Abstract
Background
We previously described the visceral antinociceptive property of α-bisabolol (BISA) in mouse models of visceral nociception induced by cyclophosphamide and mustard oil (MO). This study examined the effect of BISA in mouse models of visceral nociception induced by acetic acid, capsaicin, formalin, and the contribution of the nitric oxide system, α2, KATP, 5-HT3 and TRPV1 receptors to the effect of BISA on MO-evoked nociceptive behaviors. Mice were pretreated orally with BISA (50, 100 and 200 mg/kg) or vehicle, and the pain-related behavioral responses to intraperitoneal administration of acetic acid or intracolonic injection of MO were analyzed.
Results
BISA significantly suppressed the nociceptive behaviors in a dose-unrelated manner. The antinociceptive effect of BISA (50 mg/kg) was show to be glibenclamide resistant, but it was not blocked by pretreatment with the other antagonists tested. In the open-field test that detects sedative or motor abnormality, mice received 50 mg/kg BISA did not show any per se influence in ambulation frequency.
Conclusions
However, their precise antinociceptive mechanisms of action have not been determined.

Similar content being viewed by others
Background
Essential oils are natural products that exhibit a variety of biological properties such as analgesic, anticonvulsant, and anxiolytic ones. Such effects are often attributed to the presence of monoterpenes, which are the major chemical components of these oils [1]. Sesquiterpenes, such zerumbone [2], budlein A [3], polygodial [4], and lapidin [5], have been shown to present antinociceptive properties.
Recent studies demonstrated that the sesquiterpene α-bisabolol (BISA) presents antitumor [6], peripheral nervous blocker [7], gastroprotective [8], leishimanicidal [9], antioxidant [10], mutagenic and antimutagenic [11], and wound healing [12] properties. There are also evidences showing that BISA reduced visceral nociceptive pain evoked by intracolonic administration of mustard oil (MO) as well as that induced by intraperitoneal injection of cyclophosphamide, possibly involving the inhibition of sensitization of primary afferent neuron terminals by prostaglandins [13].
However, the mechanisms underlying these antinociceptive properties of BISA are not well understood.
Methods
Animals
Male Swiss albino mice (20–25 g) obtained from the Central Animal House of Regional University of Cariri were used. They were housed in environmentally controlled conditions (22°C, 12-h light–dark cycle), with free access to standard pellet diet (Purina, São Paulo, Brazil) and water. Animals were kept in cages with raised floors to prevent coprophagy. Before the visceral antinociceptive assays, they were fasted over a period of 15 h and were habituated to the test environment for 2 h before the experimentation. The experimental protocols were in accordance with the ethical guidelines of National Institute of Health, Bethesda, USA. The studies were performed in a blinded manner.
Acetic acid-induced visceral nociception
Abdominal constrictions were induced by intraperitoneal injection of acetic acid (0.6%). The animals were pretreated with BISA (50, 100 or 200 mg/kg, p.o.) or vehicle (2% Tween 80, 10 mL/kg, p.o.) 60-min prior to acetic acid injection. After the challenge, each mouse was placed in a separate glass funnel and the number of contractions of the abdominal muscles, together with stretching, was cumulatively counted over a period of 20 min. Antinociceptive activity was expressed as the reduction in the number of abdominal contractions, comparing the control animals with the mice pretreated with BISA.
Capsaicin-induced visceral nociception
Male mice divided into groups of eight in each were pretreated with the vehicle (2% Tween 80 in distillated water, 10 mL/kg, p.o.) or BISA (50, 100 or 200 mg/kg, p.o.). 1 h after oral and 30 min following systemic treatments, capsaicin (0.3% in a solution of PBS:Tween 80:ethanol (8:1:1)) was instilled into the colon (50 μL/animal) using a fine cannula (1.6-mm external diameter), 4-cm far from the anal sphincter. Solid petroleum jelly was applied onto the perianal region to avoid local nerve stimulation. The animals were then observed during a 30-min period for the spontaneous visceral pain-related behaviors (licking the upper abdomen, abdominal contortion and retraction, squashing the abdomen against the floor). A normal group, that received only saline intracolonically, was also included.
Formalin-induced visceral nociception
Male mice divided into groups of eight in each were pretreated with the vehicle (2% Tween 80 in distillated water, 10 mL/kg, p.o.) or BISA (50, 100 or 200 mg/kg, p.o.). 1 h after oral and 30 min following systemic treatments, formalin (10%) was instilled into the colon (10 μL/animal) using a fine cannula (1.6-mm external diameter), 4-cm far from the anal sphincter. Solid petroleum jelly was applied onto the perianal region to avoid local nerve stimulation. The animals were then observed during a 60-min period for the spontaneous visceral pain-related behaviors (licking the upper abdomen, abdominal contortion and retraction, squashing the abdomen against the floor). A normal group, that received only saline intracolonically, was also included.
MO-induced visceral nociception
To assess the antinociceptive effect of BISA against the MO-induced visceral nociception, mice in groups (n = 8) were orally treated with BISA (50, 100 or 200 mg/kg, p.o.) or vehicle (2% Tween 80 in distillated water, 10 mL/kg, p.o.) 1 h before the intracolonic administration of MO (0.75% in saline 0.9%, 50 μL/animal). A group of normal control received a similar dose of saline (10 mL/kg). Immediately following the intracolonic MO or saline administration, the mice were observed for the total number of nociceptive behaviors (licking the upper abdomen, stretching the abdomen, squashing the abdomen against the floor and retraction of the abdomen characterized for an arched position), for a 20-min period.
In order to verify the possible involvement of nitrergic, noradrenergic, KATP, 5-HT3, and TRPV1 receptors in the effect of BISA, the animals were treated with L-NAME (10 mg/kg, i.p), yohimbine (2 mg/kg, i.p), glibenclamide (5 mg/kg, i.p), ondansetron (10 mg/kg, i.p) or ruthenium red (3 mg/kg, s.c.), 30 min before the administration of BISA (50 mg/kg).
Locomotor activity (open-field test)
The open-field area was made of acrylic (transparent walls and black floor: 30 × 30 × 15 cm2) divided in nine squares of equal area. Four groups of animals (n = 6) were used. While groups 1 and 2 were treated, respectively, with vehicle (2% Tween 80, 10 mL/kg, p.o.) and BISA (50 mg/kg, p.o.), groups 3 and 4 received vehicle + MO (0.75%, 50 μL) or BISA (50 mg/kg, p.o.) + MO. The MO was given by an intracolonic route 90 following the vehicle or BISA. The numbers of squares each animal crossed with the four paws were noted during a 4-min period.
Statistical analysis
The results are expressed as mean ± SEM from six or eight mice per group. For statistical analysis, ANOVA followed by Tukey’s or Student–Newman–Keul’s post hoc test, as appropriate, were used. A p < 0.05 was considered statistically significant.
Results and discussion
Effect of BISA on acetic acid, capsaicin, formalin and MO-induced visceral pain in mice
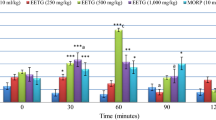
Table 1 shows the antinociceptive effect of BISA in acetic acid, capsaicin, formalin and MO. Intraperitoneal application of acetic acid (0.6%) provoked a significant increase in abdominal constrictions when compared with saline-treated normal control. In groups pretreated with BISA (50, 100 and 200 mg/kg, p.o.), acetic acid-induced abdominal constrictions were significantly inhibited in a dose-unrelated manner. Intracolonic application of capsaicin (CAP 0.3%), MO (MO 0.75%), or formalin (10%) provoked a significant increase in spontaneous pain-related behaviors when compared with saline-treated normal controls (Table 1). In groups pretreated with BISA, capsaicin, formalin and MO-induced nociceptive behaviors were significantly inhibited. L-NAME, yohimbine, glibenclamide, ondansetron, or ruthenium red failed to revert the antinociceptive effect of BISA (Tables 2, 3 and 4). Table 5 shows the effects of orally administered BISA on behavior in open-field test in mice. At the dose tested (50-mg/kg), BISA showed no significant influence on the number of crossings in open-field test.
In this study, we observed that the acetic acid, capsaicin, formalin, and MO-evoked nociceptive pain behaviors were significantly attenuated in mice pretreated with BISA, a natural sesquiterpenoid isolated from the different essential oils. It has been well established that many algogenic substances induce nociceptive pain-related behaviors in rodents following intraperitoneal or intracolonic application, and several reports reveal that naturally occurring compounds, like terpenoids, unsaturated dialdehydes and phenolic ketones can suppress these behaviors [14].
Many studies use acetic acid-induced effects as a model of visceral nociception but it lacks specificy [15]. Capsaicin, the pungent ingredient of red peppers applied topically or injected into the skin of humans or experimental animals, is known to stimulate the vanilloid receptor (Transient Receptor Potential cation channel V1 or TRPV1) located on polymodal C-fibers, but also in other tissues and initiates a complex cascade of events, including neuronal excitation and release of pro-inflammatory mediators, desensitization of receptor, and neuronal toxicity [16, 17]. The intracolonic instillation of formalin via the anus evokes differentiated behaviors, which reflect visceral nociception. All types of behavior were dose dependently inhibited by morphine, indicating that they are pain-related [18]. As shown before, BISA could significantly suppress the nociception-related behaviors against MO-induced visceral nociception [13].
Visceral pain is a prominent form of pain in many clinical conditions [15]. The results obtained in the acetic acid-, capsaicin-, formalin-, and MO-induced test models of visceral nociception are shown in Table 1. In vehicle-treated control groups of mice, acetic acid-, capsaicin-, formalin-, and MO-induced spontaneous nociception-related behaviors when compared with respective saline-treated control groups. In groups pretreated with BISA, the nociceptive behaviors induced by algogenic substances were significantly inhibited in a dose-independent manner.
In the search of the possible action mechanism of BISA, we used the acute model of visceral nociception induced by intracolonic instillation of MO, which has disease relevancy to human irritable bowel syndrome [18]. The sesquiterpene BISA could significantly suppress the pain-related behaviors against MO-induced visceral nociception, possibly regulating the functioning of primary afferent fibers. Visceral afferents express a wide range of membrane receptors (including vanilloid receptors, TRPV1) to chemical stimuli, which are involved in sensory signaling from the gut to the central nervous system [19]. When animals were pretreated with BISA and ruthenium red (a non-competitive antagonist of TRPV1) in combination, there was additive antinociception in MO test. Also, BISA 50 mg/kg failed to inhibit the visceral nociception induced by capsaicin. These observations suggest that BISA does not act as a TRPV1 agonist but may possibly induce a modulatory influence on vanilloid-receptors, which needs further clarification.
Previous studies have shown that MO can induce acute colitis and BISA has been reported to exert antiinflammatory action [13, 20]. Therefore, it is reasonable to assume that BISA suppresses the inflammatory pain. The α2-adrenoceptor agonist has been shown to induce antinociceptive effect in the experimental model of formalin-induced colitis in rats and reduce visceral hypersensitivity in clinical settings [21, 22]. Therefore, a possible involvement of α2-adrenoceptors in the antinociceptive effect of BISA in MO-model of visceral pain, using the antagonist yohimbine was investigated. Yohimbine could not reverse the antinociception produced by BISA, suggesting that α2-adrenoceptors play no role.
KATP channel openers induce cell hyperpolarization, decrease the intracellular Ca2+ level and neurotransmitter release (calcitonin gene-related peptide and substance P), that may account for antinociception [23, 24]. To verify such a possibility, we examined the effect of glibenclamide, a blocker of KATP channels on BISA antinociception. Pretreatment of mice with glibenclamide in combination with BISA showed no alteration. When mice were pretreated with L-NAME, a nitric oxide synthase inhibitor and the 5-HT3 antagonist, ondansetron, apparently a potentiated/additive response which needs further analysis through an isobolographic study.
Drugs that impair motor activity or induce sedation may give false-positive/negative results in nociceptive tests. We therefore sought to verify such effects of BISA on open-field test that detects motor incoordination [25]. The sesquiterpene failed to alter significantly the ambulation in open-field test. This result indicates that BISA exerts analgesia without causing neurological or muscular deficits.
Conclusions
Although BISA efficient diminished the acetic acid, capsaicin, formalin, and MO-evoked pain-related behaviors, its mechanism is unclear from this study and future studies are needed to verify how the sesquiterpene exerts its antinociceptive action.
References
Gonçalves JC, Alves Ade M, de Araújo AE, Cruz JS, Araújo DA: Distinct effects of carvone analogues on the isolated nerve of rats. Eur J Pharmacol 2010,645(1–3):108–112.
Sulaiman MR, Perimal EK, Zakaria ZA, Mokhtar F, Akhtar MN, Lajis NH, Israf DA: Preliminary analysis of the antinociceptive activity of zerumbone. Fitoterapia 2009,80(4):230–232.
Valério DA, Cunha TM, Arakawa NS, Lemos HP, Da Costa FB, Parada CA, Ferreira SH, Cunha FQ, Verri WA: Anti-inflammatory and analgesic effects of the sesquiterpene lactone budlein A in mice: inhibition of cytokine production-dependent mechanism. Eur J Pharmacol 2007,562(1–2):155–163.
Mendes GL, Santos AR, Malheiros A, Filho VC, Yunes RA, Calixto JB: Assessment of mechanisms involved in antinociception caused by sesquiterpene polygodial. J Pharmacol Exp Ther 2000,292(1):164–172.
Valencia E, Feria M, Díaz JG, González A, Bermejo J: Antinociceptive, anti-inflammatory and antipyretic effects of lapidin, a bicyclic sesquiterpene. Planta Med 1994,60(5):395–399.
da Silva AP, Martini MV, de Oliveira CM, Cunha S, de Carvalho JE, Ruiz AL, da Silva CC: Antitumor activity of (−)-alpha-bisabolol-based thiosemicarbazones against human tumor cell lines. Eur J Med Chem 2010,45(7):2987–2993.
Alves AM, Gonçalves JC, Cruz JS, Araújo DA: Evaluation of the sesquiterpene (−)-alpha-bisabolol as a novel peripheral nervous blocker. Neurosci Lett 2010,472(1):11–15.
Bezerra SB, Leal LK, Nogueira NA, Campos AR: Bisabolol-induced gastroprotection against acute gastric lesions: role of prostaglandins, nitric oxide, and KATP+ channels. J Med Food 2009,12(6):1403–1406.
Morales-Yuste M, Morillas-Márquez F, Martín-Sánchez J, Valero-López A, Navarro-Moll MC: Activity of (−)alpha-bisabolol against Leishmania infantum promastigotes. Phytomedicine 2010,17(3–4):279–281.
Braga PC, Dal Sasso M, Fonti E, Culici M: Antioxidant activity of bisabolol: inhibitory effects on chemiluminescence of human neutrophil bursts and cell-free systems. Pharmacology 2009,83(2):110–115.
Gomes-Carneiro MR, Dias DM, De-Oliveira AC, Paumgartten FJ: Evaluation of mutagenic and antimutagenic activities of alpha-bisabolol in the Salmonella/microsome assay. Mutat Res 2005,585(1–2):105–112.
Villegas LF, Marçalo A, Martin J, Fernández ID, Maldonado H, Vaisberg AJ, Hammond GB: (+) epi-Alpha-bisabolol [correction of bisbolol] is the wound-healing principle of Peperomia galioides: investigation of the in vivo wound-healing activity of related terpenoids. J Nat Prod 2001,64(10):1357–1359.
Leite GO, Leite LH, Sampaio Rde S, Araruna MK, de Menezes IR, da Costa JG, Campos AR: (−)-α-Bisabolol attenuates visceral nociception and inflammation in mice. Fitoterapia 2011,82(2):208–211.
Oliveira FA, Costa CL, Chaves MH, Almeida FR, Cavalcante IJ, Lima AF, Lima RC, Silva RM, Campos AR, Santos FA, Rao VS: Attenuation of capsaicin-induced acute and visceral nociceptive pain by alpha- and beta-amyrin, a triterpene mixture isolated from Protium heptaphyllum resin in mice. Life Sci 2005,77(23):2942–2952.
Lima-Júnior RC, Oliveira FA, Gurgel LA, Cavalcante IJ, Santos KA, Campos DA, Vale CA, Silva RM, Chaves MH, Rao VS, Santos FA: Attenuation of visceral nociception by alpha- and beta-amyrin, a triterpenoid mixture isolated from the resin of Protium heptaphyllum , in mice. Planta Med 2006,72(1):34–39.
Szolcsanyi J: A pharmacological approach to elucidation of the role of different nerve fiber receptor endings in mediation of pain. J Physiol 1977,73(3):251–259.
Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D: Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000,288(5464):306–313.
Laird JM, Martinez-Caro L, Garcia-Nicas E, Cervero F: A new model of visceral pain and referred hyperalgesia in the mouse. Pain 2001,92(3):335–342.
Wood JN: Recent advances in understanding molecular mechanisms of primary afferent activation. Gut 2004,53(2):ii9-ii12.
Kimball ES, Palmer JM, D’Andrea MR, Hornby PJ, Wade PR: Acute colitis induction by oil of mustard results in later development of an IBS-like accelerated upper GI transit in mice. Am J Physiol Gastrointest Liver Physiol 2005,288(6):G1266-G1273.
Miampamba M, Chery-Croze S, Chayvialle JÁ: Spinal and intestinal levels of substance P, calcitonin gene-related peptide and vasoactive intestinal polypeptide following perendoscopic injection of formalin in rat colonic wall. Neuropeptides 1992,22(2):73–80.
Blackshaw LA, Gebhart GF: The pharmacology of gastrointestinal nociceptive pathways. Curr Opin Pharmacol 2002,2(6):642–649.
Ocana M, Cendan CM, Cobos EJ, Entrena JM, Baeyens JM: Potassium channels and pain: present realities and future opportunities. Eur J Pharmacol 2004, 500: 203–219.
Lohmann AB, Welch SP: ATP-gated K+ channel openers enhance opioid antinociception: indirect evidence for the release of endogenous opioid peptides. Eur J Pharmacol 1999, 385: 119–127.
Novas ML, Wolfman C, Medina JH, De Robertis E: Proconvulsant and anxiogenic effects of n -butyl-β-carboline-3-carboxylate, on endogenous benzodiazepine binding inhibitor from brain. Pharmacol Biochem Behav 1988, 30: 331–336.
Acknowledgments
The authors are grateful to CAPES, CNPq, and Funcap for the fellowship awards and financial support by grants.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interest.
Authors’ contributions
GOL carried out the antinociceptive studies. CNF carried out the antinociceptive studies . IRAM carried out the antinociceptive studies. JGMC carried out the antinociceptive studies. ARC carried out the antinociceptive studies and draft the manuscript. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Leite, G.O., Fernandes, C.N., de Menezes, I.A. et al. Attenuation of visceral nociception by α-bisabolol in mice: investigation of mechanisms. Org Med Chem Lett 2, 18 (2012). https://doi.org/10.1186/2191-2858-2-18
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2191-2858-2-18