Abstract
Background
Pyrroles are widely distributed in nature and important biologically active molecules. The reaction of amines with 2,5-dimethoxytetrahydrofuran is a promising pathway for the synthesis of pharmacologically active pyrroles under microwave irradiation.
Results
Microwave-induced polystyrenesulfonate-catalyzed synthesis of pyrroles from amines and 2,5-diemthoxytetrahydrofuran has been accomplished with excellent yield. This method produces pyrroles with polyaromatic amines.
Conclusion
The present procedure for the synthesis of N-aromatic substituted pyrroles will find useful application in the area of pharmacologically active molecules.

Similar content being viewed by others
Background
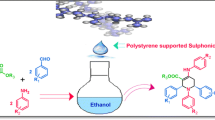
Pyrrole is one of the most significant heterocyclic structural scaffold present in a large number of biologically active molecules [1, 2] with a wide range of applications in medicinal chemistry [3]. Besides its pharmacological activity, pyrrole derivatives play a crucial role in material science [4]. The traditional methods rely on the cyclization of amines with ketones or diketones discovered by Knorr and Paal in 1880 s [5, 6]. Since then, a number of publications have appeared on this reaction, but still, this method is under active investigation because of its simplicity. Recently, a number of methods and catalysts have been reported, e.g., supercritical carbon dioxide [7], silver-salts promoted three component reaction [8], manganese(III)-catalyzed [3 + 2] annulation [9], rhodium(III)-catalyzed bond functionalization [10], palladium-induced three component reaction [11], gold(I)-catalyzed amino-Claisen rearrangement [12] and zinc chloride [13]. In contrast, many of these reported methods involve the use of expensive reagents, hazardous solvents, longer reaction times and tedious workup procedure. Therefore, it is desirable to develop an efficient and practical method for the synthesis of pyrrole derivatives. We have also reported several procedures in this area [14–19]. We report herein a simple microwave-assisted method for the synthesis of N-arylpyrroles using an aqueous solution of polystyrene sulfonate. Synthesis of pyrroles in aqueous solution is promising and demanding because of eco-friendly nature of the procedure (Figure 1).
Although the mechanism of this reaction is not investigated, we believe compound 2 (Figure 1) produces a diketo compound in the media due to acid-induced cleavage reaction. The intermediate diketo compound then undergoes reaction with aromatic amines through nucleophilic and dehydration pathways (Figure 2). It is interesting to note that dehydration occurs in the presence of aqueous acid. Pyrroles are sensitive under strong acidic conditions, and isolation of these types of products from acidic solution may prove to be problematic. However, this problem can be overcome by simply basifying the reaction mixture prior to extraction. Higher temperature (60°C) and high power microwave radiation (300 W) accelerate the reaction significantly (Table 1). The reaction proceeds well in ethanol, THF and acetonitrile.
Methods
FT-IR spectra were registered on a Bruker IFS 55 Equinox FTIR spectrophotometer (Bruker Corporation, Billerica, MA, USA) as KBr discs.1 H-NMR (600 MHz) and13 C-NMR (150 MHz) spectra were obtained at room temperature with Bruker-600 equipment (Bruker Corporation) using TMS as internal standard and CDCl3 as solvent. Analytical grade chemicals (Sigma-Aldrich Corporation, St. Louis, MO, USA) were used throughout the project. Deionized water was used for the preparation of all aqueous solutions.
Results and discussion
In continuation of our research on environmentally benign reaction and biological evaluation of various polyaromatic compounds as novel anticancer agents [20–26], we have investigated reaction between aromatic amines (1) with 2,5-diemthoxytetrahydrofuran (2) using aqueous polystyrene sulfonate. After various experimentations, we have identified that this method works well under microwave-induced reaction conditions. Monocyclic, bicyclic, tricyclic and tetracyclic aromatic amines are used, and N-aryl pyrroles are produced in good yields (Figure 1). At the beginning of the procedure, 2,5-diemthoxytetrahydrofuran (2), the amine (1) and aqueous solution of polystyrene sulfonate were mixed in ethanol. The mixture was then stirred at room temperature, and the progress of the reaction was monitored through TLC every after 30 min. After completion of the reaction, the reaction mixture was basified with aqueous-saturated sodium bicarbonate solution and extracted with dichloromethane. The organic part was then washed with brine, dried with sodium sulfate and evaporated. The yields of the products are shown in Table 2. The multi-cyclic aromatic amines needed longer reaction time compared to the amines which are more basic and superior nucleophilic reagents.
Experimental
General procedure for the synthesis of pyrroles (3)
Amine (1.0 mmol), 2, 5-dimethoxytetrahydrofuran (1.2 mmol) and polystyrene sulfonate (18 wt% solution in water) in ethanol/water (1:1) mixture was stirred at room temperature, and the progress of the reaction was monitored by TLC every 30 min. After completion of the reaction (Table 2), the reaction mixture was basified with saturated aqueous sodium bicarbonate solution and extracted with dichloromethane. The organic layer was then washed with brine, dried with sodium sulfate and evaporated to isolate the pure product.
Alternatively, amine (1.0 mmol), 2, 5-dimethoxytetrahydrofuran (1.2 mmol) and polystyrene sulfonate (18 wt% solution in water) in ethanol/water (1:1) were irradiated in an automated microwave oven (CEM Corporation, Matthews City, NC, USA). The reaction was monitored by TLC every 5 min. Depending upon the nature of the amines, the reaction was completed in different time. The result of the procedure was shown in Table 1. All the products have demonstrated satisfactory spectral and mp data with our reported compounds [16].
Conclusions
A new and simple method for the synthesis of N-substituted pyrroles in aqueous solution has been investigated with success. Based on our previous studies in this series, the compounds as reported herein may demonstrate anticancer activities.
References
Fan H, Peng J, Hamann MT, Hu J-F: Lamellarins and related pyrrole-derived alkaloids from marine organisms. Chem Rev 2008, 108: 264–287. 10.1021/cr078199m
Walsh CT, Garneau-Tsodikova S, Howard-Jones AR: Biological formation of pyrroles: nature's logic and enzymic machinery. Nat Prod Rep 2006, 23: 517–531. 10.1039/b605245m
Aiello A, D'Esposito M, Fattorusso E, Menna M, Mueller WEG, Perovic-Ottstadt S, Schroeder HC: Novel bioactive bromopyrrole alkaloids from the Mediterranean sponge Axinella verrucosa. Bioorg Med Chem 2006, 14: 17–24. 10.1016/j.bmc.2005.07.057
Domingo VM, Aleman C, Brillas E, Julia L: Diradical dications of m- and p-phenylenebis[2,5-di(2-thienyl)-1-pyrrole]: weakly coupled diradicals. J Org Chem 2001, 66: 4058–4061. 10.1021/jo001656d
Knorr L: Synthesis of pyrroline-derivatives II. Chem Ber 1884, 17: 1635–1642. 10.1002/cber.18840170220
Paal C: Synthesis of thiophen and pyrolline derivatives. Chem Ber 1885, 18: 367–371. 10.1002/cber.18850180175
Cardoso AL, Nunes RMD, Arnaut LG, Pinho M, Teresa MVD: Synthesis of pyrroles in supercritical carbon dioxide: formal [3 + 2] cycloaddition of 2-benzoyl-aziridines and allenoates. Synthesis 2011, 21: 3516–3522.
Zeng J, Bai Y, Cai S, Ma J, Liu X-W: Direct synthesis of pyrroles via a silver-promoted three-component reaction involving unusual imidazole ring opening. Chem Commun 2011, 47: 12855–12857. 10.1039/c1cc14716a
Ng EPJ, Wang Y-F, Chiba S: Manganese(III)-catalyzed formal [3 + 2] annulation of vinyl azides and β-keto acids for synthesis of pyrroles. Synlett 2011, 6: 783–786.
Stuart DR, Alsabeh P, Kuhn M, Fagnou K: Rhodium(III)-catalyzed arene and alkene c-h bond functionalization leading to indoles and pyrroles. J Am Chem Soc 2010, 132: 18326–18339. 10.1021/ja1082624
Lamande-Langle S, Abarbri M, Thibonnet J, Duchene A, Parrain J-L: Domino allylic amination/Sonogashira/heterocyclisation reactions: palladium-catalysed three-component synthesis of pyrroles. Chem Commun 2010, 46: 5157–5159. 10.1039/c0cc00500b
Saito A, Konishi T, Hanzawa Y: Synthesis of pyrroles by gold(I)-catalyzed amino-Claisen rearrangement of N-propargyl enaminone derivatives. Org Lett 2010, 12: 372–374. 10.1021/ol902716n
Wyrebek P, Sniady A, Bewick N, Li Y, Mikus A, Wheeler KA, Dembinski R: Microwave-assisted zinc chloride-catalyzed synthesis of substituted pyrroles from homopropargyl azides. Tetrahedron 2009, 65: 1268–1275. 10.1016/j.tet.2008.11.094
Banik BK, Samajdar S, Banik I: Simple synthesis of substituted pyrroles. J Org Chem 2004, 69: 213–216. 10.1021/jo035200i
Banik BK, Banik I, Renteria M, Dasgupta SK: A straightforward highly efficient Paal–Knorr synthesis of pyrroles. Tetrahedron Lett 2005, 46: 2643–2645. 10.1016/j.tetlet.2005.02.103
Bandyopadhyay D, Mukherjee S, Banik BK: An expeditious synthesis of N-substituted pyrroles via microwave-induced iodine-catalyzed reaction under solventless conditions. Molecules 2010, 15: 2520–2525. 10.3390/molecules15042520
Andoh-Baidoo R, Danso R, Mukherjee S, Bandyopadhyay D, Banik BK: Microwave-induced N-bromosuccinimide-mediated novel synthesis of pyrroles via Paal-Knorr reaction. Heterocycl Lett 2011, 1: 107–109.
Abrego D, Bandyopadhyay D, Banik BK: Microwave-induced indium-catalyzed synthesis of pyrrole fused with indolinone in water. Heterocycl Lett 2011, 1: 94–95.
Rivera S, Bandyopadhyay D, Banik BK: Facile synthesis of N via microwave-induced bismuth nitrate-catalyzed reaction under solventless conditions.-substituted pyrroles. Tetrahedron Lett 2009, 50: 5445–5448. 10.1016/j.tetlet.2009.06.002
Banik I, Becker FF, Banik BK: Stereoselective synthesis of β-lactams with polyaromatic imines: entry to new and novel anticancer agents. J Med Chem 2003, 46: 12–15. 10.1021/jm0255825
Becker FF, Banik BK: Polycyclic aromatic compounds as anticancer agents: synthesis and biological evaluation of some chrysene derivatives. Bioorg Med Chem Lett 1998, 8: 2877–2880. 10.1016/S0960-894X(98)00520-4
Becker FF, Mukhopadhyay C, Hackfeld L, Banik I, Banik BK: Polycyclic aromatic compounds as anticancer agents: synthesis and biological evaluation of dibenzofluorene derivatives. Bioorg Med Chem 2000, 8: 2693–2699. 10.1016/S0968-0896(00)00213-3
Banik BK, Becker FF: Polycyclic aromatic compounds as anticancer agents. 4. Structure-activity relationships of chrysene and pyrene derivatives. Bioorg Med Chem 2001, 9: 593–605. 10.1016/S0968-0896(00)00297-2
Banik BK, Becker FF: Synthesis, electrophilic substitution and structure-activity relationship studies of polycyclic aromatic compounds towards the development of anticancer agents. Curr Med Chem 2001, 8: 1513–1533. 10.2174/0929867013372120
Banik BK, Becker FF, Banik I: Synthesis of anticancer β-lactams: mechanism of action. Bioorg Med Chem 2004, 12: 2523–2528. 10.1016/j.bmc.2004.03.033
Bandyopadhyay D, Granados JC, Short JD, Banik BK: Polycyclic aromatic compounds as anticancer agents: evaluation of synthesis and in vitro cytotoxicity. Oncol Lett 2012, 3: 45–49.
Acknowledgment
We gratefully acknowledge the funding support from the Kleberg Foundation, Texas.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
RAVC and BOQL performed the reactions with the help of AR and DB. BKB is the PI. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Cárdenas, R.V., Leal, B.Q., Reddy, A. et al. Microwave-assisted polystyrene sulfonate-catalyzed synthesis of novel pyrroles. Org Med Chem Lett 2, 24 (2012). https://doi.org/10.1186/2191-2858-2-24
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2191-2858-2-24