Abstract
River Gomti, a tributary of river Ganga in northern India, is being polluted due to indiscriminate disposal of domestic sewage and industrial wastes that contain genotoxic chemicals. The study was conducted to evaluate the genotoxic potential of polluted water of river Gomti in two fish species, namely Channa punctatus and Mystus vittatus. The fishes were exposed in situ in nylon cages to the polluted water of river Gomti fixed near a distillery outlet located in Lucknow. The induction of DNA damage and micronuclei were determined in blood erythrocytes using comet assay and micronucleus test, respectively. The induction in micronuclei frequencies and DNA damage were found to be significantly elevated (p < 0.01) in exposed specimens after 3 days post-exposure as compared to the control, i.e. from laboratory-acclimatized fish specimens. The comparison of DNA damage between the two species indicated that C. punctatus is more sensitive to aquatic pollutants. Thus, this fish could be used as a bio-indicator of genotoxicity for bio-monitoring of water bodies. The results further revealed that the river Gomti is being contaminated with potential genotoxic and mutagenic chemicals produced from industrial and domestic activities; therefore, immediate measures are needed to reduce the inflow of pollutants in the river.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Majority of river systems are polluted due to admixture of domestic waste, industrial effluents, and many other pollutants that are adversely affecting the human health and the ecosystem of the water bodies. The major sources of contamination include waste from paper, PVC plastic, pigments and ceramic industries, battery, mining and smoldering units, and other modern industries ([Gupta et al. 2003]). The pollutant enters into the aquatic bodies through sewage and with the runoff from agricultural wastes ([Cherian and Goyer 1989]). Many of these polluting agents contain a number of chemicals that are highly persistent and have mutagenic and/or clastogenic properties ([Waters et al. 1991, 1999]). Several studies have shown that a wide range of chemical pollutants in aquatic ecosystem affect essential physiological functions in various aquatic organisms and causes adverse effects at cellular and molecular levels ([Mishra and Mohanty 2009, Li et al. 2010, 2011]).
In situ toxicity tests have been increasingly used with cladocerans, amphipods, and fishes as an effective tool in monitoring environmental quality. Furthermore, exposing test organisms in situ may reduce the uncertainties of extrapolation from the laboratory ([Hoffman et al. 1995]). In situ standard toxicity assessment in various aquatic organisms has been used extensively in river environments to assess water and sediment toxicity, and this method is recognized as a useful tool for assessing non-point source contaminants ([Chappie and Burton 1997, Tucker and Burton 1999]). According to[Burton (1991]), this technique is relatively simple and eliminates possible errors induced by the sampling and manipulation of sediments in the laboratory. Further, in situ tests with zooplankton species constitute a rapid and efficient way of evaluating environmental contamination and validating laboratory experiments ([Pereira et al. 1999]).
Genotoxic pollution of aquatic ecosystem refers to the introduction of contaminants with mutagenic, teratogenic, and/or carcinogenic potentials into its principal media and genome of the resident organisms ([Fagr et al. 2008]). Genotoxicity is a deleterious action, which affects a cell's genetic material affecting its integrity ([Smith 1996]). Genotoxicants include certain chemical compounds like heavy metals ([Matsumoto et al. 2005, Igwilo et al. 2006]), microbial toxins ([Smith 1996]), and polycyclic aromatic hydrocarbons ([Fernandez and L' Haridon 1992, Germain et al. 1993]). These genotoxicants have been reported to cause mutations because they form strong covalent bonds with the DNA, resulting in the formation of DNA adducts preventing accurate replication ([Hartwell et al. 2000]).
Since there is a growing concern over the presence of genotoxins in the aquatic environment, the development of sensitive biomarkers for detection of genotoxic effects in aquatic organisms has gained importance ([Hayashi et al. 1998]). The genotoxic effects of environmental pollutants can be monitored using a broad range of both in vitro and in vivo biomarker assays, but the comet assay is gaining popularity over others since its advantages include sensitivity for detecting low levels of DNA damage ([Gedic et al. 1992]) and the short time needed to complete a study. The micronucleus (MN) assay is another useful and popular technique for showing clastogenic and aneugenic effects ([Norppa and Falck 2003]) and has been extensively used in situ ([Al-Sabti and Metcalfe 1995]). Several studies have shown that the MN test and comet assay (CA) are the two sensitive, rapid, and extensively used methods in the detection of mutagenicity and genotoxicity of chemicals and xenobiotics under field and laboratory conditions ([Jha 2004, Pandey et al. 2006, Nagpure et al. 2008, Ventura et al. 2008, Ali et al. 2009, Kumar et al. 2010, Nwani et al. 2010]).
Fish serve as useful genetic model for the evaluation of pollution in aquatic ecosystems ([Mitchell and Kennedy 1992]) and can play significant roles in assessing potential risk associated with contamination in aquatic environment since they are directly exposed to chemicals and mutagens resulting from agricultural production via surface runoff or indirectly through the food chain of the ecosystem ([Cavas and Ergene-Gözükara 2005]). Therefore, the use of fish biomarkers as indices of the effects of pollution are of increasing importance and can permit early detection of aquatic environmental problems ([Lopez-Barea 1996, Van Der Oost et al. 2003]).
Gomti River is one of the major tributaries of river Ganga in northern India; its ecosystem is being polluted seriously due to industrialization and urbanization occurring during the past few decades ([Singh et al. 2005, Sarkar et al. 2010]). The contaminants in the river are increasing day by day, thereby causing genotoxicity in the fishes and adversely affecting their diversity; hence, they are of significance. Keeping view of the facts, the present study was conducted with the aim to investigate the genotoxic effect of polluted river water, containing various types of agricultural and industrial wastes, based on the induction of micronuclei and DNA damage in the blood erythrocytes using MN and CA tests in two native fish species, namely Channa punctatus (Bloch) and Mystus vittatus (Bloch), exposed to the most polluted river water.
Methods
Experimental site
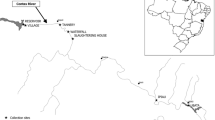
The river Gomti, a tributary of river Ganga, originates from Gomat Taal, near Madho Tanda, Pilibhit district, Uttar Pradesh (UP). It flows nearly 800 km through UP and meets the river Ganga near Saidpur in the Ghazipur district of UP. In the middle of its course, the river passes through the Lucknow urban area. The river receives a large amount of domestic and industrial pollutants as it flows through the highly populous areas of Uttar Pradesh. Various industries, namely distillery, sugar units, carpet, aircraft accessories, and yeast manufacturing, located on the riverbank ultimately release pollutants like suspended solids, oil and grease, dyes, cyanide, chromium, nickel, etc. into the river through different channels. The site selected for the study, i.e. Daliganj (N36°53'2210", E080°54'0270") in Lucknow (Figure1), receives maximum pollutants, as per the UP Pollution Control Board report (personal communication), through different channels.
Water quality assessment
River water was sampled at the 1st, 3rd, 6th, 10th, and 15th days of fish exposure. The estimation of physicochemical parameters of the water, namely temperature, pH, dissolved oxygen, conductivity, and total hardness, were analyzed by standard methods ([APHA et al. 2005]). In addition, concentrations of heavy metals cadmium and mercury, and pesticide endosulfan were also estimated.
Experimental animals and in situ exposure conditions
For in situ exposure, the specimens of freshwater air-breathing murrel fish C. punctatus (Bloch; Family: Channidae; Order: Perciformes) and freshwater catfish M. vittatus (Bloch; Family: Bagridae; Order: Siluriformes) were caught from nearby ponds with the help of local fishermen. After transportation to the laboratory, the specimens were given prophylactic treatment with 0.05% KMnO4 solution for 2 min to avoid any dermal infection and were properly maintained and acclimatized well under laboratory conditions for a period of more than a month. During acclimatization, they were fed with fish feed and boiled eggs at 5% of their body weight. The specimens of C. punctatus had an average wet weight and length of 20.46 g (range 12 to25) and 13.53 cm (range 11 to 16), respectively, whereas the values for M. vittatus were 15.59 g (range 11 to 19) and 10.41 cm (range 9 to 13).
After acclimatization, the specimens of similar length and weight were transported to selected polluted site, randomly placed in nylon cages, and transplanted in the polluted river water. The blood samplings were done at day 1, 3, 6, 10, and 15 post-exposure for MN test and CA. Four specimens per species were sampled at each sampling time. Two replicate slides per specimen were prepared for MN test and CA. Similarly, four acclimatized specimens of each species, along with in situ exposure, were used for MN test and CA, and average data of all exposure duration were considered as baseline values for statistical comparisons.
Blood collection
About 50 μl of peripheral blood was collected from the caudal vein of the specimens on each sampling time using heparinized 1-ml disposable syringes and transferred to 1-ml eppendorf tube containing 450 μl of chilled Ca++- and Mg++- free phosphate buffer saline. The tube was placed on ice for its further use in MN test, viability test, and for CA. After blood collection, the specimens were transferred to separate water tanks.
Micronucleus test
A very thin smear of peripheral blood was made onto the pre-cleaned slide, separately from each blood samples. After fixation in methanol for 20 min, the slides were allowed to air-dry and then stained with 6% Giemsa solution for 25 min. A total of 2,000 erythrocytes from each slides were examined at × 100 magnification under a Leica microscope equipped with a digital camera. Small, non-refractive, circular or ovoid chromatin bodies displaying the same staining and focusing pattern as the main nucleus were scored as micronuclei ([Al-Sabti and Metcalfe 1995]). The MN frequency (%) was calculated as
Comet assay
DNA damage including single-strand breaks and alkali-labile sites was detected by comet assay as a three-layer procedure ([Singh et al. 1988]) using conventional microscopic slides ([Klaude et al. 1996]). Cell viability test for each blood sample was carried out using the trypan blue extrusion test ([Anderson et al. 1994, Henderson et al. 1998]). The samples showing >85% cell viability were further processed for comet assay. In brief, 20 μl of blood sample was mixed with 80 μl of 0.5% low-melting-point agarose (LMPA) prepared in Ca++- and Mg++-free phosphate buffered saline (PBS) and layered on one end of a frosted glass slide, which was pre-coated with a layer of 200 μl of 1% normal agarose in PBS. The slide was again covered with a third layer of 100 μl of 0.5% LMPA and covered with cover slip. After gel solidification, the cover slip was removed, and the slide was immersed in a cold lysing solution (2.5 M NaCl, 100 mM Na2EDTA, 10 mM Tris, pH 10, and 10% DMSO and 1% Triton X-100 added fresh) and refrigerated at 4°C for 2 h. After lysis, the slide was placed on a horizontal electrophoretic chamber. The chamber was filled with fresh electrophoresis buffer (1 mM Na EDTA, 300 mM NaOH, 0.2% DMSO, and pH 13.5) to a level of approximately 0.25 cm above the slides. The slides were left in the buffer solution for 20 min for unwinding. Electrophoresis was performed using the same solution at 18 V and 300 mA for 18 min. The slides were then neutralized gently with 0.4 M Tris buffer at pH 7.5 and stained with 75-μl ethidium bromide (concentration, 20 μg/ml). Two slides per specimen were prepared, and 25 cells per slide, i.e., 200 cells per exposed duration, were scored randomly and analyzed using an image analysis system (Komet–5.5 Kinetic Imaging Ltd., Nottingham, UK) attached to a florescent microscope (Leica) equipped with appropriate filters. The parameter selected for quantification of DNA damage was percentage of tail DNA (=100 − % head DNA) as determined by the software.
Statistical analysis
The mean percentage of micronucleus frequencies was compared among species and sampling times using Mann–Whitney test. One-way analysis of variance was applied to compare the difference in mean percentage of tail DNA among the species and sampling times. p values less than 0.01 were considered statistically significant.
Results
Physicochemical parameters of river water
The physicochemical characteristics of the test water are presented in Table1. The average values of water temperature and pH were 21.8°C and 8.5, respectively. The average values of dissolved oxygen concentration and conductivity were 7.6 mg/l and 441 μM/cm, correspondingly. The mean total alkalinity and total hardness were 315 and 205 mg/l as CaCO3, respectively, during the experimental period. The estimated quantities of metals, like mercury and cadmium, and pesticide, like endosulfan and chloride, were 1.19, 3.00, 0.006, and 37.82 μg/l, respectively, observed at a level higher than the permissible limits of the BIS guideline ([Indian Standard 1982]) for a non-polluted river.
Induction of micronuclei
The observations of micronucleus present in erythrocytes of C. punctatus and M. vittatus are summarized in Table2. A significant (p < 0.01) higher micronuclei induction was observed in exposed specimens of both species from day 3 onwards as compared to the baseline values and frequency of micronuclei on day 1. A time-dependent gradual increase in the micronucleus frequency was observed in both species, whereas the induction in micronuclei in exposed specimens from day 3 to 15 was nonsignificant (Figure2). The maximum frequency of MN in the subject species was observed on day 15, with comparatively higher micronuclei induction in C. punctatus than in M. vittatus.
DNA damage
The DNA damage measured as percentage of tail DNA in the erythrocytes of the subject species is presented in Table3, which indicated that both fish species exposed to polluted river water exhibited significantly higher DNA damage (p < 0.01) as compared to baseline values. A time-dependent gradual and linear increase in DNA damage was found in both species with the advancement of the experiment. Significant (p < 0.01) increase in DNA damage was observed among all sampling intervals in C. punctatus, whereas nonsignificant increase was observed in M. vittatus from day 3 to 15. Among the two species, significant variation (p < 0.01) in DNA damage was also observed on the 1st, 3rd, and 15th day post-exposure (Figure3).
Discussion
In recent years, increasing concern about genotoxicity due to pollution in land and water has led to development of many mutagenesis test systems in bacteria, yeast, plants, and animals including fishes. Fishes are reported to be suitable organisms for ecotoxicological studies because they play different roles in the heterotrophic web, undergo bioaccumulation, respond to mutagens at low concentration such as environmental pollutants, and activate xenobiotics through the cytochrome P-450-dependent oxidative metabolism like mammals ([Goksoyr et al. 1991, Cavas and Ergene-Gözükara 2005]). In situ investigation of mutagenic pollution effects in terms of environmental bio-monitoring has been reported by many researchers ([De Flora et al. 1993, Sanchez-Galan et al. 2001]). Furthermore, ([Hayashi et al. 1998]) evaluated the monitoring systems that use aquatic organisms to assess the genotoxicity of water in the field and in the laboratory. In situ cage studies or transplantation provide precise information of the place and duration of exposure, but it may be difficult to establish in surveys of native populations ([Oikari 2006, Pellacani et al. 2006, Klobučar et al. 2010]). Transplanted animals used from the same unpolluted source can also potentially reduce inter-individual variability and decrease the influence of adaptive mechanisms ([Klobučar et al. 2003, Pellacani et al. 2006]). ([Nagpure et al. 2008]) have also reported in situ bio-monitoring studies through genotoxicity assessment in some carp species that provided important information on the increasing pollution status of the river waters.
In a field study, micronucleus assay was shown to be applicable to micronucleus-inducing agents in frogs ([Saleh and Zeytinoğlu 2001]). The micronucleus test is simple and successful among many mutagenesis assays, which are both dependent on any karyotypic characteristics and less time consuming ([Heddle et al. 1991]). According to ([Hartwell et al. 2000]) and ([Fagr et al. 2008]), the incidence of micronuclei in fish and other aquatic life serves as an index of these types of damage, and counting of micronuclei is much faster and less technically demanding than scoring of chromosomal aberrations. The comet assay has been considered as a sensitive, rapid, and reliable method of quantitatively measuring DNA damage in eukaryotic and prokaryotic cells ([Bajpayee et al. 2005]). It is increasingly being used in testing of substances such as industrial chemicals, biocides, agrochemical, food additives, and pharmaceuticals for genotoxicity testing ([Brendler-Schwaab et al. 2005]). Thus, for these reasons, comet assay and micronuclei test in fish erythrocyte seem to be promising tests in environmental genotoxicity and mutagenesis investigations. ([Andrade et al. 2004]).
According to our findings, duration-dependent linear increase in DNA damage was found in both species. The DNA damage detected in this study could have originated from DNA single-strand breaks, DNA double-strand breaks, DNA adduct formations, and DNA-DNA and DNA-protein cross-links ([Mitchelmore and Chipman 1998]) resulting from the interactions with the toxic substance in polluted water of river Gomti with DNA ([Fairbrairn et al. 1995]). Furthermore, environmental contaminants are known to modulate antioxidant defensive systems and to cause oxidative damage in aquatic organisms by the production of reactive oxygen species ([Risso-de Facerney et al. 2001, Liu et al. 2006]). A significant difference observed between C. punctatus and M. vittatus on days 1, 3, and 15 and between baseline values of DNA damage indicated that some other factors like species, age, and sex may have some influence to cause these minor variations. Similar variation in genotoxicity patterns was documented for other fish species such as cyprinids ([Smith 1990, Lemos et al. 2001, Vigano et al. 2002]). The significant increase in DNA damage at different exposure durations shows that C. punctatus is more sensitive for assessing the water quality in aquatic ecosystem.
The present investigation revealed significant increase in micronucleus frequencies observed after 3 days post-exposure in comparison to baseline values of micronuclei in unexposed fishes. The maximal MN was observed on day 15 in both fish species. A nonsignificant effect of micronucleus induction was observed between the two fish species. The present study indicated that C. punctatus and M. vittatus are sensitive fish species and could be successfully used for determination of the effect of pollutants present in river water. Similarly, they could also be used in periodical bio-monitoring and river conservation programs by in situ exposure. ([Nagpure et al. 2008]) reported similar observations in commonly available fishes, namely Labeo rohita and Puntius puntius, of the river Gomti. These findings are also in accordance with the studies conducted for basal-level range-finding for other fish species, as reviewed by genotoxicity studies using fish ([Al-Sabti and Metcalfe 1995]). The significantly higher DNA damage, as well as induction in MN frequencies in both fish species, was observed 3 days post-exposure as compared to baseline frequencies, which indicated that a minimum of 3-day, or a week, in situ exposure of fishes could be used to evaluate the status of a body of water for bio-monitoring purpose.
Our results are also supported by some other investigators' findings that assess the in situ and ex situ genotoxic and mutagenic potential of various toxic chemicals including heavy metals and pesticides in various fish species ([Mitchelmore and Chipman 1998, Pandrangi et al. 1995, Sumathi et al. 2001, Lee and Steinert 2003]). The results of this study emphasized the importance of comet assay and MN test and suggest its broader application as an early biological marker of fish exposure to genotoxic and clastogenic pollutants in aquatic environments.
Conclusions
The results of the present investigation on the genotoxic and mutagenic potential of the polluted water of river Gomti suggested a serious concern about its potential danger to aquatic organisms, especially to fish, and indirectly to human beings. However, further studies are needed to explore the biological consequences of DNA damage in aquatic organisms due to deleterious effects of polluted water of river Gomti and to formulate future strategies for safeguarding aquatic organisms and environment.
References
Ali D, Nagpure NS, Kumar S, Kumar R, Kushwaha B, Lakra WS: Assessment of genotoxic and mutagenic effects of chlorpyrifos in freshwater fish Channa punctatus (Bloch) using micronucleus assay and alkaline single-cell gel electrophoresis. Food Chem Toxicol 2009, 47: 650–656. 10.1016/j.fct.2008.12.021
Al-Sabti K, Metcalfe CD: Fish micronuclei for assessing genotoxicity in water. Mutat Res 1995, 343: 121–135. 10.1016/0165-1218(95)90078-0
Anderson D, Yu TW, Phillips BJ, Schmezer P: The effect of various antioxidants and other modifying agents on oxygen-radical-generated DNA damage in human lymphocytes in the Comet assay. Mutat Res 1994, 307: 261–271. 10.1016/0027-5107(94)90300-X
Andrade VM, Silva J, Silva FR, Heuser VD, Dias JF, Yoneama ML, Freitas TR: Fish as bioindicators to assess the effects of pollution in two southern Brazilian rivers using the Comet assay and micronucleus test. Environ Mol Mutagen 2004, 44: 459–468. 10.1002/em.20070
APHA, AWWA, WPCF: Standard methods for the examination of water and wastewater. 21st edition. American Publication of Health Association, Washington, DC; 2005.
Bajpayee M, Pandey AK, Parmar D, Marthur N, Seth PK, Dhawan A: Comet assay responses in human lymphocytes are not influenced by the menstrual cycle: a case study in healthy Indian females. Mutat Res 2005, 565: 163–172. 10.1016/j.mrgentox.2004.10.008
Brendler-Schwaab S, Hartman A, Pfuhler S, Speit G: The in vivo comet assay: use and status in genotoxicity testing. Mutagenesis 2005, 20: 245–254. 10.1093/mutage/gei033
Burton GA Jr: Assessing the toxicity of freshwater sediments. Environ Toxicol Chem 1991, 10: 1585–1627. 10.1002/etc.5620101204
Cavas T, Ergene-Gözükara S: Micronucleus test in fish cells: a bioassay for in situ monitoring of genotoxic pollution in the marine environment. Environ Mol Mutagen 2005, 46: 64–70. 10.1002/em.20130
Chappie DJ, Burton GA: Optimization of in situ bioassays with Hyalella azteca and Chironomus tentans. Environ Toxicol Chem 1997, 16: 559–564.
Cherian MG, Goyer AA: Cadmium toxicity. Comments Toxicol 1989, 3: 191–206.
De Flora S, Vigano L, D'Agostini F, Camoirano A, Bagnasco M, Bennicelli C, Melodia F, Arillo A: Multiple genotoxicity biomarkers in fish exposed in situ to polluted river water. Mutat Res 1993, 319: 167–177. 10.1016/0165-1218(93)90076-P
Fagr A, El-shehawi AM, Seehy MA: Micronucleus test in fish genome: a sensitive monitor for aquatic pollution. Afr J Biotech 2008,7(5):606–612.
Fairbrairn DW, Olive PL, O'Neill KL: The comet assay: a comprehensive review. Mutat Res 1995, 399: 37–59.
Fernandez M, L'Haridon J: Influence of lighting conditions on toxicity and genotoxicity of various PAH in the newt in vivo. Mutat Res 1992, 298: 31–41. 10.1016/0165-1218(92)90026-V
Gedic CM, Ewen SWB, Collins AR: Single cell gel electrophoresis applied to analysis of UV-C damage and its repair in human cells. Int J Radiat Biol 1992, 62: 313–320. 10.1080/09553009214552161
Germain A, Perron F, Van Coillie R: PAHs in the environment: fate, ecotoxicity and regulations. Environment Canada, Conservation and Protection, Quebec Region, Montreal, Que; 1993.
Goksoyr A, Anderson T, Buhler DR, Stegeman JJ, Williams DB, Forlin L: Immunochemical cross reactivity of β-naphthoflavone inducible cytochrome P450 (P450 IAI) in lives microsomes from different fish species and rat. Fish Physiol Chem 1991, 9: 1–13.
Gupta DK, Rai UN, Singh A, Inouhe M: Cadmium accumulation and toxicity in Cicer arietinum L. Poll Res 2003, 22: 457–463.
Hartwell LH, Hood L, Goldberg ML, Reynolds AE, Silver LM, Veres RC: Genetics: from genes to genomes. McGraw Hill Higher Education; 2000:70–98. 144–169, 179–182, 341–351 144–169, 179–182, 341–351
Hayashi M, Ueda T, Uyeno L, Wada K, Kinae N, Saotome K, Tanaka N, Takai A, Sasaki YF, Asano N, Sifuni Y, Ojimma T: Development of genotoxicity assay systems that use aquatic organisms. Mutat Res 1998, 399: 125–133. 10.1016/S0027-5107(97)00251-0
Heddle JA, Cimino MC, Hayashi M, Romagna F, Shelby MD, Tucker JD, Vanparys P, MacGregor JT: Micronuclei as an index of cytogenetic damage: past, present and future. Environ Mol Mutagen 1991, 18: 277–291. 10.1002/em.2850180414
Henderson L, Wolfreys A, Fedyk J, Boumer C, Windebank S: The ability of the comet assay to discriminate between genotoxins and cytotoxins. Mutagenesis 1998, 13: 89–94. 10.1093/mutage/13.1.89
Hoffman DJ, Rattner BA, JR Burton GA, JR Cairns J: Handbook of ecotoxicology. Lewis, Boca Raton; 1995:755.
Igwilo IO, Afonne OJ, Maduabuchi UJ, Orisakwe OE: Toxicological study of the Anambra River in Otuocha, Anambra State, Nigeria. Arch Environ Occup Health 2006,61(5):205–208. 10.3200/AEOH.61.5.205-208
ISI: Tolerance limits for inland surface waters subject to pollution (second revision). IS, New Delhi; 1982:2296–1982.
Jha AN: Genotoxicological studies in aquatic organisms: an overview. Mutat Res 2004,552(494):1–17.
Klaude M, Eriksson S, Nygren J, Ahnstrom G: The comet assay: mechanisms and technical considerations. Mutat Res 1996, 363: 89–96. 10.1016/0921-8777(95)00063-1
Klobučar GIV, Pavlica M, Erben R, Papeš D: Application of the micronucleus and comet assays to mussel Dreissena polymorpha haemocytes for genotoxicity monitoring of freshwater environments. Aquat Toxicol 2003, 64: 15–23. 10.1016/S0166-445X(03)00009-2
Klobučar GIV, Štambuk A, Pavlica Mirjana Sertić PM, Hackenberger BK, Hylland K: Genotoxicity monitoring of freshwater environments using caged carp (Cyprinus carpio). Ecotoxicology 2010, 19: 77–84. 10.1007/s10646-009-0390-6
Kumar R, Nagpure NS, Kushwaha B, Srivastava SK, Lakra WS: Investigation of the genotoxicity of malathion to freshwater teleost fish Channa punctatus (Bloch) using the micronucleus test and comet assay. Arch Environ Contam Toxicol 2010,58(1):123–130. 10.1007/s00244-009-9354-3
Lee RF, Steinert S: Use of the single cell gel electrophoresis/comet assay for detecting DNA damage in aquatic (marine and freshwater) animals. Mutat Res 2003, 544: 43–64. 10.1016/S1383-5742(03)00017-6
Lemos D, Rodel CT, Rödel PM, Terra NR, Erdtmann B: Evaluation of basal micronucleus frequency and hexavalent chromium effects in fish erythrocytes. Environ Toxicol Chem 2001, 20: 1320–1324.
Li ZH, Zlabek V, Velisek J, Grabic R, Machova J, Randak T: Enzymatic alterations and RNA/DNA ratio in intestine of rainbow trout, Oncorhynchus mykiss, induced by chronic exposure to carbamazepine. Ecotoxicology 2010, 19: 872–878. 10.1007/s10646-010-0468-1
Li ZH, Zlabek V, Turek J, Velisek J, Pulkrabova J, Kolarova J, Sudova E, Berankova P, Hradkova P, Hajslova J, Randak T: Evaluating environmental impact of STPs situated on streams in the Czech Republic: an integrated approach to biomonitoring the aquatic environment. Water Res 2011, 45: 1403–1413. 10.1016/j.watres.2010.10.032
Liu Y, Zhang Y, Liu J, Huang D: The role of reactive oxygen species in the herbicide acetachlor-induced DNA damage on Bufo raddei tadpole liver. Aquat Toxicol 2006, 78: 21–26. 10.1016/j.aquatox.2006.01.016
Lopez-Barea J: Biomarkers to detect environmental pollution. Toxicol Lett 1996, 88: 77–79.
Matsumoto ST, Janaina R, Mario SM, Maria AM: Evaluation of the genotoxic potential due to the action of an effluent contaminated with chromium, by the comet assay in CHO-K1 cultures. Caryologia 2005,58(1):40–46.
Mishra AK, Mohanty B: Effect of hexavalent chromium exposure on the pituitary-interrenal axis of a teleost, Channa punctatus (Bloch). Chemosphere 2009, 76: 982–988. 10.1016/j.chemosphere.2009.04.012
Mitchell S, Kennedy S: Tissue concentrations of organochlorine compounds in common seals from the coast of Northern Ireland. Sci Total Environ 1992, 115: 235–240.
Mitchelmore CL, Chipman JK: Detection of DNA strand breaks in brown trout (Salmo trutta) hepatocytes and blood cells using the single cell gel electrophoresis (comet) assay. Aquat Toxicol 1998, 4: 161–182.
Nagpure NS, Sharma S, Pandey S, Kumar R, Srivastava SK, Verma MS, Kapoor D: Use of comet assay for genotoxicity assessment in fishes from Gomti River. Indian J Fish 2008,55(3):285–288.
Norppa H, Falck GCM: What do human micronuclei contain? Mutagenesis 2003, 18: 221–233. 10.1093/mutage/18.3.221
Nwani CD, Lakra WS, Nagpure NS, Kumar R, Kushwaha B, Srivastava SK: Mutagenic and genotoxic effects of carbosulfan in freshwater fish Channa punctatus (Bloch) using micronucleus assay and alkaline single-cell gel electrophoresis. Food Chem Toxicol 2010, 48: 202–208. 10.1016/j.fct.2009.09.041
Oikari A: Caging techniques for field exposures of fish to chemical contaminants. Aquat Toxicol 2006, 78: 370–381. 10.1016/j.aquatox.2006.03.010
Pandey S, Nagpure NS, Kumar R, Sharma S, Srivastava SK, Verma MS: Genotoxicity evaluation of acute doses of endosulfan to freshwater teleost Channa punctatus (Bloch) by alkaline single-cell gel electrophoresis. Ecotoxicol Environ Safe 2006, 65: 56–61. 10.1016/j.ecoenv.2005.06.007
Pandrangi R, Petras M, Ralph S, Vrzoc M: Alkaline single cell gel (comet) assay and genotoxicity monitoring using bullheads and carp. Environ Mol Mutagen 1995, 26: 345–356. 10.1002/em.2850260411
Pellacani C, Buschini A, Furlini M, Poli P, Rossi C: A battery of in vivo and in vitro tests useful for genotoxic pollutant detection in surface waters. Aquat Toxicol 2006, 77: 1–10. 10.1016/j.aquatox.2005.10.010
Pereira AMM, Soares AMVM, Gonçalves F, Ribeiro R: Test chambers and test procedures for in situ toxicity testing with zooplankton. Environ Toxicol Chem 1999, 18: 1956–1964. 10.1002/etc.5620180914
Risso-de Facerney C, Devaux A, Lafaurie M, Girard JP, Bailly B, Rahmani R: Cadmium induces apoptosis and genotoxicity in rainbow trout hepatocytes through generation of reactive oxygen species. Aquat Toxicol 2001, 53: 65–76. 10.1016/S0166-445X(00)00154-5
Saleh K, Zeytinoğlu H: Micronucleus test in peripheral erythrocytes of Rana ridipunda as an indicator of environmental pollution. Ana Uni J Sci Tech 2001, 2: 77–82.
Sanchez-Galan S, Linde AR, Ayllon F, Garcia-Vazquez E: Induction of micronuclei in eel (Anquilla anquilla L.) by heavy metals. Ecotoxicol Environ Safe 2001, 49: 139–143. 10.1006/eesa.2001.2048
Sarkar UK, Gupta BK, Lakra WS: Biodiversity, ecohydrology, threat status and conservation priority of the freshwater fishes of river Gomti, a tributary of river Ganga (India). Environmentalist 2010, 30: 3–17. 10.1007/s10669-009-9237-1
Singh NP, McCoy MT, Tice RR, Schneider EL: A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 1988, 175: 184–191. 10.1016/0014-4827(88)90265-0
Singh KP, Malik A, Sinha S: Water quality assessment and apportionment of pollution sources of Gomti River (India) using multivariate statistical techniques—a case study. Anal Chim Acta 2005, 538: 355–374. 10.1016/j.aca.2005.02.006
Smith R: Erythrocytic micronuclei in wild fish from lakes Superior and Ontario that have pollution-associated neoplasia. J Great Lakes Res 1990, 16: 139–142. 10.1016/S0380-1330(90)71406-2
Smith TM: The mechanism of benzene-induced leukemia: a hypothesis and speculations on the causes of leukemia. Environ Health Perspect 1996,104(Suppl 6):1219–1225. 10.1289/ehp.961041219
Sumathi M, Kalaiselvi K, Palanivel M, Rajaguru P: Genotoxicity of textile dye effluent on fish (Cyprinus carpio) measured using the comet assay. Bull Environ Contam Toxicol 2001, 66: 407–414. 10.1007/s00128-001-0020-3
Tucker KA, Burton GA: Assessment of nonpoint-source runoff in a stream using in situ and laboratory approaches. Environ Toxicol Chem 1999, 18: 2797–2803. 10.1002/etc.5620181221
Van Der Oost R, Beyer J, Vermeulen NPE: Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharm 2003, 13: 57–149. 10.1016/S1382-6689(02)00126-6
Ventura BC, Angelis DF, Marin-Morales MA: Mutagenic and genotoxic effects of the Atrazine herbicide in Oreochromis niloticus (Perciformes, Cichlidae) detected by the micronuclei test and the comet assay. Pest Biochem Physiol 2008, 90: 42–51. 10.1016/j.pestbp.2007.07.009
Vigano L, Camoirano A, Izzotti A, D´Agostini F, Polesello S, Francisci C, De Flora S: Mutagenicity of sediments along the Po River and genotoxicity biomarkers in fish from polluted areas. Mutat Res 2002, 515: 125–134. 10.1016/S1383-5718(02)00002-5
Waters MD, Stack HF, Garrett NE, Jackson MA: The genetic activity profile database. Environ Health Perspect 1991, 96: 41–45.
Waters MD, Stack HF, Jackson MA: Genetic toxicology data in the evaluation of potential human environmental carcinogens. Mutat Res 1999, 437: 21–49. 10.1016/S1383-5742(99)00037-X
Acknowledgements
The authors are grateful to the Director of the National Bureau of Fish Genetics Resources for providing the necessary support to conduct this study and the Indian Council of Agricultural Research (ICAR Cess Fund F. No. 4(31)/2000-ASR-1; Code No. 0677001) for the financial assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
NSN conceived and coordinated the study. BK designed the experiment and carried out the in situ exposure studies. SP carried out the micronucleus test. SS carried out the comet assay. SKS conducted the in situ exposure studies. RS participated in the comet assay. RK performed the statistical analysis. AD performed the statistical analysis. BK, RS, and AD wrote the manuscript. NSN and RK edited the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Kushwaha, B., Pandey, S., Sharma, S. et al. In situ assessment of genotoxic and mutagenic potential of polluted river water in Channa punctatus and Mystus vittatus. Int Aquat Res 4, 16 (2012). https://doi.org/10.1186/2008-6970-4-16
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2008-6970-4-16