Abstract
In this work, CdO cauliflower-like nanostructure synthesized by mechanochemical method was employed to evaluate the adsorption ability of Congo red (CR) from the aqueous solution for the first time. UV-visible absorption spectroscopy was used to record the adsorption behavior. This special structure composed of nanorods and tubes with the high contact sites and surface area of 104 m2 g−1 can be operated as a capable adsorbent to absorb the dye molecules via adsorption process. The adsorption capacity of this material (0.01 g) was studied in high concentrations of CR (50 to 300 mg L−1) and represented an excellent efficiency to eliminate this toxic dye. Maximum adsorption capacity (qmax) calculated using Langmuir isotherm model, at room temperature and neutral pH, was found to be 588.24 mg g−1. Electrostatic interactions were conceived as the main adsorption mechanism, and the calculated dimensionless separation factor (R L ), 0.023, indicated a favorable adsorption process. The kinetic and thermodynamic parameters for this proceeding were evaluated and confirmed the high performance of the synthesized adsorbent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Dyes and pigments are the most common environmental pollutants widely used in various industries. Being toxic and harmful for environment and living organisms, these pollutants should be removed. A significant challenge that we deal with such as textile, food, paper, and dyeing industries has always been the wastewaters refinement of toxic contaminants before their discharge to the environment [1, 2]. There are some techniques such as coagulation, flocculation, advanced oxidation processes, membrane filtration, and adsorption in order to eliminate the contaminants from wastewaters [3, 4]. For this purpose, one of the most effective and economical methods is adsorption procedure which has attracted much attentions in recent years [5]. Many adsorbents are employed to decolorize wastewaters and remove pollutants such as magnetic materials [5], activated carbons [6], fly ash [7], and chitosan/montmorillonite nanocomposite [8]. In fact, using cadmium oxide as an adsorbent to purify the organic wastewaters like Congo red (CR) aqueous solution has not been reported yet. Congo red is a toxic and carcinogenic dye, which despite it being forbidden is still used in some developing countries in the textile industry and via their wastewaters is entered to the environment without treatment. Therefore, CR dye was selected as the model contamination to investigate the capability of the synthesized CdO nanostructure for decolorizing this dye from an aqueous solution.
Cadmium oxide, CdO, is a known n-type semiconductor with the direct band gap energy of 2.5 eV [9]. CdO is applied in solar cells [10], gas sensors [11], transparent electrodes [12], catalysts, photocatalysts [13, 14], and photodiodes [15]. Numerous structures of cadmium oxide-like nanoparticles [16], nanowires [17], nanoneedles [18], and nanocrystals [19] have been reported in nanoscale. Among many structures of this compound, cauliflower structure indicates a quick growth in the materials science owing to novel and special morphology. This structure has a great importance due to its high specific surface area and potential applications in various fields [20, 21]. We easily synthesized this particular structure using a mechanochemical method, a cost-effective process, followed by heating treatment, which has been presented in our previous work in detail [21].
In this research, we employed the cadmium oxide cauliflower-like nanostructure to remove the CR dye from the solution for the first time. The results of Langmuir isotherm pattern indicate that this compound has the highest adsorption capacity among many reports published up to now. Meanwhile, the adsorption kinetics, thermodynamics parameters, and desorption process for this adsorbent were investigated.
Results and discussion
The structural description of the prepared adsorbent
The structural and morphological characterization of the prepared adsorbent, CdO cauliflower-like nanostructure, was carried out using the techniques clearly described in our previous work [21]. A series of experiments were carried out to investigate the synthesized compound adsorption performance after confirming the purposed special morphology (shown in Figure 1a,b,c,d). The SEM images indicated the cauliflower-like microstructure of this product. By recording the TEM images, it was denoted that this structure has been constructed from the nanorod bundles. In fact, it was found that nanorods are interwoven and formed the cauliflower-like structure.
Meanwhile, the XRD pattern from the prepared product clearly showed the formation of pure cubic CdO phase with lattice constant 4.695 Å and the space group Fm 3m (Figure 2). The diffraction peaks at 2θ values of 32.90°, 38.20°, 55.20°, 65.80°, and 69.20° are in a close agreement with the 111, 200, 220, 311, and 222 planes (JCPDS-05-0640), respectively.
The surface area determination
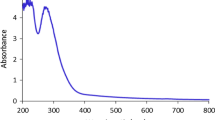
The nitrogen adsorption and desorption experiments were performed to evaluate the surface area of this product. A distinct hysteresis loop recorded at the range of 0 < P/P0 < 1 indicated the isotherm curve of type V (Figure 3). The Brunauer-Emmett-Teller (BET) surface area of 104 m2 g−1 proved the presence of high contact sites on the surface of this adsorbent. Likewise, the pore size distribution of 3.53 nm was obtained by the Barrett-Joyner-Halenda (BJH) plot using the desorption branch of the nitrogen isotherm (in the inset of Figure 3). This value introduces a mesoporous structure for the synthesized product. Therefore, there are the high surface contact sites, which can increase the adsorption percentage of dye molecules by this adsorbent in water.
Adsorption study
Effect of contact time and initial pH
The contact time effect of the adsorbent on the adsorption of CR in the range of 0.5 to 3 h was studied to determine the optimal demanded time for removing 100 mg L−1 of CR solution at neutral pH. A certain amount of adsorbent, 0.01 g of CdO nanostructure, was added into 25 ml of CR solution. The adsorption of CR molecules on the adsorbent led to a decrease in the concentration of CR with time (Figure 4). According to this diagram, the maximum removal efficiency of CR dye (nearly 100%) occurs for about 3 h, and after this time, the adsorption efficiency of CR is constant. Therefore, agitation time of 3 h was selected for further studies. The pH is a significant controlling parameter that can intensely influence the adsorption efficiency of cationic or anionic dyes onto the adsorbent sites. The effect of solution pH on the adsorption percentage of CR by the cauliflower CdO nanostrucutre was studied via adjusting the pH of aqueous medium at the range of 4 to 9 using HCl and NaOH solutions (0.01 mol L−1). The screening of these experiments indicated the highest removal efficiency of CR in a solution with pH of 6, which is close to the neutral pH (shown in Figure 5). As a result, the neutral pH was selected as the appropriate pH for per run.
In fact, adsorption proceeding at neutral pH is a beneficial performance that few adsorbents show this feature. Most reports provided by researchers to remove the CR dye are at pH 5 and 6 [22, 23].
Effect of the amount of CdO nanostructures
The effect of the amount of the adsorbent required for the maximum adsorption percentage of CR dye is shown in Figure 6. Different quantities of CdO nanostructures were investigated in the range of 0.005 to 0.015 g. Maximum percentage was specified when 0.01 g of CdO cauliflower-like nanostructure was used in 25-mL solution of CR dye with 100 mg L−1 concentration at neutral pH. Therefore, further studies were conducted on CR dye at an optimum amount of mentioned adsorbent.
Adsorption isotherm
Figure 7 illustrates the adsorption capacity of CdO cauliflower-like structure for the CR solution, which is studied by measuring the initial and final concentration of CR after 3 h of agitation in the dark. It was observed that the adsorption of CR molecules increases with an enhancement in dye concentration and inclines to reach the saturation point at higher concentrations (250, 275, and 300 mg L−1). Langmuir and Freundlich equations, the famous adsorption isotherm models, were applied to examine the relationship between the amount of CR adsorbed onto the CdO particles and its equilibrium concentration in solution. In fact, these adsorption isotherms were employed to interpret the interactions between CR molecules and adsorbent.
The linearized forms of Langmuir (Equation 1) and Freundlich (Equation 2) isotherm models are as follow:
The parameters of these two isotherm models were calculated and given in Table 1. a L (L mg−1) and K L (L g−1) are the Langmuir constants. These constants were calculated from the slope and intercept of the plot of C e /q e vs. C e shown in Figure 8a. K F (mg1−1/n L1/n g−1), and n is the Freundlich adsorption isotherm constant, which were obtained from the slope and intercept of linear plot of Log q e vs. Log C e (Figure 8b). In these equations, C e is the equilibrium concentration of the CR in the solution (mg L−1). Meanwhile, q e , the amount of CR adsorbed (mg g−1) per unit of adsorbent at equilibrium (mg g−1), is calculated by the following Equation (3) [24]:
where, C i and C f are the initial and final concentrations of CR in milligrams per liter, respectively; V is the volume of experimental solution in liters, and m is the weight of CdO cauliflower nanostructure in grams.
The results indicated that this adsorption process does not follow the Freundlich isotherm model but is in a good agreement with Langmuir model with reference to the obtained value of regression coefficients (R = 0.997). Although Langmuir’s model does not consider the variation in adsorption energy, it obviously describes the adsorption method. It is based on the physical theory that the maximum adsorption capacity includes of a monolayer adsorption [22].
The maximum adsorption capacity (milligrams per gram) is indicated by [q m = K L /a L ], and it highly depends on the number and structure of adsorption sites. In our pervious study, we reported that cauliflower structure has been composed of tubes and rods with the average size of 68 nm [21]. As long as there are unoccupied sites, adsorption process will resume with increasing CR concentrations. However, as soon as all of the adsorbent sites are filled, an additional increase in the concentrations of CR solutions does not increase the amount of CR on adsorbent [22].
The maximum adsorption capacity (qmax) for the adsorption of CR onto the CdO cauliflower structure was found to be 588.24 mg g−1. This value is the highest value among the other adsorbents, which have been reported until now (see Table 2).
In fact, the high removal efficiency of CR from aqueous solution by cauliflower-like nanostructure can be referred to the presence of enormous adsorbing sites on the surface of this structure. An electrostatic interaction is established between these adsorbing sites and dye species, which lead to remove the CR molecules from the solution in a short time.
In order to express the essential characteristics of the presented Langmuir isotherm, a dimensionless constant separation factor (R L ) was used (Equation 4):
Generally, the nature of adsorption process can be described by several terms of R L value; unfavorable (R L > 1), linear (R L = 1), favorable (0 < R L < 1), and irreversible (R L = 0). The obtained R L value of 0.023 revealed that the adsorption of CR on the CdO cauliflower-like nanostructure is a favorable process.
Adsorption kinetics
Figure 9 represents two kinetic models of pseudo-first-order (Figure 9a) and pseudo-second-order (Figure 9b) rate equations of adsorption process from CR dye onto CdO adsorbent as follow:
where, q e and q t are the amount of dye adsorbed (mg g−1) at equilibrium and time, t (min), respectively. The parameters of k1 and k2 are the rate constants of pseudo-first-order (min−1) and pseudo-second-order (g mg−1 min−1) equations, which were calculated from the linear plots of log (q e − q t ) vs. t and (t/q t ) vs. t, respectively. Table 3 shows the calculated kinetic parameters of both rate models. Comparing the correlation coefficients (R2) of the mentioned models, the results revealed that the pseudo-second-order rate model is a better fit than the pseudo-first-order according to experimental adsorption data. In fact, this good agreement to the pseudo-second-order kinetic model presents the dependence of adsorption mechanism on the adsorbate and adsorbent [26].
Adsorption thermodynamic
The thermodynamic parameter of the performed adsorption process was evaluated through calculating of free energy change (delta G, kilojoules per mole) and using the following equation (7):
Where, R is the gas constant (8.314 J mol−1 K−1), T (K) is the temperature, and Kc (mL/g) is the standard thermodynamics equilibrium constant:
The obtained value of ΔG value is −11.48 kJ mol−1 (T = 298 K). This negative value of free energy change indicates the spontaneous nature of adsorption process of CR dye on this adsorbent [25]. The mentioned result confirms the high performance of CdO cauliflower-like nanostructure to remove the CR dye from aqueous solution by using adsorption process.
Desorption procedure
The adsorbed dye molecules were easily desorbed using an appropriate amount of acetone with magnetic stirring for 1 h. Desorption efficiency of 74% was calculated by relation of (9) as follows:
It was found that such adsorbent not only possesses the high adsorption capability but also presents a good desorption efficiency for CR dye. Likewise, the implemented adsorbent was efficiently recovered by a heating treatment at 450°C for 2 h. The maximum adsorption capacity and high desorption ratio are the merits of this type of adsorbent to reduce the charges of purification treatments.
Conclusions
In the present work, the CdO cauliflower-like nanostructure was applied to remove the CR dye from aqueous solution in high concentrations (50 to 300 mg L−1). The analysis of adsorption isotherm showed that this adsorption experiment is in a well accordance with the Langmuir model. The calculated maximum adsorption capacity (qmax) for this adsorbent was observed to be 588.24 mg g−1, which is the highest value in comparison with the previous reports. It is inferred that the special structure of this compound having the numerous number of surface sites and strong interactions leads to this excellent adsorption performance and water treatment. Meanwhile, it was found that the adsorption process follows a pseudo-second-order rate model much better than a pseudo-first-order rate model. The kinetics and thermodynamics findings indicated the more efficient performance of this adsorbent to remove the CR dye from aqueous solution at a short time and without any additives. Therefore, this compound can be a promising candidate among other counterparts for water treatment.
Methods
Chemicals
Cadmium acetate dehydrate (Cd(CH3COO)2.2H2O, pure, Merck, Whitehouse Station, NJ, USA) and acetamide (CH3CONH2, pure, Merck) were provided to synthesize the CdO adsorbent. Congo red (sodium sodium 3,3′-([1,1′-biphenyl]-4,4′-diyl)bis(4-aminonaphthalene-1-sulfonate) as a pollutant model was supplied from commercial source. All the chemicals were used without further purification.
Synthesis of adsorbent
The cauliflower-like nanostructure of cadmium oxide, CdO, was synthesized using mechanochemical method similar to the process reported in our previous work [21]. Briefly, Cd(CH3COO)2 2H2O and CH3CONH2 as the starting materials with a molar ratio of 3:4 were milled to each other at the milling rate of 1,800 rpm for 30 min. Then, the resulting precursor was calcined at 450°C for 2 h in air to prepare the product. The obtained product, CdO cauliflower-like nanostructure, was employed for dye adsorption proceeding.
Adsorption experiment
The Congo red dye was selected as an adsorbate to evaluate the performance of the resulting product at the adsorption proceeding of dye from the aqueous solution. The structure of this organic azo dye is illustrated in Figure 10. Adsorption operation was performed under the following conditions: 0.01 g of the prepared CdO cauliflower nanostructures as the adsorbent was added to 25 mL of CR dye aqueous solution with a known initial concentration (in the range of 50 to 300 mg L−1) at neutral pH. The obtained suspension was stirred using a magnetic stirrer at room temperature for 3 h in the dark. Then, CR-loaded CdO powder was separated with centrifugation at 1,800 rpm for 5 min. In the specified time periods, the portions of suspension were taken away from the reaction vessel, and the concentration of the residual dye was measured using UV–vis spectrophotometer at an appropriate wavelength corresponding to the maximum absorption of CR (498 nm).
The percent removal of dye from the solution can be calculated by the following equation:
where, C0 is the initial concentration of dye, and Ci is the final concentration of dye after treatment with CdO nanostructure. The removal percentage of CR molecule increased with time due to its adsorption onto adsorbent sites. A double-beam UV spectrophotometer (Shimadzu UV-1700, Kyoto, Japan) was used to determine CR concentration in the supernatant solutions before and after adsorption.
Characterization
The prepared adsorbent, CdO cauliflower-like nanostructure, was characterized by utilizing the techniques clearly described in previous work reported in [21]. The surface area of the product was obtained by using the BET technique with Micromeritics (Gemini, Norcross, GA, USA) in the range of relative pressures from 0.0 to 1.0. Before employing, the sample was degassed at 200°C for 2 h. In addition, the pore size distribution was determined from the desorption branch of the isotherm curve using the BJH model.
References
Guo H, Lin K, Zheng Z, Xiao F, Li S: Sulfanilic acid-modified P 25 TiO 2 nanoparticles with improved photocatalytic degradation on Congo red under visible light. Dye. Pigment. 2012, 9: 1278–1284.
Ma J, Jia Y, Jing Y, Yao Y, Sun J: Kinetics and thermodynamics of methylene blue adsorption by cobalt-hectorite composite. Dye. Pigment. 2012, 93: 1441–1446. 10.1016/j.dyepig.2011.08.010
Verma AK, Dash RR, Bhunia P: A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters. J. Environ. Manag. 2012, 93: 154–168. 10.1016/j.jenvman.2011.09.012
Cheng S, Oatley DL, Williams PM, Wright CJ: Characterisation and application of a novel positively charged nanofiltration membrane for the treatment of textile industry wastewaters. Water Res. 2012, 46: 33–42. 10.1016/j.watres.2011.10.011
Rahimi R, Kerdari H, Rabbani M, Shafiee M: Synthesis, characterization and adsorbing properties of hollow Zn-Fe 2 O 4 nanospheres on removal of Congo red from aqueous solution. Desalination 2011, 280: 412–418. 10.1016/j.desal.2011.04.073
Purkait MK, Maiti A, DasGupta S, De S: Removal of Congo Red using activated carbon and its regeneration. J. Hazard. Mater. 2007, 145: 287–295. 10.1016/j.jhazmat.2006.11.021
Acemioğlu B: Adsorption of Congo red from aqueous solution onto calcium-rich fly ash. J. Colloid Interface Sci. 2004, 274: 371–379. 10.1016/j.jcis.2004.03.019
Wang L, Wang A: Adsorption characteristics of Congo Red onto the chitosan/montmorillonite nanocomposite. J. Hazard. Mater. 2007, 147: 979–985. 10.1016/j.jhazmat.2007.01.145
Grado-Caffaro MA, Grado-Caffaro M: A quantitative discussion on band-gap energy and carrier density of CdO in terms of temperature and oxygen partial pressure. Phys Lett A 2008, 372: 4858–4860. 10.1016/j.physleta.2008.04.068
Yakuphanoglu F: Nanocluster n-CdO thin film by sol–gel for solar cell applications. Appl. Surf. Sci. 2010, 257: 1413–1419. 10.1016/j.apsusc.2010.08.045
Kamble AS, Pawar RC, Patil JY, Suryavanshi SS, Patil PS: From nanowires to cubes of CdO: ethanol gas response. J. Alloys Compd. 2011, 509: 1035–1039. 10.1016/j.jallcom.2010.09.166
Gupta RK, Ghosh K, Patel R, Kahol PK: Low temperature processed highly conducting, transparent, and wide bandgap Gd doped CdO thin films for transparent electronics. J. Alloys Compd. 2011, 509: 4146–4149. 10.1016/j.jallcom.2011.01.007
Singh G, Kapoor IPS, Dubey R, Srivastava P: Synthesis, characterization and catalytic activity of CdO nanocrystals. Mater Sci Eng: B 2011, 176: 121–126. 10.1016/j.mseb.2010.10.009
Li J, Ni Y, Liu J, Hong J: Preparation, conversion, and comparison of the photocatalytic property of Cd(OH) 2 , CdO. CdS and CdSe. J. Phys. Chem. Solid 2009, 70: 1285–1289. 10.1016/j.jpcs.2009.07.014
Yakuphanoglu F, Caglar M, Caglar Y, Ilican S: Electrical characterization of nanocluster n-CdO/p-Si heterojunction diode. J. Alloys Compd. 2010, 506: 188–193. 10.1016/j.jallcom.2010.06.174
Dong W, Zhu C: Optical properties of surface-modified CdO nanoparticles. Opt. Mater. 2003, 22: 227–233. 10.1016/S0925-3467(02)00269-0
Chang Q, Chang C, Zhang X, Ye H, Shi G, Zhang W, Wang Y, Xin X, Song Y: Enhanced optical limiting properties in suspensions of CdO nanowires. Opt. Commun. 2007, 274: 201–205. 10.1016/j.optcom.2007.01.064
Liu X, Li C, Han S, Han J, Zhou C: Synthesis and electronic transport studies of CdO nanoneedles. Appl. Phys. Lett. 2003, 82: 1950–1952. 10.1063/1.1562331
Tadjarodi A, Imani M: Synthesis and characterization of CdO nanocrystalline structure by mechanochemical method. Mater. Lett. 2011, 65: 1025–1027. 10.1016/j.matlet.2010.12.054
Ren H-X, Huang XJ, Yarimaga O, Choi YK, Gu N: A cauliflower-like gold structure for superhydrophobicity. J. Colloid Interface Sci. 2009, 334: 103–107. 10.1016/j.jcis.2009.03.023
Tadjarodi A, Imani M: A novel nanostructure of cadmium oxide synthesized by mechanochemical method. Mater. Res. Bull 2011, 46: 1949–1954. 10.1016/j.materresbull.2011.07.016
Afkhami A, Moosavi R: Adsorptive removal of Congo red, a carcinogenic textile dye, from aqueous solutions by maghemite nanoparticles. J. Hazard. Mater. 2010, 174: 398–403. 10.1016/j.jhazmat.2009.09.066
Pavan FA, Dias SLP, Lima EC, Benvenutti EV: Removal of Congo red from aqueous solution by anilinepropylsilica xerogel. Dye. Pigment. 2008, 76: 64–69. 10.1016/j.dyepig.2006.08.027
Chatterjee S, Lee DS, Lee MW, Woo SH: Enhanced adsorption of congo red from aqueous solutions by chitosan hydrogel beads impregnated with cetyl trimethyl ammonium bromide. Bioresource Technol 2009, 100: 2803–2809. 10.1016/j.biortech.2008.12.035
Wang L, Li J, Wang Y, Zhao L, Jiang Q: Adsorption capability for Congo red on nanocrystalline MFe 2 O 4 (M= Mn, Fe, Co, Ni) spinel ferrites. Chem. Eng. J. 2012, 181–182: 72–79.
Ayad M, Abu El-Nasr A: Anionic dye (acid green 25) adsorption from water by using polyaniline nanotubes salt/silica composite. J Nanostruct Chem 2012, 3: 3–11.
Acknowledgements
The financial support of this study by Iran University of Science and Technology and Iranian Nanotechnology Initiative is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AT designed the related subject, managed all of the process, and conceived this study. MI carried out all of the experimental section and drafted the manuscript. HK participated in the manuscript preparation. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Tadjarodi, A., Imani, M. & Kerdari, H. Adsorption kinetics, thermodynamic studies, and high performance of CdO cauliflower-like nanostructure on the removal of Congo red from aqueous solution. J Nanostruct Chem 3, 51 (2013). https://doi.org/10.1186/2193-8865-3-51
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2193-8865-3-51