Abstract
Sulfonic acid functionalized ordered nanoporous sodium montmorillonite was easily prepared by the reaction of sodium montmorillonite with chlorosulfonic acid. The new catalyst demonstrated efficient and chemoselective methoxymethylation reaction of alcohols with formaldehyde dimethyl acetal in chloroform under reflux conditions. This reaction affords corresponding ethers in good to excellent yields. The present method offers several advantages such as short reaction times, high yields, simple procedure, mild conditions, heterogeneous nature, and reusability of the catalyst.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Selective protection and deprotection of hydroxyl groups have occupied a unique position in organic synthesis because of the fundamental importance of hydroxyl groups in multistep synthesis of complex natural products [1]. Between several methods available for the protection of the alcoholic hydroxyl groups, methoxymethylation has attracted the attention of many organic chemists. This considerable attention can be attributed to the stability of the produced methoxymethyl (MOM) ethers against reagents such as strong bases, butyl lithium, lithium aluminum hydride, and Grignard reagents as well as easy removal of the MOM moiety by acid treatment. Generally, the formation of MOM ethers is carried out by the alkylation of alcohols with excess amounts of chloromethyl methyl ether (CME) in alkaline solution [2]. However, the potent carcinogenic property of CME limits the use of this method, and MOM ethers are prepared by the reaction of alcohols or phenols with formaldehyde dimethyl acetal (FDMA), as a cheap and commercially available compound. Even though the handling of this reagent does not need special precautions and workup of the reaction mixture is not time-consuming, the low methoxymethylating power of FDMA is the main drawback for its application. To overcome this limitation, a number of catalysts such as p-toluenesulfonic acid [3], Nafion-H [4], TMSI [5], Envirocat [6], expansive graphite [2], sulfated metal oxides [7], silica sulfuric acid [8], Sc(OTf)3[9], Bi(OTf)3[10], Al(HSO4)3[11], MoO2(acac)2[12], H3PMo12O40[13], anhydrous FeCl3 dispersed on 3A molecular sieve [14], TiO2/SO42−[15], H3PW12O40[16, 17], melamine trisulfonic acid [18], benzyltriphenylphosphonium tribromide [19], and high-valent [SnIV (Br8TPP)(OTf)2)] [20] have been reported. Although these methods are improvements, most of them suffer from disadvantages such as harsh reaction conditions, long reaction times, poor yields, poor selectivity, use of toxic or expensive reagents, and use of large amounts of FDMA. Thus, the searches for new reagents and methods that utilize eco-friendly protocols are still in demand.
In recent years, clays as nanostructured materials have been widely used in organic transformations as solid acid catalysts [21–23]. The main reasons for use of clays are accessibility, easy modification, cheapness, non-corrosiveness, and recyclability. Montmorillonite (MMT) is one of the most widely used clays. Montmorillonite minerals have very small micron-sized particles, and they are extremely fine-grained and thin-layered [24]. Layers of MMT have a thickness of about 1 nm and a length of 100 nm or a little more. Broken bonds on the edge of MMT layers are common phenomena for layered silicates and lead to the free formation of hydroxyl groups [25, 26], which can be utilized for chemical modification. The first attempt may be traced back in 1941 when Berger found that hydroxyl groups of montmorillonite could be methylated with diazomethane [27]. Many scientists then used this property for different modifications on the surface of montmorillonite [28, 29].
Results and discussion
Recently, preparation of new catalysts for organic reactions, by modification of hydroxyl groups of various hetero- and homogenous compounds by sulfonic moiety, became an important part of our research program [30–33]. In continuation of these studies, we have found that chlorosulfonic acid can be used for the modification of Na+-MMT until -OH groups on the surface of this clay be converted to -SO3H (Scheme 1). The reaction is easy and clean and needs no special precautions because HCl gas evolved from the reaction vessel immediately.
On the basis of the structure of sulfonic acid-functionalized ordered nanoporous Na+-MMT (SANM), we anticipated that this reagent would act as an efficient catalyst in the reactions that need the use of acidic reagents to speed up. In our first communication, we used this reagent as an efficient catalyst for N tert-butoxycarbonylation of amines with di-tert-butyl dicarbonate [34], our procedure provided better activity with high yields compared to the heterogeneous ones in the N tert-butoxycarbonylation of amines. Next, we have shown the catalytic application of our synthesized catalyst in the trimethylsilylation of alcohols and phenols with high chemoselectivity and yields [35]. Herein, we are reporting the promoting effect of SANM in the conversion of alcohols to their corresponding MOM ethers. All reactions were performed in CHCl3 at reflux temperature under completely heterogeneous reaction conditions in good to high yields (Scheme 2, Table 1). Phenols remain intact under the same reaction conditions (entry 18 of Table 1). Therefore, the method can be useful for the chemoselective methoxymethylation of alcohols in the presence of phenols and primary alcohols in the presence of tertiary alcohols (entry 19 of Table 1).
Investigation in the reusability of the catalyst showed that SANM is reusable four times (entries 1 and 2 of Table 1).
The possible mechanism for the methoxymethylation of various alcohols in the presence of SANM as a promoter is shown in Scheme 3. On the basis of this mechanism, SANM catalyzes the reaction by electrophilic activation of FDMA and by making the central carbon of FDMA susceptible to nucleophilic attack by the alcohol. Successive elimination of MeOH results in the formation of MOM-ether derivatives and regenerates SANM in the reaction mixture [7].
To illustrate the efficiency of the present method, Table 2 compares our results with some of those reported in the literature [8–12].
Conclusions
In conclusion, we have developed a simple and efficient protocol for the methoxymethylation of various alcohols using SANM as a novel heterogeneous catalyst. Good yields of the products, short reaction times, heterogeneous nature of reaction conditions, use of relatively small amounts of FDMA, ease of preparation, stability of the reagent, recyclability, and easy workup procedure are important features of the reported method. We are exploring further applications of SANM for the other types of functional group transformations in our laboratory.
Methods
General
Chemicals were purchased from Southern Clay Products (Gonzales, TX, USA), Fluka (Buchs, Switzerland), Merck (Darmstadt, Germany), and Sigma-Aldrich (St. Louis, MO, USA) chemical companies. All of the products are known compounds and were characterized by spectral analyses, comparisons with authentic samples (IR and NMR), and regeneration of the corresponding alcohols. All yields refer to the isolated products. The purity determination of the substrate and reaction monitoring was accompanied by gas chromatography or thin-layer chromatography (TLC) on silica-gel polygram SILG-UV 254 plates.
Catalyst preparation
A 500-mL suction flask charged with 2.5 g Na+-montmorillonite (Southern Clay Products) and 10 mL CHCl3 was equipped with a constant pressure dropping funnel containing chlorosulfonic acid (0.50 g, 9 mmol) and a gas inlet tube for conducting HCl gas into water as adsorbing solution. Chlorosulfonic acid was added dropwise over a period of 30 min while the reaction mixture was stirred slowly in an ice bath (0°C). After addition was completed, the mixture was stirred for additional 30 min to remove all HCl. The mixture was then filtered, and the solid residue was washed with methanol (20 mL) and dried at room temperature to obtain SANM as white powder (2.58 g) [34, 35].
Catalyst characterization
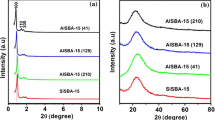
The synthesized catalyst was characterized by Fourier transform infrared spectroscopy, transmission electron microscopy, nitrogen sorption technique, X-ray diffraction, thermogravimetric analysis, and elemental analysis [34, 35].
General procedure
A mixture of the substrate (1 mmol), FDMA (6 mmol, 0.456 g), and SANM (3 mg) in CHCl3 (3 mL) was stirred at reflux temperature. The progress of the reaction was monitored by TLC. After completion of the reaction, the solvent was evaporated, and Et2O (5 mL) was added. The mixture was filtered, and the solid residue was washed with Et2O (5 mL). The filtrate was washed with a saturated solution of NaHCO3 and H2O and dried over MgSO4. Evaporation of the solvent afforded the requested MOM ether in high purity.
References
Schelhaas M, Waldmann H: Protecting group strategies in organic synthesis. Angew Chem Int Ed Engl 1996, 35: 2056–2083. 10.1002/anie.199620561
Jin TS, Li TS, Gao YT: A facile preparation of methoxymethyl ethers of primary and secondary alcohols with dimethoxymethane catalyzed by expansive graphite. Synth Commun 1998, 28: 837–841. 10.1080/00032719808006481
Yardley JP, Fletcher H: Introduction of the methoxymethyl ether protecting group. Synthesis 1976, 1976: 244–245. 10.1055/s-1976-24000
Olah GA, Husain A, Gupta BGB, Narang SC: Catalysis by solid superacids 1; 15. Facile preparation of methoxymethyl ethers. Synthesis 1981, (6):471–472.
Olah GA, Husain A, Narang SC: Iodotrimethylsilane-catalyzed preparation of methoxymethyl ethers of primary and secondary alcohols with dimethoxymethane. Synthesis 1983,1983(11):896–897. 10.1055/s-1983-30554
Bandgar BP, Hajare CT, Wadgaonkar PP: Envirocat EPZG catalyzed methoxymethylation of alcohols. J Chem Res 1996, 90–91.
Lin CH, Wan MY, Huang YM: Methoxymethylation of alcohols catalyzed by sulfated metal oxides. Catal Let. 2003, 87: 253–256. 10.1023/A:1023472029190
Niknam K, Zolfigol MA, Khorramabadi-Zad A, Zare R, Shayegh M: Silica sulfuric acid as an efficient and recyclable catalyst for the methoxymethylation of alcohols under solvent-free conditions. Catal Commun 2006, 7: 494–498. 10.1016/j.catcom.2006.01.004
Karimi B, Ma’mani L: Scandium trifluoromethanesulfonate as a recyclable catalyst for efficient methoxymethylation of alcohols. Tetrahedron Lett 2003, 44: 6051–6053. 10.1016/S0040-4039(03)01481-3
Sreedhar B, Swapna V, Sridhar C: Bismuth triflate and microencapsulated bismuth triflate: Efficient catalysts for methoxymethylation of alcohols and carboxylic acids. Catal Commun 2005, 6: 293–296. 10.1016/j.catcom.2005.02.003
Niknam K, Zolfigol MA, Shayegh M, Zare R: Metal hydrogen sulfates catalyzed methoxymethylation of alcohols under solvent-free conditions. J Chin Chem Soc 2007, 54: 1067–1073.
Kantam ML, Santhi PL: Molybdenum catalyzed methoxymethylation of alcohols. Synlett 1993, 429–430.
Zolfigol MA, Shiri M: Molybdatophosphoric acid as a catalyst for the methoxymethylation of alcohols under solvent-free conditions. Mendeleev Commun 2005, 15: 165–166. 10.1070/MC2005v015n04ABEH002064
Patney HK: Anhydrous iron(III) chloride dispersed on 3A molecular sieves, a mild and efficient catalyst for methoxymethylation of alcohols. Synlett 1992, 567–568.
Jin TS, Guo JJ, Yin YH, Zhang SL, Li TS: TiO2/SO42−, an efficient catalyst for the methoxymethylation of alcohols. J Chem Res 2002, 188–189.
Mohammadpoor-Baltork I, Moghadam M, Tangestaninejad S, Mirkhani V, Mirjafari A: H3PW12O40 - a selective, environmentally benign, and reusable catalyst for the preparation of methoxymethyl and ethoxymethyl ethers and their deprotections under mild conditions. Can J Chem 2008, 86: 831–840. 10.1139/v08-068
Mohammadpoor-Baltork I, Moghadam M, Tangestaninejad S, Mirkhani V, Mirjafari A: 12-Tungstophosphoric acid supported on inorganic oxides as heterogeneous and reusable catalysts for the selective preparation of alkoxymethyl ethers and their deprotections under different reaction conditions. Polyhedron 2008, 27: 2612–2624. 10.1016/j.poly.2008.05.002
Shirini F, Zolfigol MA, Albadi J: Melamine trisulfonic acid (MTSA): a new efficient catalyst for the chemoselective methoxymethylation of alcohols. Synth Commun 2010, 40: 910–914. 10.1080/00397910903026707
Shirini F, Imanzadeh GH, Mousazadeh SAR, Mohammadpoor-Baltork I, Abedini M: A mild and efficient method for the methoxymethylation and acetylation of alcohols promoted by benzyltriphenylphosphonium tribromide. Chin Chem Lett 2010, 21: 1187–1190. 10.1016/j.cclet.2010.04.031
Moghadam M, Tangestaninejad S, Mirkhani V, Mohammadpoor-Baltork I, Khajehzadeh M, Kosari F, Araghi M: High-valent [SnIV(Br8TPP)(OTf)2] as a highly efficient and reusable catalyst for selective methoxymethylation of alcohols and phenols: the effect of substituted bromines on the catalytic activity. Polyhedron 2010, 29: 238–243. 10.1016/j.poly.2009.07.041
Bergaya F, Lagaly G: Surface modification of clay minerals. Appl Clay Sci 2001, 19: 1–3. 10.1016/S0169-1317(01)00063-1
Vaccari A: Preparation and catalytic properties of cationic and anionic clays. Catal Today 1998, 41: 53–71. 10.1016/S0920-5861(98)00038-8
Laszlo P: Chemical reactions on clays. Science 1987, 235: 1473–1477. 10.1126/science.235.4795.1473
Giannelis EP, Krishnamoorti R, Manias E: Polymer-silicate nanocomposites: model systems for confined polymers and polymer brushes. Adv Polym Sci 1999, 138: 107–147. 10.1007/3-540-69711-X_3
Shi H, Lan T, Pinnavaia TJ: Interfacial effects on the reinforcement properties of polymer-organoclay nanocomposites. Chem Mater 1996, 8: 1584–1587. 10.1021/cm960227m
Yariv S, Cross H: Organo-clay complexes and interactions. 1st edition. CRC, New York; 2002.
Berger G: The structure of montmorillonite. Preliminary communication on the ability of clays and clay minerals to be methylated. Chem Weekblad 1941, 38: 42–43.
Deuel H, Huber G, Iberg R: Organische derivate von tonmineralien. Helv Chim Acta 1950, 33: 1229–1232. 10.1002/hlca.19500330514
Gieseking JE: The clay minerals in soils. Adv Agrononmy 1949, 1: 159–204.
Shirini F, Zolfigol MA, Mohammadi K: Silica sulfuric acid as a mild and efficient reagent for the acetylation of alcohols in solution and under solvent free conditions. Bull Korean Chem Soc 2004, 25: 325–327.
Shirini F, Zolfigol MA, Mohammadi K: Silica sulfuric acid as an efficient reagent for the synthesis of symmetrical ethers under mild and heterogeneous conditions. Phosphorous Sulfur Silicon 2003, 178: 2357–2361. 10.1080/714040949
Shirini F, Sadeghzadeh P, Abedini M: Silica sulfuric acid: a versatile reagent for oxathioacetalyzation of carbonyl compounds and deprotection of 1,3-oxathiolanes. Chin Chem Lett 2009, 20: 1457–1460. 10.1016/j.cclet.2009.07.006
Salehi P, Zolfigol MA, Shirini F, Baghbanzadeh M: Silica sulfuric acid and silica chloride as efficient reagent for organic reactions. Curr Org Chem 2006, 10: 2171–2189. 10.2174/138527206778742650
Shirini F, Mamaghani M, Atghia SV: Sulfonic acid-functionalized ordered nanoporous Na+-montmorillonite (SANM): A novel, efficient and recyclable catalyst for the chemoselective N-Boc protection of amines in solventless media. Catal Commun 2011, 12: 1088–1094. 10.1016/j.catcom.2011.03.030
Shirini F, Mamaghani M, Atghia SV: A mild and efficient method for the chemoselective trimethylsilylation of alcohols and phenols and deprotection of silyl ethers using sulfonic acid-functionalized ordered nanoporous Na+-montmorillonite. Appl Clay Sci 2012, 58: 67–72.
Acknowledgments
We are thankful to the University of Guilan Research Council for the partial support of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Shirini, F., Mamaghani, M. & Atghia, S.V. Sulfonic acid functionalized ordered nanoporous Na+ montmorillonite as an efficient and recyclable catalyst for the chemoselective methoxymethylation of alcohols. J Nanostruct Chem 3, 2 (2012). https://doi.org/10.1186/2193-8865-3-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2193-8865-3-2