Abstract
A pencil graphite electrode (PGE) electrodeposited by nanostructure polypyrrole (PPy) conducting polymer film doped with 5-sulfosalicylic acid (SSA) as sensing material (PGE/PPy/SSA-Cu) was successfully developed for the detection of Cu (II) in aqueous solutions. For the preparation of the copper-sensing electrode, electrodeposition of a polypyrrole film was carried out using cyclic voltammetry in a solution containing 10.0 mM pyrrole and 5.0 mM 5-sulfosalicylic acid. The introduced electrode in the current paper can be fabricated simply and was found to possess high selectivity, showed wide working concentration range, fast response time (<20 s), long lifetime, and very good sensitivity to Cu (II) ion. The prepared electrode exhibited a linear Nernstian response within the copper ion concentration range of 1.0 × 10−5 to 1.0 × 10−1 M with a slope of 29.60 ± 0.30 mV per decade over the pH range of 4.0 to 6.0. Under the optimized conditions, a detection limit of 5.42 μM was obtained. Applications of this electrode for determination of copper ion in real samples and as an indicator electrode for potentiometric titration of Cu2+ ion using EDTA are reported.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
The determination of copper assumes the importance in view of its widespread occurrence in environmental samples. As such, large concentrations of copper can be tolerated by human beings; however, excessive dosage and long-term exposure may cause irritation of the nose, mouth, and eyes, and it causes headache, stomachache, dizziness, vomiting, and diarrhea. Copper deficiency results in anemia while its accumulation resulting in Wilson disease [1–6].
A number of instrumental methods such as atomic absorption spectrometry (AAS), cold vapor AAS or flame atomic absorption spectrometry-electrothermal atomization, inductively coupled plasma optical emission spectroscopy, anodic stripping voltammetry, chromatography, gravimetric detection, or photometry have been proposed for its determination at low concentration level [7–11]. Most of the mentioned methods usually have sufficiently low detection limit and high selectivity but also in many cases, possess drawbacks such as high cost of equipment and expensive materials, time-consuming, and complicated operation. Thus, these methods are generally unsuitable for simple, low cost, and remote determination of copper.
Ion-selective electrodes (ISE) provide analytical procedures for such situations as they are fast, convenient, require minimum sample pretreatment, and may also be suitable for online analysis. In recent years, many substances have been used for electrode modification and determination of copper [12–20]. Conductive polymers (CPs) due to combination of tunable chemical and electronic properties of them are a promising group of compounds, which are widely applied in chemical sensors and a variety of other applications [21–23]. One type of sensors is of noble metal electrodes coated with a conductive polymer film. These electrodes, when used for potentiometric measurements, are called coated wire electrodes. This type of sensors, by eliminating the internal filling solution, provides new advantages such as good mechanical stability, simplicity, and possibility of miniaturization. The polymer layer is relatively easy to prepare and results in thick and homogenous coverage of the metal. Since this type of sensors is not selective to create a selective CP-based receptor, it requires a polymerizable and selective monomer that is also stable under the polymerization conditions. The CP's selectivity is controlled by doping the film from solution or by incorporating a recognition unit into the conjugated polymeric backbone. In terms of potentiometry, the film acts as a cation or anion exchanger and provides a cationic or anionic potentiometric response. Sensors based on conductive polymers have distinct advantages, such as simple preparation, fast response towards the analyte, and potential for miniaturization. Their lifetime is longer than classical PVC membrane electrodes because there is no receptor (ionophore) loss from the membrane due to the low solubility of the polymeric film in conventional organic and inorganic solvents [24–26]. Polypyrrole (PPy) has received the most attention owing to its convenience of preparation, high stability, and wide range of applications. The electroactive nature or the switching properties of PPy has been utilized as the basis of most proposed applications such as sensors, separation devices, rechargeable batteries, and controlled drug release. All other applications of this polymer are based on their electrical conductivity and unique electrochemical properties [27–29].
In this research, a new solid state electrode was prepared by the electrodeposition of a PPy nanolayer, which was doped with 5-sulfosalicylic acid (SSA) (Figure 1) onto the surface of pencil graphite electrode (PGE). We chose pencil graphite as the material of the electrode because it has a larger active electrode surface area and therefore is able to detect low concentrations and/or volumes of the analyte. The main original idea developed in the current research is drawn from the unique properties of the polypyrrole conducting polymer in which anions from the reaction solution can be incorporated into the polymer during electropolymerization as dopant or counterion. The incorporated anions termed as dopants can then induce its property into the polymer product. Choosing of 5-sulfosalicylic acid as dopant during electropolymerization of polypyrrole was based on its high affinity for the formation of a stable complex with Cu (II) ion [30]. Therefore, 5-sulfosalicylic acid was predicted to act as dopant during electropolymerization and as ionophore in the course of potentiometric determination of cupric ions. The thickness of the coated polymer film onto working electrode can be easily controlled by measuring the charge that passes through the cell during electropolymerization. The polymeric nanofilm (PPy/SSA) used for the preparation of the copper-sensing electrode is an electronically and ionically conducting material based on polypyrrole which allows better defined ion-to-electron transduction compared to the classical coated wire electrodes.
Results and discussion
The electrode composition effect on the potential response of the PGE/PPY/SSA-C
It is well known that the compositions of membranes have great effect on the characteristics of ISEs such as sensitivity, working concentration range, selectivity, and potential stability. Therefore, electrodes with different compositions have been prepared, and their potentiometric response characteristics were evaluated in order to find out the optimum electropolymerization conditions. The result of some investigated parameters is shown in Table 1. It was found that the best electrodeposition conditions for observing Nernstian response resulted when a cell solution containing 10-mM monomer (pyrrole), and 5.0-mM electrolyte (5-sulfosalicylic acid) was employed by cyclic voltammetry method, potential range of −1 to +1 (V), scan rate of 50 (mV/s), and cycle number of 20 (at optimal set pH = 5), (no. 13). The fabricated electrode was soaked for 1 h (optimized condition time) in 1.0 × 10−3 M Cu (II) solution before its use for potentiometric analysis. The electromotive force (EMF) values were measured using an accurate digital voltmeter.
Characterization
Scanning electron microscope (SEM) is a useful instrument for characterizing the surface morphology of the polymers. The micrographs obtained for polypyrrole used in this investigation is shown in Figure 2: (a) SEM of bare PGE, (b) PGE/PPY/Cl, (c) PGE/PPY/SSA-Cu, and (d) cross section of PGE/PPY/SSA-Cu. As the SEM pictures show, the PPy at the presence of SSA as a dopant is electrodeposited onto the pencil graphite electrode as nanoparticles which the average diameter of those is about 84 nm, and the layer thickness is about 200 nm (Figure 2c,d). These images also demonstrate that a bulk quantity of nanoparticle size on the PPy exists.
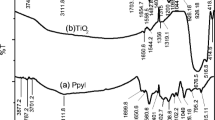
Fourier transform infrared spectroscopy (FTIR) technique was used to ascertain the presence of SSA in polymer matrix. Additional file 1: Figure S1a shows the FTIR spectrum of pure PPy. The band at 1,570.54 and 1,384.60 cm−1 may be corresponded to typical polypyrrole ring vibration and =C–H band in plane vibration, respectively. Additional file 1: Figure S1b shows that the FTIR spectrum of PPy/SSA, which exhibits absorption peaks at 1,085.49, is assigned as S=O stretching band and at 1,387.76 and 1,635.47 may be corresponded to C=C stretching bands. Also, a broad and sharp band observed at 3,443.32 may be attributed to the presence of O–H. These results confirm the incorporation of SSA within the polymer structure which has the most important role of sensing the electrode toward Cu (II) ions.
Energy-dispersive X-ray spectroscopy (EDS) is another analytical technique which is used for chemical characterization of a sample. Its characterization capabilities are due to the fundamental principle that each element has a unique atomic structure allowing unique set of peaks on its X-ray spectrum. The comparison of the EDS spectra of the polymer before and after doping with SSA is illustrated in Additional file 2: Figure S2a,b, respectively. The presence of a sharp and intense peak of sulfur in EDS spectrum appeared at 2.2 to 2.4 keV (Additional file 2: Figure S2b) which is a clear indication of incorporation of SSA into the polypyrrole film during electropolymerization.
Calibration curve and statistical data
Direct potentiometry using a working curve (a plot of EMF vs. log C) is usually used in potentiometric analysis with ion-selective electrodes. The calibration curve for analysis of Cu (II) ions was prepared using standard solutions of Cu (II) ion (pH of solution was adjusted about 5.0), and the potential readings were carried out using the cell setup with solid state Cu (II) electrode (PGE/PPy/SSA-Cu) and was compared with the result of PGE/PPy/SA (Figure 3, curves A and B, respectively).
As the results show (Figure 3, curve A), the fabricated electrode exhibits linear response over a working concentration range of 1.0 × 10−5 to 1.0 × 10−1 M with a Nernstian slope of 29.60 ± 0.30 mV per decade (for one electrode with n = 4) of copper ion concentrations. By extrapolating the linear parts of the ion-selective calibration curve, the detection limit of an ion-selective electrode can be calculated. In this work, the detection limit of the prepared sensor was 5.42 μM which was calculated by the extrapolating of the two segments of the calibration curve in Figure 3. The reproducibility of the Cu2+ electrode was also investigated. The standard deviations of the slope of four replicate constructed electrodes were ±0.50. The response characteristics of the prepared copper-sensing electrode are also summarized in Table 2.
Effect of pH
The influence of the pH of the test solution on the potential response of the electrode for a solution containing 1.0 × 10−4 M Cu (II) was considered in the pH range of 2.0 to 7.0, and the results are shown in Additional file 3: Figure S3. As can be seen, the potential remains constant over a pH range of 4.0 to 6.0. At alkali media, the potential sharply decreased due to the formation of some hydroxyl complexes of Cu (II) ions in solution. On the other hand, at pH lower than 3.5, the interference of H+ ions is more which is due to the high rate of diffusion of H+ ions from the sample solution to electrode surface, and its resulting increase in potential that indicates the protonated ionophore possesses a poor response to the Cu (II) ions and strong response to H3O+ ions in solution.
Response time
Response time is the average time required for the electrodes to reach a potential response within ±1 mV of the final equilibrium value, after successive immersions in a series of testing solutions, each having a tenfold concentration difference. In this study, the practical response time of the sensor was recorded by changing the Cu2+ concentration in solution, over a concentration range of 1.0 × 10−5 to 1.0 × 10−1 M. The potentials vs. time traces are shown in Figure 4a. It is noticed that stable potentials were attained within 20 s. This short response time is most probably due to the fast exchange kinetics of complexation-decomplexation of Cu2+ ion with the sensing material on the tested solution-polymeric surface of electrode interface. In order to examine the stability of the potential reading of the electrode, it was also put in a 1.0 × 10−3 M of copper ion. As the results show (Figure 4b), the electrode reaches its equilibrium response in a very short time (approximately 20 s) and remained constant for approximately 4 min.
Reversibility and lifetime
Reversibility factor is one of the most important parameters in assessing a sensor performance for its practical application. In order to find out the reversibility of the response, the designed electrode was exposed repetitively in the Cu (II) solutions with two different concentrations (1.0 × 10−4and 1.0 × 10−3 M). The reported potential is the mean values of at least three measurements for each concentration. The consequences observed are shown in Figure 5 as the data fascinatingly indicate that the signal of the fabricated Cu (II) electrode is interestingly highly reversible.
The lifetime of the PGE/PPy/SSA-Cu electrode was carried out by performing periodic calibration with standard solutions and calculating the response and slope over the range 1.0 × 10−5 to 1.0 × 10−1 M Cu2+ solution (Table 3). It was found that the electrode worked well over the period of 2 months without showing any significant divergence in concentration range, slope, and response time. During non-usage, the electrode was stored in the air and before use; they were re-equilibrated by dipping in 1.0 × 10−3 M Cu2+ solution for 1 h.
Potentiometric selectivity
The potentiometric selectivity coefficients, which reflect the relative response of the membrane sensor towards the primary ions over other ions present in solution, are perhaps the most essential characteristic of an ISE. To investigate the membrane electrode selectivity, its potential response was monitored in the presence of various interfering foreign cations with the help of two different procedures, namely the so-called matched potential method (MPM) and the separated solution method (SSM). The resulting selectivity coefficients are presented in Table 4. As the results present, the electrode is very selective to Cu (II) ions over all the tested interfering ions.
Effect of temperature
The standard cell potentials (E°cell) were determined at different temperatures from the respective calibration plots as the intercepts of these plots at p[Cu] = 0 and were used to determine the isothermal temperature coefficient (dEo/dt) of the cell [31]. By plotting of E°cell vs. (t − 25), a straight line is produced (Equation 1). The slope of this line was taken as the isothermal temperature coefficient of the cell.
The standard potentials of the reference (Calomel) and the prepared electrodes were calculated using Equations 2 and 3, respectively.
A plot of E°electrode vs. (t − 25) gives a straight line. The slope of this line was taken as the isothermal temperature coefficient of the prepared copper-sensing electrode. Trend of changes of PGE/PPy/SSA-Cu performance at various temperatures (20°C to 60°C) is depicted in Additional file 4: Figure S4. Results showed that the electrode exhibited good Nernstian behavior at temperature range between 20°C and 60°C. The amount of the isothermal temperature coefficient of the cell and the PGE/PPy/SSA-Cu electrode are obtained 1.26 and 1.27 (mV/°C), respectively. The small values of (dEo/dt)cell and (dEo/dt)electrode reveal the high thermal stability of the electrode within the investigated temperature range.
Effect of partially non-aqueous medium on the working of PGE/PPy/SSA-Cu electrode
The prepared electrode was investigated in partially non-aqueous media using methanol and ethanol mixture with water. Table 5 indicates that the slope remains unaltered with the addition of non-aqueous solvents. Hence, the new electrode can be successfully used in partially non-aqueous medium.
Some practical applications of the prepared copper-sensing electrode
The Cu2+ sensor was successfully used as an indicator electrode in the titration of 5.0 mL of a 1.0 × 10−2 M Cu (II) solution with 1.0 × 10−2 M of ethylenediaminetetraacetic acid (EDTA) (at pH = 5.0 acetate buffer) and vice versa. The resulting sigmoid titration curve is given in Figure 6. As it is seen, the amount of Cu (II) ions in solution can be determined with the fabricated electrode. Therefore, the PGE/PPy/SSA-Cu electrode can be used as indicator electrode in potentiometric titration of Cu (II) ions successfully.
The electrode was also successfully applied to the direct potentiometric measurement of copper (II) in a known copper solution prepared from electrical copper wire (purity >98%) using standard addition technique [28]. The experiments were run three times, and the results obtained are presented in Table 6. Statistical treatment of the data (t test) has shown that the potentiometric measurements of copper ion performed at several samples using the prepared copper-sensing electrode have high accuracy (texp < tcri).
Conclusions
The introduced indicator electrode based on polypyrrole conducting polymer can be easily prepared via simple electrochemical method (cyclic voltammetry). The copper sensor electrode fabricated in the current study showed response characteristics with Nernstian behavior over the concentration range 1.0 × 10−5 to 1.0 × 10−1 M of Cu (II) ion. The advantages such as fast response time (less than 20 s), low detection limit (5.42 μM), simplicity of preparation, low cost, high shelf lifetime (at least 60 days), high selectivity, sensitivity, accuracy, and precision as well as good dynamic linear range, high repeatability and reproducibility make this electrode potentially useful for measurement of free Cu (II) ions in real samples. The copper sensor electrode can also be used for potentiometric titration of copper ion with sharp and distinguished end point. The electrode was also used for determination of copper ion in real samples very successfully. Due to the high toxicity of copper ions to aquatic life, the finding in this study is very important from both analytical and environmental point of views.
Methods
Chemicals and apparatus
All chemicals used were of analytical grade (AR) and were prepared in distilled water. Pyrrole was obtained from Merck (Darmstadt, Germany, http://www.merckmillipore.com/chemicals) and was distilled before use. 5-sulfosalicylic acid and the nitrate or chloride salts of all cations were purchased from Merck with highest purity available and used without any further purification. Cu2+ solution (1.0 M) was prepared by dissolving AR grade CuSO4.5H2O in distilled water and was used as stock solution. Standard solutions for calibration graph were then prepared by the successive diluting of the accurately prepared stock solution, and potential readings started from the least concentrated standard. For the investigation of selectivity, interfering metal ions were prepared as 0.010 M from their pure related salts. Electrochemical synthesis of polymer was carried out with an electrochemical analyzer (SAMA 500, SAMA research center, Esfahan, Iran). A Metrohm pH meter (model 827) (Metrohm, Filderstadt, Germany, http://www.metrohmusa.com) with a combined double junction glass electrode, calibrated against two standard buffer solutions at pH 4.0 and 7.0, was used for pH measurement. The pH of the solutions was adjusted using 0.10 M HCl and NaOH solutions. All potentiometric measurements were carried out at 25°C with a cell of the following type: SCE | Cu2+ (aA) | PGE/PPy/SSA-Cu. EMF measurements were carried out using an accurate digital electronic voltmeter with high internal impedance to the nearest 0.10 mV. The readings were recorded when the potentials reached a stable and constant value (within 1 mV). The sensing material of the fabricated Cu (II) electrode obtained was characterized using FTIR, energy-dispersive X-ray spectroscopy, and SEM techniques. The surface morphology was examined using Philips XL30 scanning electron microscope (Amsterdam, The Netherlands, https://www.mems-exchange.org/equipment/E1175/ at an accelerating voltage of 20.0 kV with 10,000× magnification. The FTIR spectra of the electrode were recorded in Bruker, Madison, WI, USA (model Vertex 70) at room temperature in the region 4,000 to 500 cm−1.
Preparation of electrode and procedure
A three-electrode system was employed for electropolymerization of pyrrole in aqueous solution containing monomer. Electropolymerization was carried out by cyclic voltammetry method. A PGE as working electrode, a Pt wire as auxiliary electrode, and a double junction saturated calomel electrode were used as the reference electrodes. PGE (with 0.70 mm diameter) was cleaned with concentrated HNO3 (6.0 M) for removing any possible metal impurities. A PGE was also used for the electrodeposition of polypyrrole from a solution of pyrrole and KCl as electrolyte under the same conditions used for the PGE/PPy/SSA-Cu copper-sensing electrode termed as PGE/PPy/Cl. In order to have a good comparison, this electrode (PGE/PPy/Cl) was then used for surface morphological analysis and determination of copper ions. Since any potential stability, reversibility, and linear response for Cu2+ using the PGE/PPy/Cl electrode were not observed, all the potentiometric experiments were shown only for PGE/PPy/SSA-Cu electrode. Since salicylic acid (SA) is the metal-chelating ligand, for comparison and also further confirmation that 5-sulfosalicylic acid is indeed working as an ionophore, we also tested salicylic acid as an electrolyte for electrodeposition of polypyrrole termed as PGE/PPy/SA. The EMF (E) of the sensor electrode system dipped in the solution of Cu (II) ion was investigated given by the following equation (Equation 4):
where E (mV) is the total potential developed between the sensing and reference electrodes andk (mV) is a constant which is characteristic of the particular ISE/reference pair. It can be calculated from the linear plot of E vs. log C, and S is the electrode slope. ISEs are not actually specific but are more selective toward target or analyte ion. So, it is important to determine the selectivity of a fabricated sensor electrode toward target ion or the possible interferences from other ions. Different methods are available for determination of selectivity of an ISE. In the current study, selectivity coefficient () values for a range of common interfering ions were calculated by both the MPM and the SSM, which are recommended by IUPAC [32, 33]. According to the MPM, selectivity coefficient () is determined as follows:
where ΔA = a'A − aA, aA and a'A are the initial and final primary ion activities (or concentrations), respectively, and aB the interfering ion activity. In practice, a specified amount of primary ions (A) is added to a reference solution (primary ion with a fixed activity), and the membrane potential is measured. In a separate experiment, interfering ions (B) are successively added to an identical reference solution until the membrane potential matches the one obtained before with the primary ion. It should be noted that the values of aA and a'A for Cu2+ ion were taken as 1.0 × 10−4 and 1.0 × 10−3 M, respectively, at pH 5.0 and aB was determined experimentally. In SSM, the EMF of a cell is measured for each of two separate solutions, one containing the ion A of the activity aA and charge ZA (but not B), the other containing the ion B with charge ZB at the same activity aB = aA (but not A). If the measured values are EA and EB, respectively, then the value of may be calculated from Equation 6:
For the preparation of the calibration curve, standard solutions of Cu (II) ion were prepared with different concentrations (from 1.0 × 10−6 to 1.0 × 10−1 M), and the potentials were recorded after 20 s or less until the readings were stable. The maximum electrode potential fluctuation was less than 1.0 mV. The effect of temperature on the performance of the potentiometric electrodes was evaluated in a thermostat at different temperatures ranged from 20°C to 60°C.
Abbreviations
- CP:
-
conductive polymer
- EDS:
-
energy-dispersive X-ray spectroscopy
- ISE:
-
ion-selective electrode
- MPM:
-
matched potential method
- PGE:
-
pencil graphite electrode
- PGE/PPy/Cl:
-
polypyrrole conducting polymer doped with chloride ion
- PGE/PPy/SSA-Cu:
-
polypyrrole conducting polymer doped with 5-sulfosalicylic acid
- PPy:
-
polypyrrole
- SA:
-
sulfosalicylic acid
- SSA:
-
5-sulfosalicylic acid
- SSM:
-
separated solution method.
References
Greenwood NN, Earnshow A: Chemistry of the Elements. New York: Pergamon; 1984:628–630.
Mazloum Ardakania M, Mirhoseinib SH, Salavati-Niasari M: Copper selective electrode based on 1,8-Bis(2-hydroxynaphthaldiminato)3,6-dioxaoctane. Acta Chim. Slov 2006, 53: 197–203.
Ansari R, Fallah Delavar A, Aliakbar A, Mohammad-khah A: Solid-state Cu (II) ion-selective electrode based on polyanilin-conducting polymer film doped with copper carmosin dye complex. J. Solid State Electrochem 2012, 16: 869–875. 10.1007/s10008-011-1436-2
Jeong DC, Lee HK, Jeon S: Highly copper(II)-selective PVC membrane based on a Schiff base complex of N, N'-Bis-pyridin-2-ylmethylene-naphthalene-1,8-diamine as an ionophore. Bull. Korean Chem. Soc 2006,27(10):1593–1596.
Underwood EJ: Trace elements in human and animal nutrition. New York: Academic Press; 1962:197–204.
Kumar Mahajan R, Sood P: Novel copper (II)-selective electrode based on 2,2’:5’,2”-terthiophenein PVC matrix. Int. J. Electrochem. Sci 2007, 2: 832–847.
Neudachina LK, Oshintseva EV, Skorik YA, Vshivkovа AA: N-Aryl-3-Aminopropionic acids as selective reagents for the determination of copper in metallurgical products. J. Anal. Chem 2005,60(3):240–246. 10.1007/s10809-005-0078-x
Mashhadizadeh MH, Pesteh M, Talakesh M, Sheikhshoaie I, Ardakani MM, Karimi MA: Solid phase extraction of copper (II) by sorption on octadecyl silica membrane disk modified with a new Schiff base and determination with atomic absorption spectrometry. Spectrochim. Acta. B 2008, 63: 885–888. 10.1016/j.sab.2008.03.018
Campos RC, Santos HR, Grinberg P: Determination of Copper, Iron, Lead, Nickle in gasoline by electrothermal atomic absorption spectrometryusing three component solutions. Spectrochim. Acta. B 2002, 57: 15–28. 10.1016/S0584-8547(01)00362-7
Asan A, Andac M, Isildak I: Flow-injection spectrophotometric determination of copper(II) ion at low mu g L-1 levels using 4 benzylpiperidinedithiocarbamate. Anal. Sci 2001, 17: 1125–1127. 10.2116/analsci.17.1125
Mohadesi A, Salmanipour A, Mohammadi Z, Pourhatami A, Taher MA: Stripping voltammetric determination of copper (II) on an overoxidized polypyrrole functionalized with Nitroso-R. J. Braz. Chem. Soc 2008,19(5):956–962. 10.1590/S0103-50532008000500022
Ganjali MR, Ghafarloo A, Faridbod F, Norouzi P: Copper-selective PVC membrane sensor. Int. J. Electrochem. Sci 2012, 7: 3706–3716.
Stankovic D, Roglic G, Jovic M, Andjelkovic I, Mutic J, Manojlovic D: New potentiometric sensor for copper determination in waste waters based on a new type of modified GC electrode. Anal. Bioanal. Electrochem 2012,4(3):238–245.
Brin S, Buzuk M, Generali E, Brali M: Improving the response of copper (II) selective PVC membrane electrode by modification of N 2 S 2 donor ligand. Acta Chim. Slov 2010, 57: 318–324.
Essi M: Chalcogenide sensors for copper (II) detection in polluted waters. Chalcogenide Letters 2011,8(2):103–110.
Shokrollahi A, Abbaspour A, Ghaedi M, Haghighi AN, Kianfar AH, Ranjbar M: Construction of a new Cu2+ coated wire ion selective electrode based on 2-((2-(2-(2-(2 hydroxy-5-methoxybenzylidene amino) phenyl) disufanyl) phenylimino) methyl)-4 methoxy phenol Schiff base. Talanta 2011,84(1):34–41. 10.1016/j.talanta.2010.12.002
Maksymiuk K: Chemical reactivity of polypyrrole and its relevance to polypyrrole based electrochemical sensors. Electroanalysis 2006,18(16):1537–1551. 10.1002/elan.200603573
Mosayebzadeh Z, Ansari R, Mohammad-khah A, Arvand M: Electrochemical preparation of a copper ion selective electrode based on polypyrrole conducting polymer doped with Ponceau 4R Azo dye. Anal. Bioanal. Electrochem 2013,5(1):109–129.
Buzuk M, Brinic S, Generalic E, Bralic M: Copper (II) ion selective PVC membrane electrode based on S, S'-bis(2-aminophenyl)ethanebis(thioate). Croat Chem Acta 2009,82(4):801–806.
Jeong E, Lee HK, Ahmed MS, Seo H, Jeon S: Copper (II) selective PVC membrane electrodes based on Schiff base 1,2-Bis (E-2-hydroxy benzylidene amino) anthracene-9,10-dione complex as an Ionophore. Bull Korean Chem Soc 2010,31(2):401–405. 10.5012/bkcs.2010.31.02.401
Trojanowicz M: Application of conducting polymers in chemical analysis. Microchim Acta 2003, 143: 75–91. 10.1007/s00604-003-0066-5
Aminur Rahman MD, Kumar P, Park DS, Shim YB: Electrochemical sensors based on organic conjugated polymers. Sensors 2008, 8: 118–141. 10.3390/s8010118
Ansari R, Mosayebzadeh Z: Removal of anionic dye Eosin Y from aqueous solutions using conducting electroactive polymers. Iranian Polym J 2010,19(7):541–551.
Pankaj K, Kumar SH, Sukhjeet K: Conducting polymer based potentiometric sensors. Res. J. Chem. Environ 2012,16(3):125–133.
Gupta N, Sharma S, Ahmad Mir I, Kumar D: Advances in sensors based on conducting polymers. J. Sci. Ind. Res 2006, 65: 549–557.
Michalska A: All-solid-state ion selective and all-solid-state- reference electrodes. Electroanalysis 2012,24(6):1253–1265. 10.1002/elan.201200059
Migdalski J, Blaz T, Lewenstam A: Conducting polymer-based ion-selective electrodes. Anal. Chim. Acta 1996,322(3):141–149. 10.1016/0003-2670(95)00575-7
Ansari R, Fallah Delavar A, Mohammad-khah A: A solid state Cr(VI) ion-selective electrode based on polypyrrole. Microchimica Acta 2012,178(1–2):71–79.
Ansari R, Fallah Delavar A, Mohammad-khah A: Solid-State ion selective electrode based on polypyrrole conducting polymer nanofilm as a new potentiometric sensor For Zn2+ ion. J Solid State Electrochem 2012,16(10):3315–3322. 10.1007/s10008-012-1759-7
Charles V, Rajendra S: Composition and stability of 5-sulfosalicylate complexes of beryllium and copper. J. Am. Chem. Soc 1959,81(23):6159–6163. 10.1021/ja01532a014
Khalil S: Ion-selective electrode for the determination of trazodone in tablets. Analyst 1999, 124: 139–142. 10.1039/a808800d
Bakker E, Buhlmann P, Pretsch E: Carrier-based ion-selective electrodes and bulk optodes. 1. General characteristics. Chem. Rev 1997, 97: 3083–3132. 10.1021/cr940394a
Umezawa Y, Umezawa K, Sato H: Selectivity coefficients for ion-selective electrodes: Recommended methods for reporting K AB pot values. Pure Appl Chem 1995, 67: 507–518. 10.1351/pac199567030507
Acknowledgments
The authors appreciate the postgraduate students of the University of Guilan for the financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
RA participated in the idea and the design of the study and interpretation of the results. ZM carried out the experiments and wrote the manuscript. MA and AM conceived of the study and participated in its design and coordination. All authors read and approved the final manuscript.
Electronic supplementary material
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Ansari, R., Mosayebzadeh, Z., Arvand, M. et al. A potentiometric solid state copper electrode based on nanostructure polypyrrole conducting polymer film doped with 5-sulfosalicylic acid. J Nanostruct Chem 3, 33 (2013). https://doi.org/10.1186/2193-8865-3-33
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2193-8865-3-33