rapid synthesis and excellent yield of silver nanoparticles through this plant-mediated [9–12] biosynthesis have a time-related (2 to 4 h) advantage in comparison with microbial synthesis (24 to 120 h) [13, 14]. Therefore green chemistry approaches in nanotechnology for nanoparticle synthesis is used in medical applications, targeted drug delivery, imaging, and dye and heavy metal adsorption. The application of nanoparticles as delivery vehicles for bactericidal agents represents a new paradigm in the design of antibacterial therapeutics.
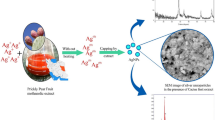
In this present investigation, we developed an inexpensive, versatile, and very reproducible method for large-scale synthesis of silver nanoparticles by reduction process using fruit extract of Vitis vinifera. This fruit extract can act both as reducing and stabilizing agents. V. vinifera is an important fruit which has phytochemicals such as resveratrol (a polyphenol), and it may have potential use in biomedical applications due to its high antibacterial activity.
Results and discussion
Visual identification
V. vinifera fruit-mediated synthesis of silver nanoparticles is a slow process, when compared with other natural plant materials. Here, the silver nanoparticles synthesis process was started in 1 h and completed in 4 h of incubation (Figure 1). This is due to the increasing concentration of silver nanoparticles as well as the particles’ growth in size [15]. The intensity of color is increased when the time of incubation was increased. The color exhibited by metallic nanoparticles is due to the coherent excitation of all “free” electrons within the conduction band, leading to an in-phase oscillation, which is known as surface plasmon resonance [9]. In V. vinifera fruit-synthesized silver nanoparticles, there are no significant changes beyond 4 h, indicating the completion of the reduction reaction. This result correlated with the earlier reports of Punica granatum[16], and Dioscorea oppositifolia[17]. At the end of reduction process, there is a dark brown color of silver nanoparticles that settled at the bottom of the conical flask.
UV-visible spectroscopic analysis
In fruit-mediated synthesis, after mixing V. vinifera fruit extract in aqueous solution of the silver ion complex, the solution slightly started to change in color from yellow to brown in 1 h of incubation at 37°C. Colloidal solutions of Ag nanoparticles show a very intense color while the time interval was increased from 1 to 4 h. This result correlated with the previous report of Chauhan et al. [16]. It is an indication of silver nanoparticles formation as the color change observed is due to the excitation of surface plasmon vibrations in silver nanoparticles [18]. The broad surface plasmon resonance band observed around 450 to 470 nm (Figure 2) indicates that the particles are large and polydispersed [18, 19].
SEM analysis
After the reduction process, the silver nanoparticles are separated by centrifugation of the solution at 15000 rpm for 15 min. Silver nanoparticles were purified by repeated centrifugation for three to four times, and the pellet was dried in hot air oven. Further, the air-dried powder was used to identify the shape and morphology of the nanoparticles. SEM characterizations of the synthesized Ag nanoparticles are shown in Figure 3. It shows relatively spherical-shaped nanoparticles formed with diameters that range from 30 to 40 nm. The nanoparticles were examined under various magnifications of ×16,000, ×25,000, and ×45,000. This result correlated with a previous report obtained using papaya fruit extract which was at a range of 25 to 50 nm [20].
EDX analysis
The fruit extract of V. vinifera- synthesized silver nanoparticles also produces a strong signal at 3 keV which reveals the presence of silver nanoparticles. The presence of the strong signal from silver (59.15%) atoms in the nanoparticles and weaker signals from oxygen (27.30%), chlorine (12.49%), and calcium (1.09%) atoms is confirmed (Figure 4). The weaker signals are recorded possibly due to elements (Cl, Ca, and O) from organic moieties like enzymes or proteins present within the extract of the pomegranate fruit [21].
X-ray diffraction analysis
The X-ray diffraction (XRD) pattern clearly shows that synthesized Ag nanoparticles formed are crystalline in nature. The fruit extract-synthesized (Vitis vinifera) Ag nanoparticles have shown five intense peaks (Figure 5) in the whole spectrum of 2θ values ranging from 30 to 80. The typical XRD pattern revealed that the sample contains a spherical structure of silver nanoparticles. A number of Bragg reflections corresponding to (0 0 4), (1 1 1), (2 0 0), (2 2 0) and (3 1 1) sets of lattice planes are observed which may be indexed, based on the FCC structure of silver. Peaks are also observed, suggesting that crystallization in the bio-organic phase occurs on the surface of the nanoparticles [22].
Fourier transform infrared spectroscopy
The fruit-synthesized (V. vinifera) silver nanoparticles obtained obvious absorption peaks at 3,314, 3,199, 1,671, 1,402, 1,399 and 1,122 cm-1(Figure 6) as well as some intensity peaks that decreased at 2,967, 2,879 and 2,362 cm-1. Figure 6 shows that bands at 3,199 to 3,314 cm-1 correspond to N-H, and O-H stretching vibrations of 1°, and 2° amines, amides, alcohol and H–bonded to phenols. The peak at 1,399 to 1,671 cm-1 indicates C-H stretching vibrations of alkene. The peak at 1,122 cm-1 represents the C-H in-plane bending of alkenes, alcohols, carboxylic acids, esters, and ethers. The weak band at 2,362 to 2,967 cm-1 corresponds to C-H stretching vibrations of alkanes.
Antibacterial activity
The inhibition zone was observed against Bacillus subtilis and Klebsiella planticola. In the quantitative assay, 10, 20, 30, 40, and 50 μ1 of Ag nanoparticles were added in five respective wells. The zone of inhibition was measured as 6, 7, 8, 9 and 10 mm respectively in diameter. The zone of inhibition measured is summarized in Table 1. From the table, it is evident that the synthesized nanoparticles are good candidates for their usage in antibacterial drugs. Similarly, the silver nanoparticles synthesized from the extracts of the plant barks of Cinnamomum camphora plants, are toxic to multi-drug-resistant bacteria, which shows their great potential effect in biomedical applications. Similar observations were found with the Allium cepa[23] and Argimone mexicana[24].
Conclusions
In the present study, we found that V. vinifera is a good source for the synthesis of silver nanoparticles. It was confirmed by the brown color formation of the extract. Silver nanoparticles were synthesized using the present method having 30 to 40-nm average mean sizes with spherical shape. The reduction of silver ions and the stabilization of silver nanoparticles were thought to occur through the participation of fruit proteins and metabolites. Most importantly, the reaction was simple and convenient to handle; it is believed that the in vitro phytosynthesis of silver nanoparticles has more advantages over other biological syntheses.
Methods
Chemicals
AgNO3 was purchased from Himedia, Mumbai, India. The bacterial cultures of B. subtilis and K. planticola were obtained from Microbial Type Culture Collection, Chandigarh, India.
Preparation of fruit extract
Ripened fruit of V. vinifera was used for the preparation of the extract. This ripened fruit weighing 25 g was thoroughly washed in distilled water and dried, cut into small pieces, crushed into 100 ml of sterile water, and filtered using Whatman filter paper no. 1; the filtrate was collected and underwent a second filtration using the same type of filter paper, but the pore size was smaller. The filtrate was collected, and this filtrate was used as the sample.
Formation of silver nanoparticle
One millimolar of 95-ml silver nitrate (0.016 g) solution was prepared and kept in a 250-ml Erlenmeyer flask. V. vinifera fruit extract (5 ml) was added to the silver nitrate solution. Ninety-five percent of the bioreduction of AgNO3 ions occurred within 4 h. The yellow colored solution which slowly turned brown indicated the formation of silver nanoparticles.
Antibacterial activity
Agar disk diffusion method was used to assay the bactericidal effect of green synthesized silver nanoparticles on Muller-Hinton agar plates against the strains of B. subtilis and K. planticola. A single colony of test strain was grown overnight in LB broth medium on a rotary shaker (200 rpm) at 35°C. After 24 h of incubation, a loop full of bacterial culture was placed on the Muller-Hinton broth medium. The silver nanoparticles are taken at different concentrations of 10 to 50 μ1 and are impregnated with a sterile disk placed over the surface of the medium to assess the bactericidal effect of the silver nanoparticles. After incubation at 35°C for 24 to 48 h, the zones of inhibition were measured.
Characterization study of synthesized silver nanoparticles
After completion of the nanoparticle synthesis process, the particle-containing solution was centrifuged to separate the nanoparticles, and this process was repeated two or three times to remove unwanted garbage. It was then dried in a hot air oven at 70°C. The synthesized nanoparticles indicated by UV-visible spectroscopy, were carried out in a Perkin-Elmer spectrophotometer (Branford, CT, USA) operating in a wavelength from 300 to 900 nm. The morphology of the nanoparticles was identified using a scanning electron microscope by Philips (Amsterdam, The Netherlands). The presence of elemental silver in the solution mixture was identified using an energy dispersive X-ray spectrophotometer, and it was operated at an accelerating voltage of 20 kV. The crystallinity of the nanoparticles was evident from the XRD measurements. FT-IR data indicate a bonding of Ag nanoparticles with the functional group of fruit extract through bridging linkage. The antimicrobial activities were done using bacteria like B. subtilis, K. planticola for biomedical application.
Authors’ information
GG completed her M.Sc. in Biotechnology in Periyar University and obtained her Ph.D. degree in Nanotechnology under the guidance of GA. Her research interests include green-meditated synthesis of nanoparticles and its agricultural applications for controlling plant diseases. KP received his B.Sc. (2005) and M.Sc. in Biotechnology (2007) from Madurai Kamaraj University and Periyar University, India, respectively. He is currently working to obtain his Ph.D. degree at Manonmaniam Sundaranar University in India. He is interested in the green and immobilized microbe-mediated synthesis process of nanoparticles and nanocomposites and their biomedical and textile industry applications. MV obtained her M.Sc. in Environmental Biotechnology in Sri Paramakalyani Centre for Environmental Sciences, Manonmaniam Sundaranar University, Alwarkurichi, Tirunelveli. She obtained her Ph.D. degree under the guidance of GA in the field of nanotechnology. Her research interests include eco-friendly synthesis of nanoparticles and its application in the environment. SR completed his M.Sc. in Biotechnology in Periyar University and obtained his Ph.D. degree in Nanotechnology under the guidance of GA. He published 11 research articles in nanoparticle synthesis. He is interested in the metallic nanoparticles synthesis using algae and algae-derived compounds and their potential applications in the biomedical field. CM completed her B.Sc. in Zoology and M.Sc. in Environmental Biotechnology in SPKCES, Manonmaniam Sundaranar University, Alwarkurichi, Tirunelveli. She achieved her Ph.D. degree under the guidance of GA in the field of nanotechnology. Her research interests include synthesis of semiconductor nanomaterials and metal nanoparticles and their biomedical applications. CK had done his master's degree and doctoral degree in the discipline of Chemistry. His research interests are in the field of green chemistry, environmental chemistry, and synthesis of nanoparticles. Currently, he works as an associate professor in MSU, Tirunelveli. GA received his M.Sc. in Applied Chemistry (1992) and Ph.D. in Environmental Biotechnology (1997) from Anna University, India. He had 10 years (1999 to 2008) of post-doctoral experiences from the National Taiwan University in Taiwan, National Institute of Advanced Industrial Science and Technology in Japan, and National Central University in Taiwan. He has received many research awards from the Indian and other country governments. He is an associate editor in five international journals. At present, he is an associate professor of Environmental Biotechnology and the leader of the Environmental Nanobiotechnology Division at SPKCES, MS University, India. His main research interest is on biosynthesis of nanoparticles and nanomaterials, nanobiocatalyst, and environmental chemistry.
References
Gardea-Torresdey JL, Parsons JG, Gomez E, Videa-Peralta J, Troiani HE, Santiago P: Formation and growth of Au nanoparticles inside live alfalfa plants. Nano Lett. 2002, 2: 397–401.
Stephen JR, Macnaughton SJ: Developments in terrestrial bacterial remediation of metal. Curr Opin Biotchnol. 1999, 10: 230–233.
Lee HJ, Yeo SY, Jeong SH: Antibacterial effect of nanosized silver colloidal solution on textile fabrics. J. Mater. Sci. 2003, 38: 2199–2204.
Mazur M: Electrochemically prepared silver nanoflakes and nanowires. Electrochem. Commun. 2004, 6: 400–403.
Askari S, Halladj R, Nasernejad B: Characterization and preparation of sonochemically synthesized silver–silica nanocomposites. Mater. Sci. Pol. 2009, 2: 27.
Mukherjee P, Ahmad A, Mandal D, Senapati S, Sainkar RS, Khan IM, Parishcha R, Ajaykumar VP, Alam M, Kumar R, Sastry M: Fungus-mediated synthesis of silver nanoparticles and their immobilization in the mycelia matrix. a novel biological approach to nanoparticle synthesis. Nano Lett. 2001, 1: 515–519.
Raveendran P, Fu J, Wallen SL: A simple and green method for the synthesis of Au, Ag, and Au-Ag alloy nanoparticles. Green Chem 2006, 8: 34–38.
Armendariz V, Gardea-Torresdey JL, Jose Yacaman M, Gonzalez J, Herrera I, Parsons JG: Gold nanoparticle formation by oat and wheat biomasses. In: Proceedings of Conference on Application of Waste Remediation Technologies to Agricultural Contamination of Water Resources. Kansas City: Marriott – Country Club Plaza; 2002. 30 July - 1 August 30 July - 1 August
Ankanna S, Prasad TK, Elumalai EK, Savithramma N: Production Of biogenic silver nanoparticles using Boswellia ovalifoliolata stem bark. Digest Journal of Nanomaterials and Biostructures 2010, 5: 369–372.
Basavaraja S, Balaji SD, Lagashetty A, Rajasab AH, Venkataraman A: Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium semitectum . Mater. Res. Bull. 2008, 43: 1164–1170.
Balaji DS, Basavaraja S, Raghunandan D, Mahesh D, Venkataraman A: Biosynthesis and stabilization of Au and Au-Ag alloy nanoparticles by fungus Fusarium semitectum . Sci. Technol. Adv. Mater. 2008, 9: 035012.
Balaji DS, Basavaraja S, Raghunandan D, Mahesh B, Prabhakar BK, Venkataraman A: Extracellular biosynthesis of functionalized silver nanoparticles by strains of Cladosporium cladosporioides fungus. Coll Surf B: Biointerfaces 2009, 68: 88.
Gardea-Torresdey JL, Gomez E, Peralta- Videa J, Parsons JG, Troiani HE, Jose- Y: Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Langmuir 2003, 13: 1357.
Shankar SS, Rai A, Ahmad A, Sastry M: Rapid synthesis of Au, Ag, and bimetallic Au core–Ag shell nanoparticles using neem ( Azadirachta indica ) leaf broth. J. Colloid Interface Sci. 2004, 275: 496–502.
Fayaz AM, Balaji K, Kalaichelvan PT, Venkatesan R: Fungal based synthesis of silver nanoparticles-an effect of temperature on the size of particles. Colloids Surf B 2009, 74: 123–6.
Chauhan S, Upadhyay MK, Rishi N, Rishi S: Phytofabrication of silver nanoparticles using pomegranate fruit seeds. International Journal of Nanomaterials and Biostructures 2011, 1: 17–21.
Maheswari RU, Lakshmi Prabha AL, Nandagopalan V, Anburaja V, Barath J: Synthesis of silver nanoparticles by using fruit extract of Dioscorea oppositifolia L. Journal of Research in Nanobiotechnology 2012, 1: 009–013.
Kamat PV, Flumiani M, Hartland GV: Picosecond dynamics of silver nanoclusters photoejection of electrons and fragmentation. J. Phys. Chem. B 1998, 102: 3123–3128.
Rani PU, Rajasekharreddy P: Green synthesis of silver -protein (core-shell) nanoparticle using Piper betle L leaf extract and its ecotoxicological studies on Daphnia magna . Colloids and surf A 2011, 389: 188–194.
Jain D, Daima HK, Kachhwaha S, Kothari SL: Synthesis of plant-mediated silver nanoparticles using papaya fruit extract and evaluation of their anti microbial activities. Digest Journal of Nanomaterials and Biostructures 2009, 4: 557–563.
Xu H, Kall M: Modeling the optical response of nanoparticle-based surface plasmon resonance sensors. Sensors and Actuators B: Chemical 2002, 4: 244–249.
Sathyavathi R, Krishna MB, Rao SV, Saritha R, Rao N: Biosynthesis of silver nanoparticles using Coriandrum sativum leaf extract and their application in nonlinear optics. Adv Sci Letters 2010, 3: 1.
Saxena A, Tripathi RM, Singh RP: Biological synthesis of silver nanoparticles by using onion ( Allium cepa ) extract and their antibacterial activity. Digest. J Nanomater Biostruct 2010, 5: 427–432.
Khandelwal N, Singh A, Jain D, Upadhyay MK, Verma HN: Green synthesis of silver nanoparticles using Argimone mexicana leaf extract and evaluation of their antimicrobial activities. Digest J Nanomater Biostruct 2010, 5: 483–489.
Acknowledgements
The authors gratefully acknowledge the DST-FIST-sponsored program of the Department of Science and Technology, New Delhi, India for funding the research development (DST/FST/ESI-101/2010) to carry out of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
GG carried out the nanoparticles synthesis and characterization. KP carried out the manuscript preparation, MV, SR and CM carried out the antibacterial activity. GA and CK supervised the research work and corrected the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Gnanajobitha, G., Paulkumar, K., Vanaja, M. et al. Fruit-mediated synthesis of silver nanoparticles using Vitis vinifera and evaluation of their antimicrobial efficacy. J Nanostruct Chem 3, 67 (2013). https://doi.org/10.1186/2193-8865-3-67
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2193-8865-3-67