Abstract
A series of Co-Cr nanoferrites having the chemical formula CoCr x Fe2 − xO4 (x = 0.0, 0.1, 0.3, 0.5, 0.7, 0.9, and 1.0) were synthesized by citrate-gel autocombustion method at a very low temperature (180°C). The X-ray diffraction analysis of as-synthesized powders and sintered powders has confirmed the formation of single-phase cubic spinel structure. The average particle size of the synthesized ferrites was 6 to 12 nm. Magnetic susceptibility measurements using Faraday magnetic susceptibility balance showed the paramagnetic nature of the ferrites. Magnetic properties of Co-Cr nanoferrites were measured using a vibrating sample magnetometer at room temperature in the applied field of 15 kOe. The saturation magnetization decreased from 33.84 to 13.83 emu/g with increase in Cr3+ concentrations, indicating the fact that the lesser magnetic Cr3+ ions substitute Fe3+ ions in the octahedral sublattice of the ferrite. With improvement in the magnetic properties, the synthesized nanoferrites become soft magnetic materials. Such materials are useful in transformer and motor cores to minimize the energy dissipation with the alternating fields associated with AC electrical applications. The coercivity of pure CoFe2O4 was larger than that of the Cr-doped cobalt ferrites.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
Nanoparticles are very important and have a distinct property, that is, they exhibit larger surface-area-to-volume ratio. This increase in surface-area-to-volume ratio leads to the change of the properties of nanoparticles better than that of the bulk particles [1]. Transition metal oxide nanoparticles represent a broad class of materials that have been investigated extensively due to their interesting catalytic, electronic, and magnetic properties relative to those of the bulk counterparts, and the wide scope of their potential applications [2]. Among these materials, ferrites have attracted immense attention of the scientific community because of their novel properties and technological applications especially when the size of the particles approaches to nanometer scale [3]. As magnetic materials, nano-sized ferrites cannot be replaced by any other magnetic material because they are relatively inexpensive, stable, and have a wide range of technological applications [4]. The spinel ferrites have remarkable magnetic and electrical properties. Among them, CoFe2O4 is interesting because of its perfect chemical properties, thermal stability, high electrical resistivity, and excellent magnetic properties [5]. Nanocrystalline CoFe2O4 with such properties have potential applications in high frequency devices, memory cores, recording media, and in biomedical field [6].
The properties of ferrite materials are sensitive to the grain size and also strongly influenced by the distribution of metallic ions among crystallographic crystal lattice sites. These in turn are sensitive to the method used to prepare those materials [7]. Various methods used to prepare nano-sized ferrites are co-precipitation [8], micro-emulsion procedures [9], microwave plasma [10], mechanical milling [11], and sol-gel autocombustion method [12]. The sol–gel auto combustion method in particular is one of the most useful and attractive techniques for the synthesis of nano-sized ferrite materials. This is because of its advantages such as good stoichiometric control and the production of ultrafine particles with a narrow size distribution in a relatively short processing time at a very low temperature.
Substitution of other metals for Fe in CoFe2O4 has been proposed as a method to tailor the magnetic and magneto-elastic properties for sensor applications [13]. Chromium ions (Cr3+) with antiferromagnetic nature are known for achieving good control over magnetic parameters in developing technologically important materials. The substitution of Cr3+ ions for Fe3+ ions will alter magnetic properties marked by similar to that of nonmagnetic substitution.
The present work reports the synthesis of nano-sized chromium-substituted cobalt ferrites by citrate-gel autocombustion method and consequent changes on their magnetic properties. Co-Cr nanoferrites were synthesized with particle size ranging from 6 to 12 nm, which will have a great effect on its magnetic properties. Magnetic properties of these ferrites were reported here.
Results and discussions
X-ray diffraction studies
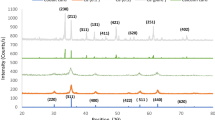
X-ray diffraction (XRD) was performed on the as-synthesized powders as well as on the powders calcined at 500°C, and the XRD patterns of all the samples were shown in Figure 1a,b. Obtained XRD patterns and crystalline phases were identified by comparison with reference data from the ICSD card no. 22–1086 for cobalt ferrites (CoFe2O4) and have been indexed. It confirms the formation of a homogeneous well-defined single-phase cubic spinel structure without any impurity peak belonging to the space group Fd3m (ICSD reference). The strongest reflection comes from the (311) plane that indicates the spinel phase.
From the XRD patterns of the as-synthesized powders, it is clear that the as-burnt powder is also in single phase with a spinel structure which indicates that the ferrite can be directly formed after the autocombustion of the gel without heat treatment. The broad peaks in the XRD patterns indicate a fine particle nature of the particles.
Figure 1a,b clearly shows that the positions of the reflection peaks for as-burnt powders are almost identical to the corresponding peaks for the calcined material. This implies that the basic structure of the nanoparticles is essentially same as that of the bulk material. The average particle sizes of the as-synthesized Co-Cr ferrites and calcined Co-Cr ferrites for different compositions were calculated from Scherrer formula [14] using the maximum intensity peak (311) and were shown in Table 1.
From the table, it is clear that nano-sized Co-Cr ferrite powders can be directly synthesized by Citrate-gel auto combustion method. After calcination at 500°C for 4 h, the reflection peaks of the samples become sharper and narrower, indicating the improvement of crystallinity. Comparing Figure 1a,b it was found that there were no differences between the same compositional samples, except for the relative intensity. Similar behavior was reported in by Toksha et al. [15].
Magnetic susceptibility using faradays balance
The magnetic susceptibility is a dimensionless proportionality constant that indicates the degree of magnetization of a material in response to an applied magnetic field. Magnetic susceptibility of heat-treated Co-Cr nanoferrites at room temperature is measured using very sensitive instrument known as a Faraday magnetic susceptibility millibalance. In the Faraday balance, the sample is placed in between the magnetic poles, and the force depends only on the total mass of the material present. The force is measured as a weight change, using a magnetic balance. Working of the Faraday balance is clearly described by Carlin [16]. To calibrate the field gradient (the force experienced by a standard sample), mercury tetra thiocyanato cobaltate was measured which is known to have a gram susceptibility of 16.44 × 10−6 cgs units at 20°C [17]. The diamagnetic materials are weakly repelled by an external magnetic field, resulting in a negative reading. Paramagnetic materials are attracted to an external magnetic field and give a positive reading.
The gram magnetic susceptibility (χg) of synthesized samples may be calculated from the following equation.
where χg is the gram susceptibility of compound,
(χg)s is gram susceptibility of 16.44 × 10−6 cgs units at 20°C, and
Ws is the weight of the standard sample in the absence of magnetic field,
Wc denotes the weight of the compound in the absence of magnetic field,
Δ ws the change in weight of the standard sample after the applied magnetic field, and
Δ wc the change in weight of the compound after the applied magnetic field.
The molar magnetic susceptibility (χm) values were then calculated from the gram magnetic susceptibility values using the following equation and were tabulated in Table 2.
The effective magnetic moment of the samples can be calculated from the gram magnetic susceptibility using the following equation and were tabulated in Table 2.
where μeff stands for effective magnetic moment in Bohr magnetons (BM),
χm is the molar magnetic susceptibility, and
T is the absolute temperature.
It is reported that the χm value ranges between 10−5 and 10−6 for the paramagnetic substances and between −10−5 and −10−6 for the diamagnetic substances.
From the Table 2 it is clear that the molar susceptibility values of Co-Cr ferrites of various compositions are of the order of 10−6, indicating the paramagnetic nature of Co-Cr ferrite system. Further, the effective magnetic moment calculated for various samples shows that μeff decreases with the increase in the concentration of dopant Cr3+. This indicates that CoFe2O4 is super paramagnetic in nature. Its behavior has changed to paramagnetic by Cr substitution (due to antiferromagnetic nature of Cr). This behavior was confirmed by studying hysteresis loops from VSM measurements.
Magnetic properties using VSM
The magnetic measurements of various compositions of heat-treated Co-Cr nanoferrites were made using vibrating sample magnetometer at room temperature in 15 kOe. Figure 2 shows the magnetic hysteresis loops for all Co-Cr ferrite samples (calcined at 500°C) obtained from VSM measurements. Hysteresis loop gives the relation between the magnetization (M) and the applied field (H).
Magnetic parameters extracted from the hysteresis loops are saturation magnetization (Ms; maximum value of magnetization), remanent magnetization (Mr; magnetization at zero field), and coercivity (Hc; magnetic field required to reduce the magnetization of that material to zero after the magnetization of the sample has been driven to saturation). These magnetic parameters are used to characterize the magnetic properties of materials.
The magnetic parameters of all the samples of Co-Cr ferrites were calculated from the individual M-H loops and were tabulated in Table 3. The M-H loops of CoFe2O4 and CoCrFeO4 were shown in Figure 3.
From Table 3, it is clear that Ms at room temperature decreased from 33.8448 to 13.8347 emu/g with the increase in Cr3+ ion concentration in the Co-Cr ferrites. This decrease in Ms value may be due to the fact that the Fe3+ (magnetic moment 5 μB) are replaced by lesser magnetic Cr3+ ions (magnetic moment 3 μB) in the octahedral (B) sites of the ferrite sublattice. As the concentration of the Cr3+ ion increases, it decreases the Fe3+(B)/Fe2+(A) ratio, that is, A-B super exchange interaction decreases [18].
Hc which is a measure of the magnetic field strength required for overcoming anisotropy to flip the magnetic moments is clearly affected by the chromium substitution. It can be seen from Table 3 that the value of Hc decreased from 1,954.66 Oe to 298.57 kOe up to the ferrite sample with Cr composition x = 0.7 and then increased for x = 0.9 and 1.0. The decrease in coercivity with increase in Cr3+ concentrations may be due to the decrease in anisotropy field which in turn decreases the domain wall energy [19]. The increase in coercivity for ferrites with composition x = 0.9 and 1.0 might be due to the increase in magnetic crystalline anisotropy. The coercivity of the nanoferrites has a contribution from their finite size namely surface anisotropy [20]. Normally, for a given composition of ferrite, when the crystal size is less, the coercivity will be more as the surface becomes much more dominant [21]. Higher Coercivity of CoFe2O4 in the present case may also be related to the particle size effect.
From the values of Hc and Ms, anisotropy constant (K) can be calculated [22] and tabulated in Table 4. It is seen that the value of K decreases with increase of Cr3+ concentrations.
The magnetic moment per formula unit in Bohr magneton (μB) was calculated [23] and tabulated in Table 4. Magnetic moment values were found to decrease with increase in Cr3+ concentration which is attributed to greater occupancy of Cr3+ at B sites.
From all these results, it is clear that the increase of Cr concentration decreases the magnetization, and the material is being converted into a soft magnetic material. Hysteresis curves also show that CoFe2O4 has large area inside the hysteresis loop, where as CoCrFeO4 has a smaller area inside the hysteresis loop. This indicates the fact that the increase in Cr substitution has made the material magnetically soft.
The present study confirms that soft magnetic Co-Cr nanoferrite materials that were synthesized can be easily magnetized and demagnetized. Such materials are desirable for transformer and motor cores to minimize the energy dissipation with the alternating fields associated with AC electrical applications.
Conclusions
Based on the discussion above, we have drawn the following conclusions:
-
A series of CoCr x Fe2 − xO4 (x = 0.0, 0.1, 0.3, 0.5, 0.7, 0.9, and 1.0) nanoparticles have been successfully synthesized by citrate-gel autocombustion technique at a low sintering temperature.
-
The XRD pattern confirms the cubic spinel structure for all compositions of both as-synthesized powders and heat-treated ferrite powders.
-
The crystallite size of the synthesized Co-Cr ferrites was from 6 to 12 nm which is a novelty of this paper.
-
Magnetic susceptibility measurements indicated the formation of super paramagnetic nano-sized ferrites and with increase in Cr composition superparamagnetic behavior has changed to paramagnetic behavior (soft magnetic materials).
-
The incorporation of Cr+3 ions in Co ferrites resulted in decrease of saturation magnetization, coercivity, and magnetic moment because the replacement of Fe+3 by Cr+3 ions weakens the sublattice interaction and lowers the magnetic moments of the unit cells.
-
Magnetic measurements from VSM confirmed the formation of magnetically soft materials.
-
These characteristics of ferrites are desirable for their utility in transformer and motor cores to minimize the energy dissipation with the alternating fields associated with AC electrical applications.
Methods
Synthesis
The starting materials for the synthesis of CoCr x Fe2 − xO4 (x = 0.0, 0.1, 0.3, 0.5, 0.7, 0.9 and 1.0) using citrate-gel autocombustion method were cobalt nitrate, ferric nitrate, chromium nitrate, citric acid, ammonia (99% pure; S D Fine-Chem Limited, Mumbai, India). The synthesis of Co-Cr nanoferrites by citrate-gel autocombustion technique was clearly reported in our earlier publication [14]. The synthesized powders were ground in agate mortar and pestle and calcined in a muffle furnace at 500°C for 4 h to obtain spinel phase.
Characterization
The structural characterization of the as-synthesized and heat-treated powders was carried out by Philips X-ray diffractometer (model 3710; Koninklijke Philips N.V., The Netherlands) using Cu Kα radiation (λ = 1.5405 Å) at room temperature by continuous scanning in the range of 2 to 85 θ° to analyze the phase and crystallite size.
The magnetic susceptibility of the different compositions of Co-Cr ferrites at room temperature was studied by Faraday magnetic susceptibility millibalance (model-7550).
The magnetic properties of synthesized Co-Cr nanoferrites were studied using vibrating sample magnetometer (VSM) (Lakeshore 665, Lake Shore Cryotronics, Inc., Westerville, USA) at room temperature in the applied field of 15 kOe.
Authors′ contributions
All the authors were involved with the whole research work presented here. The author MR has synthesized the nano particles and performed various experiments. DR has helped in interpreting the data and communicating the article to the journal. PV has provided required facilities for measurements and discussed the obtained data. All the authors read and approved the final manuscript.
Authors′ information
M. Ragashuda, M.Sc., M. Phil. Associate Professor. Department of Chemistry, Jaya Prakash Narayan College of Engineering, Mahabubnagar, Andhrapradesh, India. Prof. D. Ravinder, Ph.D. Professor and Head, Department of Physics, Nizam College, Basheerbagh, Osmania University, Hyderabad, Andhrapradesh, India. Prof. P. Veerasomaiah, Professor of Chemistry, Osmania University, Hyderabad, Andhrapradesh, India.
References
Vanaja M, Gnanajobitha G, Paulkumar K, Rajeshkumar S, Malarkodi C, Annadurai G: Phytosynthesis of silver nanoparticles by Cissus quadrangularis : influence of physicochemical factors. J. Nanostruct. Chem. 2013, 3: 17. 10.1186/2193-8865-3-17
Farhadi S, Pourzare K, Sadeghinejad S: Simple preparation of ferromagnetic Co 3 O 4 nanoparticles by thermal dissociation of the [CoII (NH3) 6 ](NO 3 ) 2 complex at low temperature. J. Nanostruct. Chem. 2013, 3: 16. 10.1186/2193-8865-3-16
Subhash C, Srivastava BK, Anjali K: Magnetic behaviour of nano-particles of Ni 0.5 Co 0.4 Fe 2 O 4 prepared using two different routes. Indian J. Pur. Appl. Phy 2004, 42: 366–367.
Costa MM, GFM PJ, Sombra ASB: Dielectric and impedance properties, studies of the lead doped (PbO)-CO 2 Y type hexa ferrite (Ba 2 CO 2 Fe 12 O 22 (CO 2 Y)). Int. J. Mater. Chem. Phy. 2010, 123: 35–39. 10.1016/j.matchemphys.2010.03.026
Muzquiz-Ramos EM, Cortes-Hernandez DA, Herrera-Romero OA, Escobedo-Bocardo JC: Preparation and properties of CoFe 2 O 4 synthesized by the modified citrate-gel method. Mater Sci Forum 2010, 644: 39–42.
Pervaiz E, Gul IH: Enhancement of electrical properties due to Cr3+ substitution in Co-ferrite nanoparticles synthesized by two chemical techniques. J. Magn. Magn. Mater. 2012, 324: 3696–3703.
Bahout MM, Bertrand S, Pena O: Synthesis and characterization of Zn 1 −x Ni x Fe 2 O 4 spinels prepared by a citrate precursor. J. Solid. State. Chem. 2005, 178: 1080–1086. 10.1016/j.jssc.2005.01.009
Kim YI, Kim D, Lee CS: Synthesis and characterization of CoFe 2 O 4 magnetic nanoparticles prepared by temperature controlled coprecipitation method. Phys B: Condensed Matter 2003, 337: 42–51. 10.1016/S0921-4526(03)00322-3
Liu C, Zou B, Rondinone AJ, Zhang ZJ: Chemical control of superparamagnetic properties of magnesium and cobalt spinel ferrite nanoparticles through atomic level magnetic couplings. Chem. Soc. 2000, 122: 6263–6267. 10.1021/ja000784g
Chau JLH, Hsu MK, Kao CC: Microwave plasma synthesis of Co and SiC-coated Co nanopowders. Mater. Lett. 2006, 60: 947–951. 10.1016/j.matlet.2005.10.054
Deng HM, Ding J, Shi Y, Liu XY, Wang J: Ultrafine zinc oxide powders prepared by precipitation/mechanical milling. J. Mater. Sci. 2001, 36: 3273–3276. 10.1023/A:1017902923289
Pradeep A, Priya Darshini P, Chandra Sekharan G: Production of single phase nano size NiFe 2 O 4 particles using sol–gel auto combustion route by optimizing the preparation conditions. Mater. Chem. Phys. 2008, 112: 572–576. 10.1016/j.matchemphys.2008.05.090
Zheng H, Wang J, Lofland SE, Ma Z, Mohaddes-Ardabili L, Zhao T, Salamanca-Riba L, Shinde SR, Ogale SB, Bai F, Viehland D, Jia YG, Schlom D, Wutting M, Roytburd A, Ramesh R: Multiferroic BaTiO 3 -CoFe 2 O 4 nanostructures. Science 2004, 303: 661–663. 10.1126/science.1094207
Raghasudha M, Ravinder D, Veerasomaiah P: Characterization of chromium substituted cobalt nano ferrites synthesized by citrate-gel auto combustion method. Adv. Mat. Phy. Chem. 2013, 3: 89–96. 10.4236/ampc.2013.32014
Toksha BG, Sagar E, Shisath , Mane ML, Patange SM, Jadhav SS, Jadhav KM: Autocombustion high-temperature synthesis, structural, and magnetic properties of CoCr x Fe 2- x O 4 (0≤ x ≤1.0). J. Phys. Chem. 2011, 115: 20905–20912.
Carlin RL: Magnetochemistry. Germany: Springer; 1986.
Figgis BN, Nyholm RS: A convenient solid for calibration of gouy magnetic susceptibility apparatus. J. Chem. Soc. 1958, 4: 4190–4216.
Gul IH, Maqsood A: Structural, magnetic and electrical properties of cobalt ferrites prepared by the sol–gel route. J. Alloy. Comp. 2008, 465: 227–231. 10.1016/j.jallcom.2007.11.006
Gul IH, Abbasi A, Amin F, Anis-ur-Rehman M, Maqsood A: Structural, magnetic and electrical properties of Co 1− x Zn x Fe 2 O 4 synthesized by co-precipitation method. J. Magn. Magn. Mater. 2007, 311: 494. 10.1016/j.jmmm.2006.08.005
Shirsath SE, Jadhav SS, Toksha BG, Patange SM, Jadhav KM: Influence of Ce4+ ions on the structural and magnetic properties of NiFe 2 O 4 . J. Appl. Phy. 2011, 110: 013914. 10.1063/1.3603004
Batoo KM, Kumar S, Lee CG, Alimuddin : Study of dielectric and ac impedance properties of Ti doped Mn ferrites. Curr Appl Phy. 2009, 9: 1397–1406. 10.1016/j.cap.2009.03.012
Mane DR, Birajdar DD, Shirsath SE, Telugu RA, Kadam RH: Structural and magnetic characterizations of Mn-Ni-Zn ferrite nanoparticles. Phys. Status. Solidi. A. 2010, 207: 2355–2363. 10.1002/pssa.201026079
Singhal S, Chanda K: Cation distribution and magnetic properties in chromium-substituted nickel ferrites prepared using aerosol route. J. Solid. State. Chem. 2007, 180: 296–300. 10.1016/j.jssc.2006.10.010
Acknowledgments
MRS is thankful to chairman K.S. Ravi Kumar, Jayaprakash Narayan College of Engineering for his support in establishing the lab for the synthesis of the samples. DR is grateful to Prof. T.L.N. Swamy, principal of Nizam College, for his encouragement to carry out this research work. The authors are thankful to Prof. C. Gyana Kumari, head of the Department of Chemistry, Osmania University, Hyderabad for her encouragement in carrying out research activities. The authors are also thankful to Prof. M. Vithal, Department of Chemistry, Osmania University for his support in the characterization of the samples.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Raghasudha, M., Ravinder, D. & Veerasomaiah, P. Magnetic properties of Cr-substituted Co-ferrite nanoparticles synthesized by citrate-gel autocombustion method. J Nanostruct Chem 3, 63 (2013). https://doi.org/10.1186/2193-8865-3-63
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2193-8865-3-63