Abstract
Background
The classical Paal-Knorr reaction is one of the simplest and most economical methods for the synthesis of biologically important and pharmacologically useful pyrrole derivatives.
Results
Polystyrenesulfonate-catalyzed simple synthesis of substituted pyrroles following Paal-Knorr reaction has been accomplished with an excellent yield in aqueous solution. This method also produces pyrroles with multicyclic polyaromatic amines.
Conclusions
The present procedure for the synthesis of N-polyaromatic substituted pyrroles will find application in the synthesis of potent biologically active molecules.

Similar content being viewed by others
Background
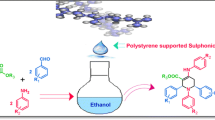
Pyrroles have demonstrated important different biological activities in several areas [1]. On this basis, diverse methods for the synthesis of substituted pyrroles are known [2]. For example, Conjugate addition reaction has been developed for the preparation of pyrroles [3]. Pyrroles can also be prepared from transition metals [4], reductive coupling reaction [5], aza-Wittig reaction [6], and other multi-step reactions [7]. However, Paal-Knorr reaction is the most reliable methods for the synthesis of pyrroles [8]. Clay-induced [9] reaction and microwave irradiation method [10] have been used for the synthesis of pyrroles. Several synthetic procedures from our laboratory have also been reported [11–16]. In this article, we report simple synthesis of substituted pyrroles using an aqueous solution of polystyrenesulfonate in ethanol. Unlike many methods, synthesis of pyrroles in aqueous solution is new and challenging (Figure 1).
Results and discussion
Synthesis of pyrroles with polyaromatic amines has not been reported. High-power microwave irradiation or considerable amounts of acids under anhydrous conditions are always necessary in the Paal-Knorr reaction. Therefore, mild reaction conditions that can overcome some of the shortcomings of previous methods are necessary. In continuation of our research on environmentally benign reaction and biological evaluation of various polyaromatic compounds as novel anticancer agents [17–22], we have investigated Paal-Knorr reaction using aqueous polystyrenesulfonate. After various experimentations, we have identified polystyrenesulfonate as a good catalyst for the preparation of pyrroles starting from amines and 1,4-diketo compound. Several amines including monocyclic, bicyclic, tricyclic, and tetracyclic aromatic amines were used. The other starting material was commercially available 2,5-hexanone (acetonylacetone) (Figure 1). At the beginning of the procedure, the diketo compound (2), the amine (1) and polystyrenesulfonate were added in ethanol. The mixture was then stirred at room temperature for 2 h-overnight depending upon the nature of the aromatic amines. The reaction mixture was basified with aqueous sodium bicarbonate solution and extracted with dichloromethane. The organic layer was then washed with brine, dried with sodium sulphate and evaporated. The yields of the products are shown in the Table 1. The less basic aromatic amines needed longer reaction time although the yields are comparable to the more basic amino compounds.
Conclusions
In conclusion, a new procedure for the synthesis of N-substituted pyrroles has been developed. Because of the simplicity of the procedure, products can be isolated very easily. The compounds reported herein will be tested against a number of cancer cells in vitro. This reaction will be applicable to the synthesis of various organic compounds of medicinal interests.
Methods
General
FT-IR spectra were registered on a Bruker IFS 55 Equinox FTIR spectrophotometer as KBr discs. 1H NMR (600 MHz) and 13C-NMR (150 MHz) spectra were obtained at room temperature with Bruker-600 equipment using TMS as internal standard and CDCl3 as solvent. Analytical grade chemicals (Sigma-Aldrich Corporation) were used throughout the project. Deionized water was used for the preparation of all aqueous solutions.
General procedure for the synthesis of pyrroles (3)
Amine (1.0 mmol), 2,5-hexanedione (1.2 mmol) and polystyrene sulfonate (18 wt. % solution in water) in water/ethanol (1:1) mixture was stirred at room temperature as specified in Table 1 and the progress of the reaction was monitored by TLC every 30 min. After completion of the reaction (Table 1) the reaction mixture was basified with aqueous sodium bicarbonate solution and extracted with dichloromethane. The organic layer was then washed with brine, dried with sodium sulphate and evaporated to isolate the pure product.
Authors' information
MB is a high school research participant; BM is an undergraduate research participant and AR is a graduate student.
References
De Leon CY, Ganem B: A new approach to porphobilinogen and its analogs. Tetrahedron 1997, 53: 7731–7752. 10.1016/S0040-4020(97)00469-9
Gilchrist TL: Synthesis of aromatic heterocycles. J Chem Soc Perkin Trans 1998, 1: 615–628.
Dieter RK, Yu H: A facile synthesis of polysubstituted pyrroles. Org Lett 2000, 2: 2283–2286. 10.1021/ol006050q
Iwasawa N, Maeyama K, Saitou M: Reactions of propargyl metallic species generated by the addition of alkynyllithiums to fischer-type carbene complexes. J Am Chem Soc 1997, 119: 1486–1487. 10.1021/ja962173n
Furstner A, Weintritt H, Hupperts A: A new titanium-mediated approach to pyrroles: First synthesis of lukianol a and lamellarin- o -dimethyl ether. J Org Chem 1995, 60: 6637–6641. 10.1021/jo00125a068
Katritzky A, Jiang J, Steel PJ: 1-Aza-1,3-bis(triphenylphosphor- ranylidene)propane: A novel: CHCH 2 N: Synthon. J Org Chem 1994, 59: 4551–4555. 10.1021/jo00095a034
Arcadi A, Rossi E: Synthesis of functionalized furans and pyrroles through annulation reactions of 4-pentynones. Tetrahedron 1998, 54: 15253–15272. 10.1016/S0040-4020(98)00953-3
Cooney JV, McEwen WE: Synthesis of substituted pyrroles by intramolecular condensation of a Wittig reagent with the carbonyl group of a tertiary amide. J Org Chem 1981, 46: 2570–2573. 10.1021/jo00325a027
Ruault P, Pilard J-F, Touaux B, Boullet FT, Hamelin J: Rapid generation of amines by microwave irradiation of ureas dispersed on clay. Synlett 1994, 6: 935–936.
Danks TN: Microwave assisted synthesis of pyrroles. Tetrahedron Lett 1999, 40: 3957–3960. 10.1016/S0040-4039(99)00620-6
Banik BK, Samajdar S, Banik I: Simple synthesis of substituted pyrroles. J Org Chem 2004, 69: 213–216. 10.1021/jo035200i
Banik BK, Banik I, Renteria M, Dasgupta SK: A straightforward highly efficient Paal-Knorr synthesis of pyrroles. Tetrahedron Lett 2005, 46: 2643–2645. 10.1016/j.tetlet.2005.02.103
Bandyopadhyay D, Mukherjee S, Banik BK: An expeditious synthesis of N -substituted pyrroles via microwave-induced iodine-catalyzed reaction under solventless conditions. Molecules 2010, 15: 2520–2525. 10.3390/molecules15042520
Andoh-Baidoo R, Danso R, Mukherjee S, Bandyopadhyay D, Banik BK: Microwave-induced N -bromosuccinimide-mediated novel synthesis of pyrroles via Paal-Knorr reaction. Heterocycl Lett 2011,1(special, July):107–109.
Abrego D, Bandyopadhyay D, Banik BK: Microwave-induced indium-catalyzed synthesis of pyrrole fused with indolinone in water. Heterocycl Lett 2011, 1: 94–95.
Rivera S, Bandyopadhyay D, Banik BK: Facile synthesis of N -substituted pyrroles via microwave-induced bismuth nitrate-catalyzed reaction under solventless conditions. Tetrahedron Lett 2009, 50: 5445–5448. 10.1016/j.tetlet.2009.06.002
Banik I, Becker FF, Banik BK: Stereoselective synthesis of β-Lactams with Polyaromatic Imines: entry to new and novel anticancer agents. J Med Chem 2003, 46: 12–15. 10.1021/jm0255825
Becker FF, Banik BK: Polycyclic aromatic compounds as anticancer agents: synthesis and biological evaluation of some chrysene derivatives. Bioorg Med Chem Lett 1998, 8: 2877–2880. 10.1016/S0960-894X(98)00520-4
Becker FF, Mukhopadhyay C, Hackfeld L, Banik I, Banik BK: Polycyclic aromatic compounds as anticancer agents: synthesis and biological evaluation of dibenzofluorene derivatives. Bioorg Med Chem 2000, 8: 2693–2699. 10.1016/S0968-0896(00)00213-3
Banik BK, Becker FF: Polycyclic aromatic compounds as anticancer agents. 4. Structure-activity relationships of chrysene and pyrene derivatives. Bioorg Med Chem 2001, 9: 593–605. 10.1016/S0968-0896(00)00297-2
Banik BK, Becker FF: Synthesis, electrophilic substitution and structure-activity relationship studies of polycyclic aromatic compounds towards the development of anticancer agents. Curr Med Chem 2001, 8: 1513–1533.
Banik BK, Becker FF, Banik I: Synthesis of anticancer β-lactams: Mechanism of action. Bioorg Med Chem 2004, 12: 2523–2528. 10.1016/j.bmc.2004.03.033
Acknowledgements
We gratefully acknowledged the funding support from the Kleberg Foundation, Texas.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
MB performed the reactions with the help of BR and AR. DB advised to use the catalyst with some initial help to run the project. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Banik, M., Ramirez, B., Reddy, A. et al. Polystyrenesulfonate-catalyzed synthesis of novel pyrroles through Paal-Knorr reaction. Org Med Chem Lett 2, 11 (2012). https://doi.org/10.1186/2191-2858-2-11
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2191-2858-2-11