Abstract
Background
Spinocerebellar ataxia type 7 (SCA7) is a genetic disorder characterized by degeneration of the motor and visual systems. Besides neural deterioration, these patients also show functional connectivity changes linked to the degenerated brain areas. However, it is not known if there are functional connectivity changes in regions not necessarily linked to the areas undergoing structural deterioration. Therefore, in this study we have explored the whole-brain functional connectivity of SCA7 patients in order to find the overall abnormal functional pattern of this disease. Twenty-six patients and age-and-gender-matched healthy controls were recruited. Whole-brain functional connectivity analysis was performed in both groups. A classification algorithm was used to find the discriminative power of the abnormal connections by classifying patients and healthy subjects.
Results
Nineteen abnormal functional connections involving cerebellar and cerebral regions were selected for the classification stage. Support vector machine classification reached 92.3% accuracy with 95% sensitivity and 89.6% specificity using a 10-fold cross-validation. Most of the selected regions were well known degenerated brain regions including cerebellar and visual cortices, but at the same time, our whole-brain connectivity analysis revealed new regions not previously reported involving temporal and prefrontal cortices.
Conclusion
Our whole-brain connectivity approach provided information that seed-based analysis missed due to its region-specific searching method. The high classification accuracy suggests that using resting state functional connectivity may be a useful biomarker in SCA 7.
Similar content being viewed by others
Background
Spinocerebellar ataxia 7 (SCA7) is an Autosomal Dominant Cerebellar Ataxia (ADCAs) caused by the expansion of the cytosine-adenine-guanine (CAG) trinucleotide in the codon region of the chromosome 3p21 encoding the protein ataxin 7 [1]. SCA7 is considered one of the rarest forms of genetic ADCAs [2]. Clinically, SCA7 is characterized by the combination of cerebellar ataxia and macular degeneration and is the only spinocerebellar ataxia that causes permanent blindness [3–5]. The brain degeneration associated with SCA7 has been relatively well documented, featuring severe neuronal loss in a broad range of cerebellar and cerebral regions [6–11]. Previous work using resting state fMRI (rsfMRI) to explore the effect of such degeneration on the pattern of functional connectivity found hyper/hypo connectivity changes between degenerate and non-degenerate areas [12].
By measuring the temporal synchronization among distant brain areas, the rsfMRI technique [13] proves to be a powerful tool for delineating the brain’s functional connectivity and has been successfully applied to study functional disruption patterns of intrinsic neural networks in various neurodegenerative disorders [14, 15]. These abnormal patterns can be helpful for improving our understanding of the pathophysiological mechanisms underlying neurodegenerative disorders and might represent a possible functional biomarker to measure the effects of putative therapeutic approaches. Using different methods, several studies have used this new information to classify brain disorders such as Alzheimer’s disease, mild cognitive impairment, major depression and autism, among others [16–20]. In recent years, there has been an increasing interest in using multivariate pattern analysis methods to distinguish patients from healthy controls by means of structural or functional brain images [16, 21–26], where the use of support vector machines (SVM) [27] arises as the most popular classifier due to its good performance and reliability against noise [18, 28].
In this study, we systematically delineated the functional changes associated with SCA7 using a whole-brain approach in 26 SCA7 patients. Then, we used the abnormal pattern of functional connectivity as a classification feature to discriminate between patients and healthy controls. Based on our previous work [12], we expected that the most discriminative functional connections would be between the cerebellum and the visual and motor cortices, however, a whole-brain approach could reveal new information about other brain regions not explored before.
Results
Abnormal functional connections
Nineteen abnormal functional connections met our threshold criteria. These included regions in the bilateral cerebellum, inferior/middle/superior temporal gyri, left precuneus, left occipital gyrus, left fusiform gyrus and inferior/middle/superior frontal gyrus (Table 1 and Table 2). The most affected functional connections in SCA7 were a hypoconnectivity between the right cerebellum crus II and the left middle frontal gyrus and a hyperconnectivity between the left superior temporal pole and the right inferior frontal gyrus in the triangular part. See Figure 1 for a representative image of selected connections. Moreover, our analysis revealed a synchrony decrease within the cerebellar cortex and between cerebellar and frontal regions, as well as a synchrony increase between temporal and several brain regions including precuneus, hippocampus and middle occipital and inferior frontal gyri (Table 3). The connection between the left middle frontal gyrus and the right superior frontal gyrus showed a negative correlation with the Scale for the Assessment and Rating of Ataxia (SARA) score and the symptoms onset (Additional file 1: Figure S1).
Highest differences between patients with SCA7 and healthy controls. Nodes represent the AAL regions involved in each of 19 abnormal functional connections. Node color indicates the anatomical location and line color indicates the abnormality. Row a) shows the connection with decreased functional connectivity, row b) shows the connections with increased functional connectivity and row c) indicates two connections showing the higher differences between SCA7 and healthy control (p < 0.0001, see Table 1). Note that for side views translucent nodes are located in the opposite hemisphere.
Classification results
Performance metrics reported high classification accuracy. After training the SVM classifier using the 19 abnormal functional connections previously selected, the classification accuracy reached 92.3% with 95% sensitivity and 89.6% specificity in a 10-fold cross validation.
Discussion
In this work we explored the whole-brain functional connectivity in a large SCA7 population and demonstrated that patients can be distinguished from healthy controls using resting state fMRI with an excellent classification accuracy and sensitivity (92.3%, 95%). Moreover, our results showed that the majority of the abnormal functional connections used for classification involved regions commonly affected by SCA7 [12], including, the cerebellar and visual cortex. However, our analysis also found changes in regions not previously reported as the bilateral inferior/middle temporal gyri, right hippocampus and the triangular/opercular parts of the inferior frontal gyrus. This new information is relevant to better understand the degenerative process of SCA7. Furthermore, changes in functional connectivity might be used as potential biomarkers to test drugs that could prevent or decrease this process in the early-stage patients.
Disruption of fronto-cerebellar network
In our previous work we found a correlation between the CAG expansion and the functional connectivity between the anterior cerebellum and the left superior frontal gyrus [12]. In that work we used a seed-based approach focused in the most degenerated regions. However, this approach restricted the search to a few specific areas. The whole-brain approach used here expands the search thorough the brain revealing a set of connections showing decreased synchrony between the cerebellum and the frontal cortex. It is well known that frontal regions are involved in motor control and planning [29–31] which also play an important role in the integration of sensory and mnemonic information, as well as regulation of intellectual function and action [32, 33]. Particularly, reduction in the metabolism of inferior/middle frontal gyri has been associated with loss of speech production, resulting in dyspraxia and dysarthria [34]. Additionally, these regions were also reported to be related to readiness and showed activity increase before the execution of self-initiated motor acts [35]. The widespread synchronicity decrease observed between the cerebellum and the frontal cortex suggests a communication disruption within the fronto-cerebellar network. Future studies should explore if the disruption of this connectivity contributes to the motor and cognitive impairments observed in these patients.
Increase in synchrony in temporal lobes
Hyperconnectivity was found in temporal regions including inferior/middle/superior gyri. Several studies have indicated the involvement of inferior temporal cortices and precuneus in object and spatial vision [36, 37] whose abnormal functioning could produce specific disruptions in visually guided movements [38]. Moreover, the hippocampus and prefrontal regions also have been related with visual working memory [39]. Besides, these areas are also involved in language processes such as comprehension of complex semantics and encoding of concrete words [40–42]. Given previous reports that an increase in functional connectivity may allow structurally damaged brain regions to remain functional [43–45], the enhanced connectivity involving the multi-sensory integration regions observed in this study may reflect a compensating effort for the visual loss and/or speech deficits associated with SCA7 [46].
Classification of functional connectivity
Multivariate classification of functional connectivity is gaining popularity due to good outcomes in discriminating between patients and healthy volunteers [21–26]. In this exploratory analysis we parcellated the brain by using the Automated Anatomical Labeling atlas (AAL) [47], dividing it into 116 regions based in the brain cytoarchitecture. This allowed us to retrieve the regional functional connectivity across the whole-brain. A possible issue resulting from this parcellation could be the size differences across regions. However, given the exploratory nature of this analysis, the good classification accuracy that we obtained, and the great acceptance of this atlas we believe that its use was appropriate. A different parcellation using isometric regions would mix signals from different anatomical regions, and would also increase the number of regions and therefore the noise/signal ratio. In the same way, several techniques have been proposed for the feature selection stage [48]. Our choice was simple but reported good classification levels (92.3% accuracy and 95% sensitivity), demonstrating that the connections selected were highly discriminating of this disease. Future work to classify between different types of spinocerebellar ataxia would require a finer parcellation and comprehensive feature selection.
Limitations
In this work we used a univariate approach as a feature selection method to select a small number of high discriminative abnormal connections across the patient’s brain. The selected subset of connections reached high classification accuracy between patients and healthy controls, proving their high discriminative power. However, this approach is limited by its own nature, comparing voxel by voxel. Different alternatives try to address this problem using multivariate approaches as principal component analysis and Independent component Analysis among others. These multivariate methods convert multidimensional vectors into statistically independent components, assuming that the number of component representing the data are less than the original dimensionality reducing the data space by discarding the components with high variance [48, 49]. However, and due to this study design and the well-defined structural degeneration in SCA7 we choose to use the univariate approach. Another limitation is the lack of significant correlation with behavioral scores. Only one connection showed a significant correlation between the functional connectivity and SARA and symptoms onset. There were no significant correlations between these variables and other connections, but there were trends. This outcome can be associated to several variables, for an instance, changes in connectivity appears early in the disease progression and are followed by structural degeneration in a slow fashion [50, 51], these difference in the velocity of the progressive degeneration could affect the correlation between variables. Taking into account that SARA score measures the motor impairments as a result of cerebellar dysfunction, and these changes develop slowly compared with the connectivity changes, the absence of a good correlation between those variables is not surprising. Something similar could have happened with the CAG expansion and the symptoms onset. In future work we will address those issues by analyzing longitudinal data of the same group of patients and by using multivariate approaches as well as behavioral/clinical data, in order to better describe changes of functional connectivity over time.
Conclusion
The use of functional connectivity measurements is a powerful tool that helps in the discrimination of neurodegenerative diseases. In this work, we demonstrated that by using whole-brain functional connectivity to classify SCA7 patients and healthy controls, a 92.3% precision accuracy was reached. At the same time our results indicate that SCA7 patients are losing synchrony within the cerebellum and between the cerebellum and different cerebral regions, like the frontal cortices. Besides, the increasing synchrony of the multi-sensory integration regions might reflect a compensatory mechanism against neurodegeneration in motor and visual systems. This outcome provides novel and relevant information about the functional changes underlying the degenerative process of SCA7 and can be helpful to better understand this rare disease. More research comparing the functional connectivity between different types of spinocerebellar ataxias will help to understand the neurodegenerative idiosyncrasies of each particular type.
Methods
Subjects
Twenty six patients with a molecular diagnosis of spinocerebellar ataxia type 7 [52] participated in this study (11 female, mean age 39.4, complete information for each patient is provided in Table 1). The control group consisted of 26 age- and gender-matched normal controls in absence of any neurological diseases or psychiatric disorders. Motor impairment of patients was tested using the Scale for the Assessment and Rating of Ataxia (SARA) [53]. All procedures were conducted in accordance with the international standards laid down in the 1964 Declaration of Helsinki carried out by the Institutional Committees on human experimentation. All participants gave written, informed consent before entering the study.
Image acquisition
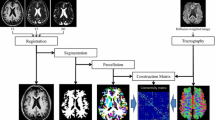
Images were acquired at the Instituto Nacional de Psiquiatria “Ramon de la Fuente Muñiz” using a 3.0 T Achieva MRI scanner (Phillips Medical Systems, Eindhoven, Holland). The anatomical acquisition consisted of a 3D T1 Fast Field-Echo sequence, with TR/TE = 8/3.7 ms, FOV 256 × 256 mm and an acquisition and reconstruction matrix of 256 × 256, resulting in an isometric resolution of 1 × 1 × 1 mm. Resting state fMRI images were collected using Echo Planar Imaging (EPI) single shot sequence with TR = 2000 ms, TE = 35 ms, and 120 whole-brain volumes with 34 slices. Final isometric resolution of rsfMRI images was 3 × 3 × 4 mm without gaps. During functional MRI acquisition, subjects in all groups were instructed to keep their eyes closed, to think about nothing in particular, and to stay awake. Five dummy scans were performed at the beginning of each functional acquisition to allow magnetization to reach a steady state.
Resting state fMRI preprocessing
RsfMRI preprocessing included brain extraction, time shifting, motion correction, spatial smoothing (6 mm full width at half maximum Gaussian kernel), linear trend removal and temporal filtering (band pass 0.01-0.08 Hz) using FSL (FMRIB, Oxford University, UK). Nuisance sources of variance including white matter, CSF, and global mean signal were removed using regression [54]. Moreover, to further control motion artifacts, volumes with a threshold of signal change < 0.5% and a frame-wise displacement < 0.5 mm was discarded [55]. After rigid alignment of rsfMRI images to its structural image for each subject, spatial normalization of rsfMRI images to MNI template was achieved using the transformation field acquired during the structural image registration step [56–58]. Automated Anatomical Labeling atlas (AAL) [47] was used to parcellate the whole brain into 116 regions (45 bilateral cortical regions, 9 bilateral cerebellar regions and 8 vermis regions).
Whole-brain functional connectivity analysis
Using MATLAB R212b (The Mathworks, Inc.), the mean time course of each AAL-defined region was obtained and Pearson’s correlation coefficient was calculated between all pairs of regions over the entire brain. A regional functional connectivity matrix was obtained (116 × 116 symmetric matrix) independently for SCA7 patients and healthy controls. Two-tailed two-group t-tests were performed for all pair-wise functional connections between SCA7 group and normal controls to detect whole-brain abnormal functional connections. Is well known that reducing the number of classification features accelerates computation and diminishes noise [24, 48, 59]. To this end, we remove the 116 diagonal values and only selected connections with a p value < 0.001 uncorrected in the lower triangular part of the symmetric matrix. In this step the multiple comparison correction is not required because this is just a feature selection method for the classification step [18]. Finally, we used each connection functional connectivity values and the behavioral data (CAG expansion, symptoms onset and SARA) to calculate the correlation between those variables.
Support vector machine classification
In order to discriminate between SCA7 group and healthy controls we used the abnormal functional connections to feed the SVM classifier (linear kernel and sequential minimal optimization). In order to test the performance of this approach we used a cross-validation technique, in which, the data is split in k-folds to test k times the classifier using different instances to train and test. Classification accuracy, sensitivity (percentage of patients correctly classified) and specificity (percentage of controls correctly classified) were calculated based on a 10 fold cross-validation [48].
Abbreviations
- SCA7:
-
Spinocerebellar Ataxia type 7
- ADCA:
-
Autosomal Dominant Cerebellar Ataxia
- CAG:
-
Cytosine-adenine-guanine
- rsfMRI:
-
Resting state functional MRI
- SVM:
-
Support Vector Machines
- EPI:
-
Echo Planar Imaging
- AAL:
-
Automated Anatomical Labeling
- MNI:
-
Montreal Neurological Institute
- SARA:
-
Scale for the Assessment and Rating of Ataxia.
References
Garden GA, La Spada AR: Molecular pathogenesis and cellular pathology of spinocerebellar ataxia type 7 neurodegeneration. Cerebellum 2008, 7: 138–149. 10.1007/s12311-008-0027-y
Hugosson T, Granse L, Ponjavic V, Andreasson S: Macular dysfunction and morphology in spinocerebellar ataxia type 7 (SCA 7). Ophthalmic Genet 2009, 30: 1–6. 10.1080/13816810802454081
Miller RC, Tewari A, Miller JA, Garbern J, Van Stavern GP: Neuro-ophthalmologic features of spinocerebellar ataxia type 7. J Neuroophthalmol 2009, 29: 180–186. 10.1097/WNO.0b013e3181b1b3f8
Paulson HL: The spinocerebellar ataxias. J Neuroophthalmol 2009, 29: 227–237. 10.1097/WNO0b013e3181b416de
Michalik A, Martin JJ, Van Broeckhoven C: Spinocerebellar ataxia type 7 associated with pigmentary retinal dystrophy. Eur J Hum Genet 2004, 12: 2–15. 10.1038/sj.ejhg.5201108
Masciullo M, Modoni A, Pomponi MG, Tartaglione T, Falsini B, Tonali P, Silvestri G: Evidence of white matter involvement in SCA 7. J Neurol 2007, 254: 536–538. 10.1007/s00415-006-0274-0
Enevoldson TP, Sanders MD, Harding AE: Autosomal dominant cerebellar ataxia with pigmentary macular dystrophy. A clinical and genetic study of eight families. Brain 1994,117(Pt 3):445–460.
Gouw LG, Digre KB, Harris CP, Haines JH, Ptacek LJ: Autosomal dominant cerebellar ataxia with retinal degeneration: clinical, neuropathologic, and genetic analysis of a large kindred. Neurology 1994, 44: 1441–1447. 10.1212/WNL.44.8.1441
Dohlinger S, Hauser TK, Borkert J, Luft AR, Schulz JB: Magnetic resonance imaging in spinocerebellar ataxias. Cerebellum 2008, 7: 204–214. 10.1007/s12311-008-0025-0
Bang OY, Lee PH, Kim SY, Kim HJ, Huh K: Pontine atrophy precedes cerebellar degeneration in spinocerebellar ataxia 7: MRI-based volumetric analysis. J Neurol Neurosurg Psychiatry 2004, 75: 1452–1456. 10.1136/jnnp.2003.029819
Alcauter S, Barrios FA, Diaz R, Fernandez-Ruiz J: Gray and white matter alterations in spinocerebellar ataxia type 7: an in vivo DTI and VBM study. NeuroImage 2011, 55: 1–7. 10.1016/j.neuroimage.2010.12.014
Hernandez-Castillo CR, Alcauter S, Galvez V, Barrios FA, Yescas P, Ochoa A, Garcia L, Diaz R, Gao W, Fernandez-Ruiz J: Disruption of visual and motor connectivity in spinocerebellar ataxia type 7. Mov Disord 2013, 28: 1708–1716. 10.1002/mds.25618
Biswal B, Yetkin FZ, Haughton VM, Hyde JS: Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 1995, 34: 537–541. 10.1002/mrm.1910340409
Greicius M: Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol 2008, 21: 424–430.
Buckner RL: Human functional connectivity: new tools, unresolved questions. Proc Natl Acad Sci U S A 2010, 107: 10769–10770. 10.1073/pnas.1005987107
Craddock RC, Holtzheimer PE 3rd, Hu XP, Mayberg HS: Disease state prediction from resting state functional connectivity. Magn Reson Med 2009, 62: 1619–1628. 10.1002/mrm.22159
Veer IM, Beckmann CF, van Tol MJ, Ferrarini L, Milles J, Veltman DJ, Aleman A, van Buchem MA, van der Wee NJ, Rombouts SA: Whole brain resting-state analysis reveals decreased functional connectivity in major depression. Front Syst Neurosci 2010, 4: 41. doi 10.3389/fnsys.2010.00041
Zeng LL, Shen H, Liu L, Wang L, Li B, Fang P, Zhou Z, Li Y, Hu D: Identifying major depression using whole-brain functional connectivity: a multivariate pattern analysis. Brain 2012, 135: 1498–1507. 10.1093/brain/aws059
Jie B, Zhang D, Gao W, Wang Q, Wee CY, Shen D: Integration of network topological and connectivity properties for neuroimaging classification. IEEE Trans Biomed Eng 2014, 61: 576–589.
Nielsen JA, Zielinski BA, Fletcher PT, Alexander AL, Lange N, Bigler ED, Lainhart JE, Anderson JS: Multisite functional connectivity MRI classification of autism: ABIDE results. Front Hum Neurosci 2013, 7: 599.
Kloppel S, Stonnington CM, Chu C, Draganski B, Scahill RI, Rohrer JD, Fox NC, Jack CR Jr, Ashburner J, Frackowiak RS: Automatic classification of MR scans in Alzheimer's disease. Brain 2008, 131: 681–689. 10.1093/brain/awm319
Desikan RS, Cabral HJ, Hess CP, Dillon WP, Glastonbury CM, Weiner MW, Schmansky NJ, Greve DN, Salat DH, Buckner RL, Fischl B, Alzheimer's Disease Neuroimaging I: Automated MRI measures identify individuals with mild cognitive impairment and Alzheimer's disease. Brain 2009, 132: 2048–2057. doi 10.1093/brain/awp123 10.1093/brain/awp123
Ardekani BA, Tabesh A, Sevy S, Robinson DG, Bilder RM, Szeszko PR: Diffusion tensor imaging reliably differentiates patients with schizophrenia from healthy volunteers. Hum Brain Mapp 2011, 32: 1–9. 10.1002/hbm.20995
Shen H, Wang L, Liu Y, Hu D: Discriminative analysis of resting-state functional connectivity patterns of schizophrenia using low dimensional embedding of fMRI. NeuroImage 2010, 49: 3110–3121. 10.1016/j.neuroimage.2009.11.011
Tang Y, Cao F, Wang L, Tan L: [Multivoxel pattern analysis of schizophrenia by resting-state functional magnetic resonance imaging]. Zhong nan da xue xue bao Yi xue ban 2013, 38: 26–30.
Liu F, Guo W, Fouche JP, Wang Y, Wang W, Ding J, Zeng L, Qiu C, Gong Q, Zhang W, Chen H: Multivariate classification of social anxiety disorder using whole brain functional connectivity. Brain Struct Funct 2013, 1–15.
Vapnik VN: An overview of statistical learning theory. IEEE Trans Neural Netw 1999, 10: 988–999. 10.1109/72.788640
Meier TB, Desphande AS, Vergun S, Nair VA, Song J, Biswal BB, Meyerand ME, Birn RM, Prabhakaran V: Support vector machine classification and characterization of age-related reorganization of functional brain networks. NeuroImage 2012, 60: 601–613. 10.1016/j.neuroimage.2011.12.052
Garavan H, Ross TJ, Stein EA: Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc Natl Acad Sci U S A 1999, 96: 8301–8306. 10.1073/pnas.96.14.8301
Konishi S, Nakajima K, Uchida I, Kikyo H, Kameyama M, Miyashita Y: Common inhibitory mechanism in human inferior prefrontal cortex revealed by event-related functional MRI. Brain 1999,122(Pt 5):981–991.
Miller EK, Cohen JD: An integrative theory of prefrontal cortex function. Annu Rev Neurosci 2001, 24: 167–202. 10.1146/annurev.neuro.24.1.167
Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW: Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci 2003, 6: 115–116. 10.1038/nn1003
MacDonald AW 3rd, Cohen JD, Stenger VA, Carter CS: Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 2000, 288: 1835–1838. 10.1126/science.288.5472.1835
Tyrrell PJ, Kartsounis LD, Frackowiak RS, Findley LJ, Rossor MN: Progressive loss of speech output and orofacial dyspraxia associated with frontal lobe hypometabolism. J Neurol Neurosurg Psychiatry 1991, 54: 351–357. 10.1136/jnnp.54.4.351
Pedersen JR, Johannsen P, Bak CK, Kofoed B, Saermark K, Gjedde A: Origin of human motor readiness field linked to left middle frontal gyrus by MEG and PET. NeuroImage 1998, 8: 214–220. 10.1006/nimg.1998.0362
Takayama Y, Sugishita M, Fukuyama H, Akiguchi I: Localization in impaired spatial vision. Clin Neurol Neurosurg 1995, 97: 249–252. 10.1016/0303-8467(95)00040-Q
Courtney SM, Ungerleider LG, Keil K, Haxby JV: Object and spatial visual working memory activate separate neural systems in human cortex. Cereb Cortex 1996, 6: 39–49. 10.1093/cercor/6.1.39
Prado J, Clavagnier S, Otzenberger H, Scheiber C, Kennedy H, Perenin MT: Two cortical systems for reaching in central and peripheral vision. Neuron 2005, 48: 849–858. 10.1016/j.neuron.2005.10.010
Ranganath C, Cohen MX, Dam C, D'Esposito M: Inferior temporal, prefrontal, and hippocampal contributions to visual working memory maintenance and associative memory retrieval. J Neurosci 2004, 24: 3917–3925. 10.1523/JNEUROSCI.5053-03.2004
Jessen F, Heun R, Erb M, Granath DO, Klose U, Papassotiropoulos A, Grodd W: The concreteness effect: evidence for dual coding and context availability. Brain Lang 2000, 74: 103–112. 10.1006/brln.2000.2340
Just MA, Newman SD, Keller TA, McEleney A, Carpenter PA: Imagery in sentence comprehension: an fMRI study. NeuroImage 2004, 21: 112–124. 10.1016/j.neuroimage.2003.08.042
Wallentin M, Ostergaard S, Lund TE, Ostergaard L, Roepstorff A: Concrete spatial language: see what I mean? Brain Lang 2005, 92: 221–233. 10.1016/j.bandl.2004.06.106
Rytsar R, Fornari E, Frackowiak RS, Ghika JA, Knyazeva MG: Inhibition in early Alzheimer's disease: an fMRI-based study of effective connectivity. NeuroImage 2011, 57: 1131–1139. 10.1016/j.neuroimage.2011.05.029
Qiu A, Tuan TA, Woon PS, Abdul-Rahman MF, Graham S, Sim K: Hippocampal-cortical structural connectivity disruptions in schizophrenia: an integrated perspective from hippocampal shape, cortical thickness, and integrity of white matter bundles. NeuroImage 2010, 52: 1181–1189. 10.1016/j.neuroimage.2010.05.046
Liang P, Wang Z, Yang Y, Jia X, Li K: Functional disconnection and compensation in mild cognitive impairment: evidence from DLPFC connectivity using resting-state fMRI. PloS one 2011, 6: e22153. 10.1371/journal.pone.0022153
Horton LC, Frosch MP, Vangel MG, Weigel-DiFranco C, Berson EL, Schmahmann JD: Spinocerebellar ataxia type 7: clinical course, phenotype-genotype correlations, and neuropathology. Cerebellum 2013, 12: 176–193. 10.1007/s12311-012-0412-4
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M: Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage 2002, 15: 273–289. 10.1006/nimg.2001.0978
Pereira F, Mitchell T, Botvinick M: Machine learning classifiers and fMRI: a tutorial overview. NeuroImage 2009, 45: S199-S209. 10.1016/j.neuroimage.2008.11.007
Afshin-Pour B, Grady C, Strother S: Evaluation of spatio-temporal decomposition techniques for group analysis of fMRI resting state data sets. NeuroImage 2014, 87: 363–382.
Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD: Neurodegenerative diseases target large-scale human brain networks. Neuron 2009, 62: 42–52. 10.1016/j.neuron.2009.03.024
Zhou J, Gennatas ED, Kramer JH, Miller BL, Seeley WW: Predicting regional neurodegeneration from the healthy brain functional connectome. Neuron 2012, 73: 1216–1227. 10.1016/j.neuron.2012.03.004
Magana JJ, Gomez R, Maldonado-Rodriguez M, Velazquez-Perez L, Tapia-Guerrero YS, Cortes H, Leyva-Garcia N, Hernandez-Hernandez O, Cisneros B: Origin of the spinocerebellar ataxia type 7 gene mutation in mexican population. Cerebellum 2013, 12: 902–905. 10.1007/s12311-013-0505-8
Weyer A, Abele M, Schmitz-Hubsch T, Schoch B, Frings M, Timmann D, Klockgether T: Reliability and validity of the scale for the assessment and rating of ataxia: a study in 64 ataxia patients. Mov Disord 2007, 22: 1633–1637. 10.1002/mds.21544
Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME: Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci U S A 2006, 103: 10046–10051. 10.1073/pnas.0604187103
Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE: Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage 2012, 59: 2142–2154. 10.1016/j.neuroimage.2011.10.018
Jenkinson M, Smith S: A global optimisation method for robust affine registration of brain images. Med Image Anal 2001, 5: 143–156. 10.1016/S1361-8415(01)00036-6
Jenkinson M, Bannister P, Brady M, Smith S: Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage 2002, 17: 825–841. 10.1006/nimg.2002.1132
Rueckert D, Sonoda LI, Hayes C, Hill DL, Leach MO, Hawkes DJ: Nonrigid registration using free-form deformations: application to breast MR images. IEEE Trans Med Imaging 1999, 18: 712–721. 10.1109/42.796284
Dosenbach NU, Nardos B, Cohen AL, Fair DA, Power JD, Church JA, Nelson SM, Wig GS, Vogel AC, Lessov-Schlaggar CN, Barnes KA, Dubis JW, Feczko E, Coalson RS, Pruett JR, Barch DM, Petersen SE, Schlaggar BL: Prediction of individual brain maturity using fMRI. Science 2010, 329: 1358–1361. doi DOI 10.1126/science.1194144 10.1126/science.1194144
Acknowledgements
We thank the patients and their families as well as control volunteers for their participation. This research was supported by the program UNAM-DGAPA-PAPIIT IN221413-3 to JFR.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
CRHC participated in the design of the study, performed the data analysis and wrote the manuscript; VG participated in the coordination and the study and applied the behavioral test to patients; CMV participated in the design and coordination of the study; JFR participated in the design and coordination of the study and helped draft the manuscript. All authors read and approved the final manuscript.
Electronic supplementary material
40673_2013_2_MOESM1_ESM.pdf
Additional file 1: Figure S1: Significant correlations between functional connectivity and behavioral scores in the connections of left middle frontal gyrus and the right superior frontal gyrus. (PDF 97 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Hernandez-Castillo, C.R., Galvez, V., Morgado-Valle, C. et al. Whole-brain connectivity analysis and classification of spinocerebellar ataxia type 7 by functional MRI. cerebellum ataxias 1, 2 (2014). https://doi.org/10.1186/2053-8871-1-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2053-8871-1-2