Abstract
The nanodiamonds (NDs) were produced on glass substrates by plasma-enhanced chemical vapor deposition (PECVD) method. Acetylene (C2H2) diluted in H2 were used as the reaction gasses as source of carbon and diluting gasses, respectively. The NDs have structures with high quality and density obtained by the selective Ni catalyst. The synthesized NDs have cubic structures with an average diameter of about 300 nm. This research focuses on the evolution of nickel catalyst under hydrogen/acetylene gasses at 320°C. The presence of the ND structure was confirmed by Raman spectroscopy, X-ray diffraction, and scanning electron microscopy analyses. It was found that the selective catalyst played an important role in producing ND structures by PECVD method on glass substrate in this research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
The revolutionary discovery of nanodiamonds (NDs) in 1980s led to intense research activities and efforts in the domain of carbon science and nanostructures [1, 2]. The fascinating properties of these unique materials have opened a great number of potential applications [3–5] of these nanostructures. Despite these stunning technical progresses, there is still much struggle in the development of a synthesis method suitable for experimental and commercial applications [6–8].
A leading candidate is the chemical vapor deposition (CVD) technique. CVD methods [9] are widely utilized techniques to fabricate nanostructures such as the ND structures in large quantities, and much progress has been made on the yield, the synthesis costs, or the purity of the products. CVD diamond growth includes steps of nucleation and formation of a continuous nanostructure, and surface morphology is closely related to these stages [6–8].
Nucleation and growth of NDs are induced by the decomposition of carbon-containing gasses (C2H2, CH4, etc.) over a Ni catalyst at experimental conditions. A key reaction step for ND growth seems to be the diffusion of carbon through the Ni catalyst, and their catalytic action depends on the type of precursors, the type of substrate, and the reaction gasses used.
In the synthesis of cubic NDs, each carbon atom is coordinated tetrahedrally by four others, and the resulting structure is a cubic structure. This nanostructure is a consequence of the sp3 hybridization of the bonding orbital. In fact, diamond is the hardest material known and is a wide-gap semiconductor [4, 9–13].
One of the major issues in the growth of high-quality diamond by CVD is the choice of a suitable substrate. Many substrates can be used for the growth of diamond, such as Si, Mo, W, Ti, and Nb. Si is the most commonly used substrate in many researches. For example, the Fujishima group utilized Si wafers as the substrate in their research, which is needed for the efficient growth of diamond [14]. Single-crystal growth of diamond is being carried out by a growing number of research groups [15], in which diamond was used as the substrate material, but in this research, the synthesis of diamond was carried out using glass-coated Ni nanoparticle catalyst as the desired substrate. Most recently, the Carnegie groups reported high-quality synthetic single-crystal diamond in desired research [16]. The single-crystal CVD diamonds have been used by Element Six and Sumitomo in electronics applications and by Apollo Diamond as gems [17–20]. High growth rate of diamond fabricated by low-pressure microwave plasma CVD is the most promising technology for fabricating low-cost and high-quality large diamond [21].
The feasibility of the plasma-enhanced chemical vapor deposition (PECVD) system to synthesize NDs using the Ni nanoparticle catalyst has been investigated with acetylene diluted in hydrogen. The NDs show cubic structures and result from the etching of carbon network on the selective catalyst and continuous precipitation on the glass substrate. In order to elucidate the nature of the Ni catalyst, the synthesis uses an atomic force microscopy (AFM) system, and the samples quenched after different pyrolysis times were investigated by X-ray diffraction (XRD), Raman spectroscopy, and scanning electron microscopy (SEM) which have been intensively employed for characterizing CVD diamond.
Results and discussion
Figure 1 shows the ND with body-centered cubic structure. The process of synthesis of NDs by PECVD method involves Ni catalyst deposition and growth of ND structures in specific conditions. Transition Ni nanoparticles are applied as catalyst for ND growth. Here, this metal was deposited on the glass substrate by a physical technique such as sputtering by PECVD method. Proper sputtering technique of the substrates was very important for the successful synthesis of NDs in our research. Ni with the capability of decomposing the hydrocarbon of C2H2 and ND formation is employed as the catalyst in the PECVD process. However, it has been found that only the C2H2 molecules are accounted for the decomposing ability of Ni, but not for ND formation. It was possible to render the substrate surfaces covered with grains of Ni catalyst of a certain size. The morphology of the Ni nanoparticle catalyst utilized in the synthesis of NDs is studied by the AFM technique.
Figure 2 shows a typical AFM image of the Ni nanoparticles deposited on the glass substrate. The deposited Ni catalyst is densely packed and of uniform diameter over the glass substrate. The Ni grain morphologies determine the quality of NDs grown on the substrate surface. Single-crystalline diamonds seem to be structured by the aggregation of nanoparticles.
The morphology of the structures was characterized by SEM technique. The SEM micrographs in Figure 3 show the cubic morphology of the single-crystal diamond, which grew on the deposited Ni nanoparticles on the glass substrate by PECVD method. In this figure, the SEM images show the ND structures synthesized with a high density and uniform diameter around 300 nm on the substrate surface. It is noticeable that Ni nanoparticles on the surface play an important role in the formation of nucleation sites; the density and distribution of Ni nanoparticles affect the synthesis and the type of NDs. In fact, Ni nanoparticles served as the well-defined nucleation sites to allow the growth of aligned NDs by PECVD system directly on the substrate surface, and the decomposition of the hydrocarbon of C2H2 and H2 gasses is more sufficient that it led to less deposition of amorphous carbon. The Ni nanoparticles can act typically as catalyst under specific conditions for ND nucleation resulting in growth. The diffraction of carbon through the Ni metal particle is indicated by an increase of the metal cell parameters identified.
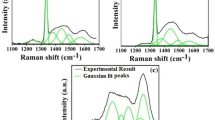
Raman spectroscopy is the most extensive and useful tool utilized to characterize CVD diamond to distinguish between different allotropes of carbon structures [22]. Figure 4 shows the Raman spectra of the ND structures grown using Ni catalyst on the glass substrate. In addition, a diamond peak around 1,332 cm-1 for the sp3 bonded carbon and a peak around 1,580 cm-1 for the sp2 bonded carbon in this structure were mainly observed. The spectra indicate the existence of good diamond phase with high quality.
XRD is a technique as powerful as Raman spectroscopy which provides a fingerprint of the presence of diamond phase. In addition to Raman spectroscopy, XRD can be utilized to confirm that diamond synthesis has been achieved by the Ni catalyst. Figure 5 demonstrates the single shape for cubic diamond. In many studies, it can be seen that the (111) plane is faster in a cubic structure for diamond, while the (100) plane is the fastest one in an octahedral structure of diamond [23]. The ND structures synthesized on glass substrate as-coated with Ni catalyst were determined by X-ray diffraction. Figure 5 illustrates the XRD spectra of the deposited ND structures produced at 320°C temperature and using the selective catalyst. The diffraction peaks show the presence of carbon material. From XRD spectra of the sample, one peak at around 43.70°C was ascribed to the (111) plane of the cubic diamond structures.
Conclusion
In summary, NDs were successfully synthesized with Ni catalyst by PECVD method on the glass substrate. In this research, the effect of the Ni catalyst and substrate on ND synthesis was investigated. It was found that the reaction of the catalyst has significant effect on the growth of NDs. Ni plays a crucial role in the PECVD synthesis of NDs, and therefore, improving the desired characteristics of catalyst will enhance the obtained ND quality as well as the process yield. The SEM images show that NDs can be grown using Ni as the selective catalyst with high density and surface morphology of the structures. The type of diamond synthesized in this research has cubic structures. From the Raman and XRD analyses of the NDs, it was proved that the Ni catalyst affected the quality and purity of the ND growth. The results were more promising than the experiments with Ni catalyst.
Methods
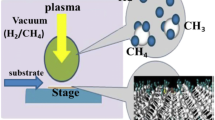
This process deals with many fundamental aspects of CVD, such as gas phase chemistry, complex heat and nucleation, surface chemistry, and diffusion as shown in Figure 6, that were utilized for all experiments in this field. Synthesis of NDs has been carried out by PECVD technique. Figure 7 describes a direct current (DC)-PECVD apparatus consisting of an electrode and a substrate with a heater. The electrode and substrate holder placed on the heater are made of Cu, while the substrate on the holder is made of glass. The substrate is set underneath the electrode. The Ni nanoparticle catalyst is deposited with the DC-PECVD system, and the sputtering of Ni nanoparticles is controlled by deposition time (120 min). The system is operated at a DC power of PDC = 600 W. The etching step utilized only acetylene gas, and at the growth step, the gas mixture of acetylene (C2H2) and hydrogen (H2) is filled during the discharge, where the flow of gas mixture ratio is expressed by PC2H2/PH2 = 30%. The glass substrate is heated up to a temperature T = 320°C, which is measured by a thermocouple directly connected to the copper substrate holder. After which, the gas mixture of C2H2 and H2 is fed and then the total pressure is kept at 15 Torr. The investigated desired optimum condition growth to control ND structures is shown in Table 1. Three most fundamental techniques, Raman spectroscopy, XRD, and SEM, are most intensively used to identify diamond structures. AFM is utilized for the morphological and structural investigation of NDs.
References
Gracio J, Fan QH, Madaleno JC: Diamond growth by chemical vapour deposition. J. Phys. 2010, 43: 374017.
Qi X, Qin C, Zhong W, Au C, Ye X, Du Y: Large-scale synthesis of carbon nanomaterials by catalytic chemical vapor deposition: a review of the effects of synthesis parameters and magnetic properties. Materials 2010, 3: 4142–4174. 10.3390/ma3084142
Luo SY, Kuo JK, Yeh B, Sung CY, Dai CW, Tsai TJ: The tribology of nanocrystalline diamond. Mater. Chem. Phys. 2001, 72: 133–135. 10.1016/S0254-0584(01)00422-9
Huang LY, Xu KW, Lu J, Guelorget B: Analysis of nano-scratch behavior of diamond-like carbon films. Surf. Coat. Technol. 2002, 154: 232–236. 10.1016/S0257-8972(02)00007-5
Ferreira NG, Silva LLG, Corat EJ: Electrochemical activity of boron-doped diamond electrodes grown on carbon fiber cloth. Diam. Relat. Mater. 2002, 11: 657–661. 10.1016/S0925-9635(02)00039-0
Chayahara A, Mokuno Y, Tsubouchi N, Yamada H: Development of single-crystalline diamond wafers. Synthesiology 2011,3(4):259–267.
Hemley RJ, Chen Y-C, Yan C-S: Growing diamond crystals by chemical vapor deposition. Elements 2005, 1: 105–108. 10.2113/gselements.1.2.105
Zhao N, Cui Q, He C, Shi C, Li J, Li H, Du X: Synthesis of carbon nanostructures with different morphologies by CVD of methane. Mater. Sci. Eng. A. 2007, 460–461: 255–260.
Hoffman A: Mechanism and properties of nanodiamond films deposited by the DC-GD-CVD process. J. Springer 2005, 192: 125–142.
Chow L, Zhou D, Hussain A, Kleckley S, Zollinger S, Wang H: Chemical vapor deposition of novel carbon materials. J Thin Solid Films 2000, 368: 193–197. 10.1016/S0040-6090(00)00763-X
Tinchev SY, Dyulgerska YP, Nikolova P, Grambole D, Kreissig U, Babeva TZ: Optical properties of PECVD deposited DLC films prepared with air addition. J. Optoelectronics Adv. Mater. 2006,8(1):308–311.
Cheung CL, Kurtz A, Park H, Leiber CM: Diameter-controlled synthesis of carbon nanotubes. J. Phys. Chem. B 2002, 106: 2429–2433. 10.1021/jp0142278
Schirru F, Kisielewicz K, Nowak T, Marcczewska B: Single crystal diamond detector for radiotherapy. J. Phys. D: Appl. Phys. 2010, 43: 265101. 10.1088/0022-3727/43/26/265101
Fazaneh F, Faal Hamedani N: Preparation of carbon nanotubes by CVD process over nanoparticles of Ni-Ce-Zr mixed oxides. J. Sci. Islam. Repub. Iran 2008,19(2):119–123.
Biener J, Ho DD, Wild C, Woerner E, Biener MM, El-dasher BS, Hicks DG, Eggert JH, Celliers PM, Collins GW, Teslich NE Jr, Kozioziemski BJ, Haan SW, Hamza AV: Diamond spheres for inertial confinement fusion. Nucl. Fusion 2009, 49: 112001. 10.1088/0029-5515/49/11/112001
Martineau PM, Lawson SC, Taylor AJ, Quinn SJ, Evans DJF, Crowder MJ: Identification of synthetic diamond grown using chemical vapor deposition (CVD). Gems and Gemology 2004,40(1):2–25. 10.5741/GEMS.40.1.2
Yan CS, Mao HK, Li W, Qian J, Zhao Y, Hemley RJ: Ultrahard diamond single crystals from chemical vapor deposition. Physica Status Solidi (A) 2004,201(4):25–27. 10.1002/pssa.200409033
Wang W, Moses T, Linares RC, Shigley JE, Hall M, Bulter JE: Gem-quality synthetic diamonds grown by a chemical vapor deposition (CVD) method. Gems and Gemology 2003,39(4):268–283. 10.5741/GEMS.39.4.268
Okushi H: High quality homoepitaxial CVD diamond for electronic devices. Diamond Relat. Mater. 2001,10(3–7):281–288.
Isberg J, Hammersberg J, Johansson E, Wikstrom T, Twitchen DJ, Whitehead AJ, Scarsbrook GA: High carrier mobility in single-crystal plasma-deposited diamond. Science 2002,297(5587):1670–1672. 10.1126/science.1074374
Charles SJ, Butler JE, Feygelson BN, Newton MF, Carroll DL, Steeds JW, Darwish H, Mao HK, Yan CS, Hemley RJ: Characterization of nitrogen doped chemical vapor deposited single crystal diamond before and after high pressure, high temperature annealing. Physica Status Solidi (A) 2004,201(11):2473–2485. 10.1002/pssa.200405175
Yan C, Yogesh K, Vohrat H, Russell J: Very high growth rate chemical vapor deposition of single-crystal diamond. Appl. Phys. Sci. 2002,99(20):12523–12525.
Aislinn H, Sirk C, Donald R: Electrochemical synthesis of diamond like carbon films. J. Electrochem. Soc. 2008, 155: 45–55. 10.1149/1.2814144
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
KZ and MAM both contributed to this research work. Both authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Zare, K., Malkeshi, M.A. Effect of catalyst on growth of diamond by plasma-enhanced chemical vapor deposition. J Nanostruct Chem 3, 27 (2013). https://doi.org/10.1186/2193-8865-3-27
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2193-8865-3-27