Abstract
Background
Herpes simplex virus type-1 (HSV-1) is the primary cause of facial lesions (mouth, lips, and eyes) in humans. The widespread use of acyclovir and nucleoside analogues has led to emergence of HSV strains that are resistant to these drugs. Recently, non-nucleoside anti-HSV compounds have received considerable attention. 1,6-Naphthyridines are a class of heterocyclic compounds that exhibit a broad spectrum of biological activities such as inhibitor of HIV-1 integrase, HCMV, FGF receptor-1 tyrosine kinase, and the enzyme acetylcholinesterase. We previously reported the synthesis, SAR studies, and evaluation anti-HSV-1 activity of 3H-benzo[b]pyrazolo[3,4-h]-1,6-naphthyridines. In the course of our search for new 1,6-naphthyridines derivatives with potential activity against HSV-1, we have synthesized and evaluated new 3H-benzo[b]pyrazolo[3,4-h]-1,6-naphthyridines (1a-k) and 3H-pyrido[2,3-b]pyrazolo[3,4-h]-1,6-naphthyridines (2a-c).
Results
A known synthetic approach was used for preparing new 3H-benzo[b]pyrazolo[3,4-h]-1,6-naphthyridines (1a-k) and 3H-pyrido[2,3-b]pyrazolo[3,4-h]-1,6-naphthyridines (2a-c), starting from ethyl 4-chloro-1-phenyl-1H-pyrazolo[3,4-b]pyridine-5-carboxylate (7). All compounds were identified by FTIR, 1H NMR, and mass spectrometry. The antiviral effect on HSV-1 virus replication was determined.
Conclusions
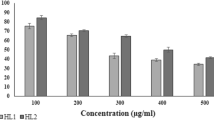
The compounds 1d, 1f, 1g, and 1h exhibited the highest anti-HSV-1 activity. In general, 3H-benzo[b]pyrazolo[3,4-h]-1,6-naphthyridines were more effective inhibitors than their corresponding 3H-pyrido[2,3-b]pyrazolo[3,4-h]-1,6-naphthyridines. The compound 1h reduced the virus yield in 91% at 50 μM and exhibited a low cytotoxicity (CC50 600 μM).

Similar content being viewed by others
Background
Herpes simplex virus type-1 (HSV-1) is a large enveloped virus containing double-stranded DNA genomes of approximately 152 kb in size. HSV-1 is the primary cause of facial lesions (mouth, lips, and eyes) in humans [1, 2]. Most of clinical anti-herpes virus compounds are nucleoside analogues, such as acyclovir (ACV), which is the most common drug used on treatment of HSV infections [3–5]. However, the widespread use of these compounds has been associated with the emergence of drug-resistant HSV strains [5]. The discovery of new non-nucleoside anti-HSV-1 agents with different mechanisms of action could offer an additional strategy against drug resistance of viruses. Several examples of non-nucleoside inhibitors have been proposed as candidate drugs for the treatment of herpes [3, 6–11].
1,6-Naphthyridines are a class of heterocyclic compounds that exhibit a broad spectrum of biological activities such as inhibitor of HIV-1 integrase [12–15], HCMV [16, 17], FGF receptor-1 tyrosine kinase [18], and the enzyme acetylcholinesterase [19]. Many routes for the syntheses of 1,6-naphthyridines derivatives have previously been reported [20–24].
Recently, our research group reported the synthesis, SAR studies, and evaluation anti-HSV-1 activity of 3H-benzo[b]pyrazolo[3,4-h]-1,6-naphthyridines derivatives I (Figure 1) [25]. In the course of our search for new 1,6-naphthyridines derivatives with potential activity against HSV-1, we have synthesized and evaluated new 3H-benzo[b]pyrazolo[3,4-h]-1,6-naphthyridines (1a-k) and 3H-pyrido[2,3-b]pyrazolo[3,4-h]-1,6-naphthyridines (2a-c) (Scheme 1).
Results and discussion
Chemistry
A known synthetic approach was used for preparing the 3H-benzo[b]pyrazolo[3,4-h]-1,6-naphthyridines (1a-k) and 3H-pyrido[2,3-b]pyrazolo[3,4-h]-1,6-naphthyridines (2a-c), starting from ethyl 4-chloro-1-phenyl-1H-pyrazolo[3,4-b]pyridine-5-carboxylate (7) (Scheme 1) [26–28]. In the first step, ethyl α-carboethoxy-β-(5-pyrazolylammonium)acrylate (8) was prepared by the condensation between 5-amino-1-phenyl-1H-pyrazole (9) and diethyl ethoxymethylenemalonate, in ethanol. The cyclization of the acrylate 8 was carried out by refluxing in phosphorus oxychloride to afford 4-chloro1-phenyl-1H-pyrazolo[3,4-b]pyridine-5-carboxylate (7) in 75% yield [26–28]. Nucleophilic displacement of the chlorine atom in compound 7 by aromatic amines gave ethyl 4-(arylamino)-1-phenyl-1H-pyrazolo[3,4-b]pyridine-5-carboxylates (5a-k) in yields 52-82% [26, 29]. Similarly, aminopicolines were used to obtain ethyl 4-[(methylpyridin-2-yl)amino]-1-phenyl-1H-pyrazolo[3,4-b]pyridine-5-carboxylates (6a-c) in yields 50-60%. These were achieved by heating at 140°C without solvents for 2-4 h an equimolar mixture of the appropriate aniline or aminopicoline and the compound 7. However, better results were obtained when these reactions were carried out in solvents such as DMF [25]. Subsequent hydrolysis of the esters 5a-k and 6a-c afforded the corresponding 4-(arylamino)-1-phenyl-1H-pyrazolo[3,4-b]pyridine-5-carboxylic acids (3a-k) and 4-[(methylpyridin-2-yl)amino]-1-phenyl-1H-pyrazolo[3,4-b]pyridine-4-carboxylic acids (4a-c), in high yields, 86-93 and 80-93%, respectively [28]. For producing 3H-benzo[b]pyrazolo[3,4-h]-1,6-naphthyridines (1a-k) and 3H-pyrido[2,3-b]pyrazolo[3,4-h]-1,6-naphthyridines (2a-c), the respective carboxylic acids 3a-k and 4a-c were cyclized with phosphorus oxychloride at 110°C over a period of 3 h [25, 30]. The tetracyclic compounds 1a-k and 2a-c were isolated in 60-70% yield.
Biological evaluation
The targets 3H-benzo[b]pyrazolo[3,4-h]-1,6-naphthyridines (1a-k) and 3H-pyrido[2,3-b]pyrazolo[3,4-h]-1,6-naphthyridines (2a-c) were evaluated for inhibition of HSV-1 replication in infected Vero cells. Results are shown in Table 1. Compounds 1d, 1f, 1g, and 1h exhibited the highest anti-HSV-1 activity. Compound 1h reduced the virus yield in 91% at 50 μM. In general, 3H-benzo[b]pyrazolo[3,4-h]-1,6-naphthyridines (1a-k) were more effective inhibitors than their corresponding 3H-pyrido[2,3-b]pyrazolo[3,4-h]-1,6-naphthyridines (2a-c).
Compounds with nearly the same antiviral effects were evaluated for cytotoxicity in Vero cells. EC50 and the selectivity index (SI) were determined in parallel. Several of the new compounds prevented the cytopathic effect of HSV-1 in Vero cells, at micromolar concentrations, and were minimally toxic to Vero cells resulting in a good SI. The MTT assay indicated that compound 1h exhibited a low cytotoxicity (CC50 600 μM). Trypan blue and MTT showed similar results (data not shown). ACV results have been included for comparison purposes (Table 2).
Conclusions
In summary, a new series of 3H-benzo[b]pyrazolo[3,4-h]-1,6-naphthyridines (1a-k) and 3H-pyrido[2,3-b]pyrazolo[3,4-h]-1,6-naphthyridine (2a-c) were synthesized and some of them were potent anti-HSV-1 agents. The compounds 1d, 1f, 1g, and 1h exhibited the highest anti-HSV-1 activity, being the 3H-benzo[b]pyrazolo[3,4-h]-1,6-naphthyridine derivatives, in general, more effective inhibitors than their corresponding 3H-pyrido[2,3-b]pyrazolo[3,4-h]-1,6-naphthyridines. The compound 1h reduced the virus yield in 91% at 50 μM and exhibited a low cytotoxicity (CC50 600 μM). The mechanism of antiviral activity of these compounds is under investigation.
Experimental
Melting points were determined on a Fisatom 430D and are uncorrected. 1H NMR spectra were recorded on a Varian Unity Plus spectrometer for 300 MHz, with tetramethylsilane as the internal standard. Chemical shifts (δ) are reported in parts per million (ppm) and the coupling constants (J) in Hertz (Hz). Fourier transform infrared absorption spectra were recorded in a Perkin-Elmer Spectrum One FTIR spectrophotometer. The solid samples were measured using potassium bromide (KBr) pellets. Thin-layer chromatography was performed on Uniplates (silica gel). All chemicals were reagent grade. High-resolution mass spectral analysis was recorded using a Finingan MAT 711A.
General procedures for the synthesis of 3H-benzo[b]pyrazolo[3,4-h]-1,6-naphthyridine derivatives (1a-k), and 3H-pyrido[2,3-b]pyrazolo[3,4-h]-1,6-naphthyridine derivatives (2a-c)
The key intermediate ethyl 4-chloro-1-phenyl-1H-pyrazolo[3,4-b]pyridine-5-carboxylate (7) was prepared according to literature [26–28]. An equimolar mixture of 7 (4 mmol) and anilines or aminopicolines in 10 mL DMF was heated under reflux for 2-4 h. The reaction mixture, after cooling, was poured into 50 mL of ice-water. The precipitated was filtered, dried, and recrystallized from a mixture of ethanol and water. The compounds obtained 5a-k and 6a-c were reacted with 10 mL NaOH (20%) and 10 mL of ethanol under reflux for 1-3 h. On cooling to room temperature, the mixture was acidified with diluted hydrochloric acid (1:3), and the precipitate was filtered and recrystallized from DMF and water. A mixture of the acids 3a-k and 4a-c (1 mmol), and phosphorus oxychloride (5 mL) was heated under reflux for 3 h. The reaction mixture was inverted over crushed ice. In some cases the excess of phosphorus oxychloride was removed under reduced pressure before inverting over crushed ice and neutralized. The new compounds 1a-k and 2a-c were isolated in yields 60-70%. The resulting precipitate was collected and purified by flash column chromatography (FC, silica gel). The structures of the compounds were elucidated by FTIR, 1H NMR, and mass spectrometry.
(1a) 6-chloro-3-phenyl-3H-benzo[b]pyrazolo[3,4-h]-1,6-naphthyridine
Yield 70%; mp 259-260°C; IR (KBr, cm-1) νmax C-H 3084, C = C 1596, C = N 1502; 1H NMR (DMSO-d6, 300 MHz) δ 8.92 (1H, s, H-1), 8.40 (1H, d, J = 8.1 Hz, H-10), 7.95 (1H, dd; J = 8.1 Hz, H-9), 7.70 (1H, dd, J = 8.1 Hz, H-8), 7.81 (1H, d, J = 8.1 Hz, H-7), 9.40 (1H, s, H-5), 8.33 (2H, d, J = 7.5 Hz, H-2', H-6'), 7.50 (2H, t, J = 7.5 Hz, H-3', H-5'), 7.52 (1H, t, J = 7.5 Hz, H-4'); EI (70eV) m/z (%): M+ 330.00761 (100).
(1b) 6-chloro-3-phenyl-9-methoxy-3H- benzo[b]pyrazolo[3,4-h]-1,6-naphthyridine
Yield 68%; mp > 300°C; IR (KBr, cm-1) νmax C-H 3083, C = C 1595, C = N 1504; 1H NMR (DMSO-d6, 300 MHz) δ 8.99 (1H, s, H-1), 7.26 (1H, s, H-10), 7.78-7.56 (5H, m, H-3', H-4', H-5', H7, H-8), 9.36 (1H, s, H-5), 8.31 (2H, d, J = 8.0 Hz, H-2', H-6'), 4.07 (3H, s, Ar-OCH3); EI (70 eV) m/z (%): M+. 360.07037 (100).
(1c) 6-chloro-3-phenyl-9-methyl-3H-benzo[b]pyrazolo[3,4-h]-1,6-naphthyridine
Yield 65%; mp > 300°C; IR (KBr, cm-1) νmax C-H 3083, C = C 1595, C = N 1503; 1H NMR (DMSO-d6, 300 MHz) δ 8.99 (1H, s, H-1), 7.79-7.57 (6H, m, H-3', H-4', H-5', H7, H-8, H-10), 9.42 (1H, s, H-5), 8.40 (2H, d, J = 8.4 Hz, H-2', H-6'), 1.39 (3H, s, Ar-CH3); EI (70eV) m/z (%): M+. 344.81022 (100).
(1d) 6,9-dichloro-3-phenyl-3H-benzo[b]pyrazolo[3,4-h]-1,6-naphthyridine
Yield 60%; mp > 300°C; IR (KBr, cm-1) νmax C-H 3082, C = C 1598, C = N 1503; 1H NMR (DMSO-d6, 300 MHz) δ 9.08 (1H, s, H-1), 7.99-7.53 (6H, m, H-3', H-4', H-5', H7, H-8, H-10), 9.38 (1H, s, H-5), 8.41 (2H, d, J = 8.4 Hz, H-2', H-6'); EI (70 eV) m/z (%): M+. 364.01693 (100).
(1e) 6,8-dichloro-3-phenyl-3H-benzo[b]pyrazolo[3,4-h]-1,6-naphthyridine
Yield 68%; mp > 300°C; IR (KBr, cm-1) νmax C-H 3084, C = C 1598, C = N 1503; 1H NMR (DMSO-d6, 300 MHz) δ 8.97 (1H, s, H-1), 7.87 (1H, d, J = 8.1 Hz, H-10), 7.89 (1H, d, J = 8.1 Hz, H-9), 8.22 (1H, s, H-7), 9.31 (1H, s, H-5), 8.27 (2H, d, J = 8.1 Hz, H-2', H-6'), 7.46 (2H, dd, J = 7.5 Hz, H-3', H-5'), 7.65 (1H, t, J = 7.5 Hz, H-4'); EI (70 eV) m/z (%): M+. 364.01789 (100).
(1f) 6-chloro-3-phenyl-9-nitro-3H-benzo[b]pyrazolo[3,4-h]-1,6-naphthyridine
Yield 60%; mp > 300°C; IR (KBr, cm-1) νmax C-H 3084, C = C 1596, C = N 1503; 1H NMR (DMSO-d6, 300 MHz) δ 8.92 (1H, s, H-1), 8.84 (1H, s, H-10), 8.05 (1H, d, J = 7.5 Hz, H-8), 8.02 (1H, d, J = 7.5 Hz, H-7), 9.40 (1H, s, H-5), 8.31 (2H, d, J = 7.5 Hz, H-2', H-6'), 7.52 (2H, dd, J = 7.5 Hz, H-3', H-5'), 7.71 (1H, t, J = 7.5 Hz, H-4'); EI (70 eV) m/z (%): M+. 375.03743 (100).
(1g) 6-chloro-3-phenyl-8-nitro-3H-benzo[b]pyrazolo[3,4-h]-1,6-naphthyridine
Yield 60%; mp 280-281°C; IR (KBr, cm-1) νmax C-H 3100, C = C 1592, C = N 1500; 1H NMR (DMSO-d6, 300 MHz) δ 8.90 (1H, s, H-1), 8.12 (1H, d, J = 7.8 Hz, H-10), 8.80 (1H, d, J = 7.8 Hz, H-9), 8.89 (1H, s, H-7), 9.42 (1H, s, H-5), 8.30 (2H, d, J = 7.5 Hz, H-2', H-6'), 7.51 (2H, dd, J = 7.5 Hz, H-3', H-5'), 7.68 (1H, t, J = 7.5 Hz, H-4'); EI (70 eV) m/z (%): M+. 375.70261 (100).
(1h) 6-chloro-3-phenyl-9-fluoro-3H-benzo[b]pyrazolo[3,4-h]-1,6-naphthyridine
Yield 62%; mp 275-277°C; IR (KBr, cm-1) νmax C-H 3100, ν C = C 1600, ν C = N 1503; 1H NMR (DMSO-d6, 300 MHz) δ 9.34 (1H, s, H-1), 8.45 (1H, s, H-10), 7.83-7.62 (1H, m, H-8), 9.02 (1H, d, J = 8.4 Hz, H-7), 9.49 (1H, s, H-5), 8.20 (2H, d, J = 7.5 Hz, H-2', H-6'), 7.25 (2H, dd, J = 7.5 Hz, H-3', H-5'), 7.41 (1H, t, J = 7.5 Hz, H-4'); EI (70 eV) m/z (%): M+. 348.04188 (100).
(1i) 6-chloro-3-phenyl-8-fluoro-3H-benzo[b]pyrazolo[3,4-h]-1,6-naphthyridine
Yield 65%; mp 278-279°C; IR (KBr, cm-1) νmax C-H 3051, C = C 1598, C = N 1503; 1H NMR (DMSO-d6, 300 MHz) δ 9.06 (1H, s, H-1), 8.03 (1H, d, J = 7.5 Hz, H-10), 8.05 (1H, m, H-9), 8.02 (1H, d, J = 8.4 Hz, H-7), 9.37 (1H, s, H-5), 8.32 (2H, d, J = 7.5 Hz, H-2', H-6'), 7.51 (1H, t, J = 7.5 Hz, H-4'), 7.69 (2H, dd, J = 7.5 Hz, H-3', H-5'); EI (70 eV) m/z (%): M+. 348.09473 (100).
(1j) 9-bromo-6-chloro-3-phenyl-3H-benzo[b]pyrazolo[3,4-h]-1,6-naphthyridine
Yield 60%; mp > 300°C; IR (KBr, cm-1) νmax C-H 3052, C = C 1593, C = N 1503; 1H NMR (DMSO-d6, 300 MHz) δ 9.05 (1H, s, H-1), 8.09 (1H, s, H-10), 7.90-7.50 (5H, m, H-3', H-4', H-5', H7, H-8), 9.30 (1H, s, H-5), 8.30 (2H, d, J = 7.5 Hz, H-2', H-6'); EI (70 eV) m/z (%): M+. 409.96178 (100).
(1k) 8-bromo-6-chloro-3-phenyl-3H-benzo[b]pyrazolo[3,4-h]-1,6-naphthyridine
Yield 68%; mp > 300°C; IR (KBr, cm-1) νmax C-H 3085, C = C 1596, C = N 1501; 1H NMR (DMSO-d6, 300 MHz) δ 8.95 (1H, s, H-1), 7.92-7.52 (5H, m, H-3', H-4', H-5', H7, H-10), 8.42 (1H, d, J = 8.4 Hz, H-9), 9.40 (1H, s, H-5), 8.30 (1H, d, J = 7.5 Hz, H-2', H-6'); EI (70 eV) m/z (%): M+. 409,67991 (100).
(2a) 6-chloro-3-phenyl-7-methyl-3H-pyrido[2,3-b]pyrazolo[3,4-h]-1,6-naphthyridine
Yield 60%; mp 230-232°C; IR (KBr, cm-1) νmax C-H 3085, C = C 1596, C = N 1501; 1H NMR (DMSO-d6, 300 MHz) δ 8.72 (1H, s, H-1), 8.26 (1H, d, J = 7.5 Hz, H-9), 7.68 (1H, d, J = 7.5 Hz, H-8), 9.44 (1H, s, H-5), 8.26 (2H, dd, J = 7.5 Hz, H-2', H-6'), 7.50 (1H, t, J = 7.5 Hz, H-4'), 7.68 (2H, dd, J = 7.5 Hz, H-3', H-5'), 1.37 (3H, s, Ar-CH3); EI (70eV) m/z (%): M+. 345.74391 (100).
(2b) 6-chloro-3-phenyl-8-methyl-3H-pyrido[2,3-b]pyrazolo[3,4-h]-1,6-naphthyridine
Yield 65%; mp 255-256°C; IR (KBr, cm-1) νmax C-H 3084, C = C 1596, C = N 1502; 1H NMR (DMSO-d6, 300 MHz) δ 8.72 (1H, s, H-1), 8.92 (1H, s, H-9), 7.91 (1H, s, H-7), 9.58 (1H, s, H-5), 8.42 (2H, d, J = 7.5 Hz, H-2', H-6'), 7.59 (1H, t, J = 7.5 Hz, H-4'), 7.78 (2H, dd, J = 7.5 Hz, H-3', H-5'), 1.42 (3H, s, Ar-CH3); EI (70 eV) m/z (%): M+. 345.78660 (100).
(2c) 6-chloro-3-phenyl-9-methyl-3H-pyrido[2,3-b]pyrazolo[3,4-h]-1,6-naphthyridine
Yield 62%; mp 247-249°C; IR (KBr, cm-1) νmax C-H 3084, C = C 1596, C = N 1502; 1H NMR (DMSO-d6, 300 MHz) δ 8.83 (1H, s, H-1), 7.67 (1H, d, J = 7.5 Hz, H-8), 7.93 (1H, d, J = 7.5 Hz, H-7), 9.41 (1H, s, H-5), 8.39 (2H, d, J = 7.5 Hz, H-2', H-6'), 7.56 (1H, t, J = 7.5 Hz, H-4'), 7.75 (2H, dd, J = 7.5 Hz, H-3', H-5'), 1.39 (3H, s, Ar-CH3); EI (70 eV) m/z (%): M+. 345.76572 (100).
Biological assays
Compounds were tested as inhibitor of HSV replication in Vero (African green monkey kidney, obtained from the American Type Culture Collection) cells. They were grown in DMEM (Gibco Laboratories) supplemented with 2% heat-inactivated fetal bovine serum (purchased from Fazenda Pig), 8% calf serum (purchased from Centro Pan-Americano de Febre Aftosa), 2.25% sodium bicarbonate, 500 U/mL penicillin, 100 μg/mL streptomycin, and 2.5 μg/mL amphotericin B. HSV-1 (ACR-29 strain) was kindly provided by Marcia Wigg (Universidade Federal do Rio de Janeiro, Brazil) and was routinely propagated in Vero cells. Virus stocks were stored at -70°C until use. ACV was purchased from Sigma (A 4669). It was dissolved in sterile deionized water and further diluted in culture medium. MTT was purchased from Sigma. Virus infectivity was measured by a dilution method using a 96-well microtitre plate and expressed as 50% tissue culture infections dose (TCID50). Cells grown in 96-well microtitre were inoculated with virus at input 1 PFU (plaque-forming unit)/cell for 2 h at 37°C. After virus adsorption, virus inoculum was replaced by a culture medium containing quinolone acyclonucleobases carboxylic acid and their correspondent esters at the concentration of 50 μM. Control cultures were incubated with media without compounds. After 3 days of incubation at 37°C in 5% CO2 atmosphere, the culture medium was harvested and the virus titre of each sample was determined in terms of 50% tissue culture dose (TCID 50/mL) by endpoint dilution.
Cytotoxicity
The cytotoxicity of the compounds was tested in Vero cells using two methods, namely, MTT and trypan blue dye exclusion assay. Monolayers of uninfected cells were incubated with culture medium containing different concentrations of compounds for 72 h at 37°C. The medium was then removed, the cells trypsinized and viable cells counted by trypan blue dye exclusion test. The 50% cytotoxic concentration (CC50) was calculated by linear regression analysis of the dose-response curves generated from these data. In the second method, monolayer of Vero cells in 96-multiwell plates were incubated with MTT (5 μg/mL) at 37°C for 4 h. After this period, SDS 10% and 0.01 N HCl were added to each well and incubated overnight. The plates were read using an automatic plate reader with a 540-nm test wavelength and a 690-nm reference wavelength. Plaque reduction assay was performed utilizing Vero cells at a density of 3 × 105 infected with various dilutions of the supernatant from a yield reduction assay for 1 h at 37°C and 5% CO2. After adsorption, the plates were washed and the medium was replaced with DMEM containing methylcellulose 1% and fetal bovine serum 5%. After incubation for 72 h, the monolayers were fixed with 1% formaldehyde in PBS, methylcellulose removed, and cell stained with a 0.1% solution of crystal violet in 70% methanol. The virus yield assay was performed as follows. Confluent Vero cells were washed with PBS and infected with HSV-1 at moi of 1 PFU/cell for 1 h at 37°C. The infected cells were washed with PBS and covered with a culture medium containing either no compounds or a different concentration of compounds. 20 h after adsorption, cells were lysed by freezing and thawing (three times), and the supernatant consisting of culture medium and lysed cells was obtained by centrifugation at 400g for 10 min at 4°C. Virus titre was determined by the plaque assay in Vero cells as described above. Data were statistically analyzed by Student's t-test for a significance level of p < 0.05.
Abbreviations
- DMEM:
-
Dulbecco's modified Eagle's medium
- DMF N :
-
N-dimethylformamide
- HCMV:
-
human cytomegalovirus
- HSV-1:
-
herpes simplex virus type-1
- MTT:
-
(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- PBS:
-
phosphate buffered saline
- PFU:
-
plaque-forming unit
- SDS:
-
sodium dodecyl sulphate.
References
James SH, Kimberlin DW, Whitley RJ: Antiviral therapy for herpesvirus central nervous system infections: neonatal herpes simplex virus infection, herpes simplex encephalitis, and congenital cytomegalovirus infection. Antiviral Res 2009, 83: 207–213. 10.1016/j.antiviral.2009.04.010
Knizewski Ł, Kinch L, Grishin NV, Rychlewski L, Ginalski K: Human herpesvirus 1 UL24 gene encodes a potential PD-(D/E)XK endonuclease. J Virol 2006, 80: 2575–2577. 10.1128/JVI.80.5.2575-2577.2006
Kang IJ, Wang LW, Hsu TA, Yueh A, Lee CC, Lee YC, Lee CY, Chao YS, Shih SR, Chern JH: Isatin-b-thiosemicarbazones as potent herpes simplex virus inhibitors. Bioorg Med Chem Lett 2011, 21: 1948–1952. 10.1016/j.bmcl.2011.02.037
Jerome KR: The road to new antiviral therapies. Clin Appl Immunol Rev 2005, 5: 65–76. 10.1016/j.cair.2004.10.002
Morfin F, Thouvenot D: Herpes simplex virus resistance to antiviral drugs. J Clin Virol 2003, 26: 29–37. 10.1016/S1386-6532(02)00263-9
Mohamed SF, Flefel EM, Amr AEE, El-Shafy DNA: Anti-HSV-1 activity and mechanism of action of some new synthesized substituted pyrimidine, thiopyrimidine and thiazolopyrimidine derivatives. Eur J Med Chem 2010, 45: 1494–1501. 10.1016/j.ejmech.2009.12.057
Ju HQ, Xiang YF, Xin BJ, Pei Y, Lu JX, Wang QL, Xia M, Qian CW, Ren Z, Wang SY, Wang YF, Xing GW: Synthesis and in vitro anti-HSV-1 activity of a novel Hsp 90 inhibitor BJ-B11. Bioorg Med Chem Lett 2011, 21: 1675–1677. 10.1016/j.bmcl.2011.01.098
Jordão AK, Ferreira VF, Souza TML, Faria GGS, Machado V, Abrantes JL, Souza MCBV, Cunha AC: Synthesis and anti-HSV-1 activity of new 1,2,3-triazole derivatives. Bioorg Med Chem 2011, 19: 1860–1865. 10.1016/j.bmc.2011.02.007
Gudmundsson KS, Johns BA, Weatherhead J: Pyrazolopyrimidines and pyrazolotriazines with potent activity against herpesviruses. Bioorg Med Chem Lett 2009, 19: 5689–5692. 10.1016/j.bmcl.2009.08.009
Lowden CT, Bastow KF: Anti-Herpes simplex virus activity of substituted 1-hydroxyacridones. J Med Chem 2003, 46: 5015–5020. 10.1021/jm030206l
Hammond JL, Koontz DL, Bazmi HZ, Beadle JR, Hostetler SE, Kini GD, Aldern KA, Richman DD, Hostetler KY, Mellors JW: Alkylglycerol prodrugs of phosphonoformate are potent in vitro inhibitors of nucleoside-resistant human immunodeficiency virus type 1 and select for resistance mutations that suppress zidovudine resistance. Antimicrob Agents Chemother 2001, 45: 1621–1628. 10.1128/AAC.45.6.1621-1628.2001
Ramkumar K, Serrao E, Odde S, Neamati N: HIV-1 integrase inhibitors: 2007–2008 update. Med Res Rev 2010, 30: 890–954. 10.1002/med.20194
Johns BA, Weatherhead JG, Allen SH, Thompson JB, Garvey EP, Foster SA, Jeffrey JL, Miller WH: 1,3,4-Oxadiazole substituted naphthyridines as HIV-1 integrase inhibitors. Part 2: SAR of the C5 position. Bioorg Med Chem Lett 2009, 19: 1807–1810. 10.1016/j.bmcl.2009.01.089
Melamed JY, Egbertson MS, Varga S, Vacca JP, Moyer G, Gabryelski L, Felock PJ, Stillmock KA, Witmer MV, Schleif W, Hazuda DJ, Leonard Y, Jin L, Ellis JD, Young SD: Synthesis of 5-(1-H or 1-alkyl-5-oxopyrrolidin-3-yl)-8-hydroxy-[1,6]-naphthyridine-7-carboxamide inhibitors of HIV-1 integrase. Bioorg Med Chem Lett 2008, 18: 5307–5310. 10.1016/j.bmcl.2008.08.038
Egbertson MS, Moritz HM, Melamed JY, Han W, Perlow DS, Kuo MS, Embrey M, Vacca JP, Zrada MM, Cortes AR, Wallace A, Leonard Y, Hazuda DJ, Miller MD, Felock PJ, Stillmock KA, Witmer MV, Schleif W, Gabryelski LJ, Moyer G, Ellis JD, Jin L, Xu W, Braun MP, Kassahun K, Tsou NN, Young SD: A potent and orally active HIV-1 integrase inhibitor. Bioorg Med Chem Lett 2007, 17: 1392–1398. 10.1016/j.bmcl.2006.11.080
Falardeau G, Lachance H, Pierre AS, Yannopoulos CG, Drouin M, Bédard J, Chan L: Design and synthesis of a potent macrocyclic 1,6-napthyridine anti-human cytomegalovirus (HCMV) inhibitors. Bioorg Med Chem Lett 2005, 15: 1693–1695. 10.1016/j.bmcl.2005.01.050
Chan L, Jin H, Stefanac T, Lavallée JF, Falardeau G, Wang W, Bédard J, May S, Yuen L: Discovery of 1,6-Naphthyridines as a novel class of potent and selective human cytomegalovirus inhibitors. J Med Chem 1999, 42: 3023–3025. 10.1021/jm9902483
Thompson AM, Connolly CJC, Hamby JM, Boushelle S, Hartl BG, Amar AM, Kraker AJ, Driscoll DL, Steinkampf RW, Patmore SJ, Vincent PW, Roberts BJ, Elliott WL, Klohs W, Leopold WR, Showalter HDH, Denny WA: 3-(3,5-Dimethoxyphenyl)-1,6-naphthyridine-2,7-diamines and related 2-urea derivatives are potent and selective inhibitors of the FGF receptor-1 tyrosine kinase. J Med Chem 2000, 43: 4200–4211. 10.1021/jm000161d
Vanlaer S, Voet A, Gielens C, De Maeyer M, Compernolle F: Bridged 5,6,7,8-tetrahydro-1,6-naphthyridines, analogues of huperzine a: synthesis, modeling studies and evaluation as inhibitors of acetylcholinesterase. Eur J Org Chem 2009, 2009: 643–654.
Brown DJ (Ed): Chemistry of heterocyclic compounds: the naphthyridines John Wiley & Sons, New Jersey; 2008.
Jachak MN, Bagul SM, Kazi MA, Toche RB: Novel synthetic protocol toward pyrazolo[3,4- h ]-[1,6]naphthyridines via Friedlander condensation of new 4-aminopyrazolo[3,4- b ]pyridine-5-carbaldehyde with reactive α-methylene ketones. J Het Chem 2011, 48: 295–300. 10.1002/jhet.242
Rote RV, Bagul SM, Shelar DP, Patil SR, Toche RB, Jachak MN: Synthesis of benzo[3,4- h ][1,6]naphthyridines via Friedlander condensation with active methylenes. J Het Chem 2011, 48: 301–307. 10.1002/jhet.391
Toche RB, Pagar BP, Zoman RR, Shinde GB, Jachak MN: Synthesis of novel benzo[h][1,6]naphthyridine derivatives from 4-aminoquinoline and cyclic b-ketoester. Tetrahedron 2010, 66: 5204–5211. 10.1016/j.tet.2010.04.085
Chandra A, Singh B, Upadhyay S, Singh RM: Copper-free Sonogashira coupling of 2-chloroquinolines with phenyl acetylene and quick annulation to benzo[b][1,6]naphthyridine derivatives in aqueous ammonia. Tetrahedron 2008, 64: 11680–11685. 10.1016/j.tet.2008.10.010
Bernardino AMR, Castro HC, Frugulhetti ICPP, Loureiro NIV, Azevedo AR, Pinheiro LCS, Souza TML, Giongo V, Passamani F, Magalhães UO, Albuquerque MG, Cabral LM, Rodrigues CR: SAR of a series of anti-HSV-1 acridone derivatives, and a rational acridone-based design of a new anti-HSV-1 3H-benzo[b]pyrazolo[3,4-h]-1,6-naphthyridine series. Bioorg Med Chem 2008, 16: 313–321. 10.1016/j.bmc.2007.09.032
Leal B, Afonso IF, Rodrigues CR, Abreu PA, Garrett R, Pinheiro LCS, Azevedo AR, Borges JC, Vegi PF, Santos CCC, Silveira FCA, Cabral LM, Frugulhetti ICPP, Bernardino AMR, Santos DO, Castro HC: Antibacterial profile against drug-resistant Staphylococcus epidermidis clinical strain and structure-activity relationship studies of 1H-pyrazolo[3,4-b]pyridine and thieno[2,3-b]pyridine derivatives. Bioorg Med Chem 2008, 16: 8196–8204. 10.1016/j.bmc.2008.07.035
Mello H, Echevarria A, Bernardino AM, Cavalheiro MC, Leon LL: Antileishmanial pyrazolopyridine derivatives: synthesis and structure-activity relationship analysis. J Med Chem 2004, 47: 5427–5432. 10.1021/jm0401006
Bernardino AMR, Azevedo AR, Pinheiro LCS, Borges JC, Carvalho VL, Miranda MD, Meneses MDF, Nascimento M, Ferreira D, Rebello MA, Silva VAGG, Frugulhetti ICPP: Synthesis and antiviral activity of new 4-(phenylamino)/4-[(methylpyridin-2-yl)amino]-1-phenyl-1 H -pyrazolo[3,4- b ]pyridine-4-carboxylic acids derivatives. Med Chem Res 2007, 16: 352–369. 10.1007/s00044-007-9035-6
Pinheiro LCS, Borges JC, Oliveira CD, Ferreira VF, Romeiro GA, Marques IP, Abreu PA, Frugulhetti ICPP, Rodrigues CR, Albuquerque MG, Castro HC, Bernardino AMR: Synthesis of new 4-(phenylamino)thieno[2,3-b]pyridines and derivatives of the novel benzo[b]thieno[3,2-h]-1,6-naphthyridine tetracyclic system. Arkivoc 2008, 14: 77–87.
Bernardino AMR, Pinheiro LCS, Rodrigues CR, Loureiro NI, Castro HC, Rangel AL, Lopes JS, Borges JC, Carvalho JM, Romeiro GA, Ferreira VF, Frugulhetti ICPP, Santos MAV: Design, synthesis, SAR, and biological evaluation of new 4-(phenylamino)thieno[2,3-b]pyridine derivatives. Bioorg Med Chem 2006, 14: 5765–5770. 10.1016/j.bmc.2006.03.013
Acknowledgements
This study was supported by the following Brazilian agencies and governmental institutions: Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Bernardino, A.M., Azevedo, A.R., Pinheiro, L.C. et al. Synthesis and anti-HSV-1 evaluation of new 3H-benzo[b]pyrazolo[3,4-h]-1,6-naphthyridines and 3H-pyrido[2,3-b]pyrazolo[3,4-h]-1,6-naphthyridines. Org Med Chem Lett 2, 3 (2012). https://doi.org/10.1186/2191-2858-2-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2191-2858-2-3