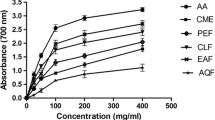
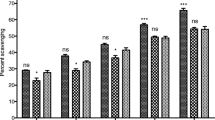
Abstract
Background
The aim of this study was to evaluate acetylcholinesterase inhibitory activity of some commonly used herbal medicine in Iran to introduce a new source for management of Alzheimer’s disease. A total of 18 aqueous-methanolic extract (1:1; v/v) from the following plants: Brassica alba, Brassica nigra, Camellia sinensis, Cinchona officinalis, Citrus aurantifolia, Citrus x aurantium, Ferula assafoetida, Humulus lupulus, Juglans regia, Juniperus sabina, Myristica fragrans, Pelargonium graveolens, Pistacia vera, Punica granatum, Rheum officinale, Rosa damascena, Salix alba, and Zizyphus vulgaris were prepared and screened for their acetylcholinesterase inhibitory activity using in vitro Ellman spectrophotometric method.
Results
According to the obtained results, the order of inhibitory activity (IC50 values, μg /ml) of extracts from highest to the lowest was: C. sinensis (5.96), C. aurantifolia (19.57), Z. vulgaris (24.37), B. nigra (84.30) and R. damascena (93.1).
Conclusions
The results indicated and confirmed the traditional use of these herbs for management of central nervous system disorders. C. sinensis showed the highest activity in inhibition of acetylcholinesterase. However, further investigations on identification of active components in the extracts are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Finding
Alzheimer’s disease (AD) is a an age related neurodegenerative disorder with clinical characteristic and pathological features associated with loss of neurons in certain brain areas leading to impairment of memory, cognitive dysfunction, behavioral disturbances, deficits in activities of daily living, which eventually leads to death [1–3]. In 2010, approximately 35 million people worldwide were suffering from AD and this number is believed to reach 65.7 million by 2030 [4].
Although the underlying pathophysiological mechanisms are not clear, AD is firmly associated with impairment in cholinergic pathway, which results in decreased level of acetylcholine in certain areas of brain [1, 2, 5, 6].
The management of AD focuses on slowing disease progression, symptomatic treatment, maintaining functional status and improving quality of life, and decreasing caregiver stress [5]. Acetylcholine, a neurotransmitter, which is hydrolyzed by acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE) is considered to play an important role in the pathology of AD [1, 2, 7].
The treatment of AD has progressed and shifted since the late 1970s to a transmitter replacement strategy. Elevation of acetylcholine levels in brain through the use of AChE inhibitors has been accepted as the most effective treatment strategy against AD [3, 8]. Therefore, AChE and/or BuChE inhibitors have become the drug of choice in management of AD [9]. Several AChE inhibitors named as “cognitive enhancers “are being investigated for the symptomatic treatment of Alzheimer’s disease but few have been approved by the Food and Drug Administration in the United States [2, 8, 10, 11]. The few drugs that have received regulatory approval to this date include: donepezil, rivastigmine and galantamine, all three working through increasing the concentration of acetylcholine at the neurotransmitter sites or acts by regulating activity at nicotinic receptors [2, 5].
However, studies investigating the use of medications for AD have not been consistently supportive [12–17]. Various side effects of medications reported in clinical trials include: nausea, vomiting, diarrhea, syncope and bradycardia. Consequently, a need for development and utilization of alternative anticholinesterase compounds with fewer side effects leads to investigation on plants as a possible source of treatment [18–26]. Plants have been used since antiquity in the treatment of various diseases including cognitive disorders, such as AD.
Considering the importance of plant-driven compounds in drug discovery, the present study was undertaken to evaluate the anticholinesterase activity of a number of selected medicinal plants with various ethnobotanical uses, aiming to discover new candidates for anticholinesterase activity to be used in management of AD.
Material and methods
Plant materials
Eighteen medicinal plants, which are listed in Table 1 were chosen randomly from local herbal market in September 2010, Tehran and identified by Dr. Faraz Mojab. Voucher specimens were kept in the Herbarium of Faculty of Pharmacy, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Chemicals
Acetylthiocholine iodide (ATCI), 5,5’–dithio-bis-2-nitrobenzoic acid (DTNB), bovine serum albumin (BSA) and electric eel AChE (AChE; EC 3.1.17; lyophilized, 500 U/vial solid, 65U/mg) were purchased from Sigma (St. Louis, MO). Physostigmine was used as the standard drug. Buffers and other chemical were of extra pure analytical grade. The following buffers were used: Buffer A: 50 mM Tris–HCl, pH 8, containing 0.1% BSA; Buffer B: 50 mM Tris- HCl, pH 8 containing 0.1 M NaCl, 0.02 M MgCl2 × 6H2O.
Preparation of extracts
Each plant sample was individually powdered and 1 g of each sample was extracted by maceration method under shaking at room temperature with aqueous methanol (20 mL; 1:1 v/v) for 24 h. After filtration, organic layer was distilled under reduced pressure at 25°C and then freeze-dried to dryness. The crude extracts were stored at -20°C until analysis. Determination of fifty percent inhibitory concentrations (IC50) at 100 μg/mL dissolved in aqueous methanol was accurately defined.
Anticholinesterase inhibitory activity
Ellman’s method was employed for determination of AChE inhibitory activity [26–28]. Acetylthiocholine was used as a substrate and hydrolysis of acetylthiocholine was determined by monitoring the formation of the yellow 5-thio-2-nitrobenzoate anion as a result of the reaction with 5,5’–dithio-bis-2-nitrobenzoic acid with thiocholine, catalyzed by enzymes at a wavelength of 412 nm.
Briefly, 25 μl of 15 mM ATCI, (43 mg/10 mL in Millipore water), 125 μl of 3 mM DTNB, (11.9 mg/10 mL buffer B), 50 μl of buffer A and 20 μl of plant extract at concentration of 100 μ g/ml were added to 96 well plates and the absorbance was measured at 412 nm every 13 s for five times. After adding 25 μl of 0.22 U/ml enzyme, (0.34 mg AChE dissolved in 100 mL buffer A), the absorbance was read again every 13 seconds for five times. The absorbance was measured using a Synergy H4 Hybrid Multi-Mode Microplate Reader (BioTek Instruments Inc., United States). Percentage of inhibition was calculated by comparing rates of the sample with the blank (aqueous methanol), control samples contained all components except the tested extract. Physostigmine was used as positive control. Then, the mean ofthree measurements for each concentration was determined (n = 3). Inhibitory concentration (IC50 value) was calculated according to Michaelis–Menten model by using EZ-Fit. Enzyme Inhibition Kinetic Analysis program (EZ-Fit: Enzyme Kinetics Software, Perrella Scientific Inc., Amshert, USA).
Statistical method
The assays were conducted in triplicate (n = 3) and after calculating the mean ± SD, the results were compared using Student’s t-test. A P value of less than 0.05 was considered significant.
Results and discussion
Eighteen plant species belonging to 16 plant families (Anacardiaceae, Apiaceae, Brassicaceae, Cannabaceae, Cupressaceae, Geraniaceae, Juglandaceae, Lythraceae, Myristicaceae, Polygonaceae, Rhamnaceae, Rosaceae, Rubiaceae, Rutaceae, Salicaceae, and Theaceae) were obtained from Tehran local herbal market and a total of 18 extracts were screened for AChE inhibitory activity using Ellman’s spectrophotometric method in 96-well microplate. Table 1 gives the names of the plants investigated, their families, their traditional uses, and acetylcholinesterase inhibitory concentration (IC50) respectively. IC50 of physostigmine (positive control) was estimated 0.093 μM against AChE. At the test extracts concentration (100 μg/ml), physostigmine showed complete inhibition of enzyme activity.
Camellia sinensis
Results showed that AChE inhibitory concentration (IC50) of leaves of C. sinensis (5.96 μg/ml) was less than the inhibitory concentration (IC50) of the other tested extracts (Table 1). An inhibitory activity has been reported for green leaves of C. sinensis previously [29]. The occurrence of flavonoid saponins, polyphenols, and catechins is well documented in different preparations of C. sinensis. Quantitative concentrations of these compounds in tea infusion might be different due to impact of preparation method, cultivar, and agricultural divergences, which might explain the observed difference in AChE inhibitory activity between different types of C. sinensis[30].
Citrus aurantifolia
In this study C. aurantifolia presented an IC50 inhibitory effect of AChE in concentration of 19.57 (μ g/ml). Report by Chaiyana and Okonogi [31] revealed inhibition of cholinesterase by essential oil of leaf and fruit peel of C. aurantifolia. Phytochemical investigations of C. aurantifolia revealed the occurrence of limonene, l-camphor, citronellol, o-cymene and 1,8-cineole as the major constituents [31]; A group of compounds reported to have AChE inhibitory activity [31]. Essential oils from same family have been reported to posses promising AChE inhibitory activity [32]. However, there is no AChE inhibitory activity in blooms of C. aurantifolia.
Zizyphus vulgaris
The extract from Z. vulgaris fruit showed moderate AChE inhibitory activity (IC50 = 24.37 μg/ml) in this study, which is similar to the results of previous studies [33, 34]. The AChE inhibitory activity of Z. vulgaris can be explained by the presence of alkaloids, saponins, and flavonoids in the extract [33].
Rosa damascena
We found AChE inhibitor activity from R. damascena floret extract (IC50 = 93.10 μg/ml) that is not in supported in previous investigations [25]. The R. damascena floret jam is used traditionally as a sweetener to tea to increase memory and wellness.
Brassica nigra
There is limited data on seeds of B. nigra. This study shows that seeds of B. nigra present AChE inhibitory activity with an IC50 of 135.0 (IC50 = 93.10 μg/ml). This finding suggests a moderate inhibitory activity for B. nigra seeds. Though further investigation in active components of the extract is needed.
The promising finding of the study shows that most of the plant-extracts screened in this study had some degrees of inhibitory activity against AChE (Table 1), but five species showed the most active inhibitory property (C. sinensis, C. aurantifolia, Z. vulgaris, B. nigra, and R. damascena) and had lowest inhibitory concentration below 100 μg/ml ranging between 5.96 to 93.10 against electric eel AChE. The extract of the herbs alone or in combination of other herbal productions such as essential oil could be considered in herbal remedies of AD management. Since the most strong synthetic or natural product driven AChE inhibitors are known to contain nitrogen, the promising activity of reported medicinal plants could be due to their high alkaloidal contents [35–40]. Alkaloids are the major compounds isolated from plants and show inhibitory activity for AChE [35–40] but only one out of each five most potent species contains alkaloids.
The search performed using Chemical Abstracts, Biological Abstracts and Scopus database shows that AChE inhibitory activity is not only limited to alkaloids but also other compounds such as flavonoid, coumarins and essential oils are reported to have AChE inhibitory activity [35–41]. The finding of this study shows that the four most active herbal extracts contained not only nitorgenic compound such as alkaloid but also these extracts also contain rich components of saponin, flavonoid, and essential oils [29–34]. The new area of interest in research involves both AChE inhibitors and BuChE inhibitors after recognition of BuChE activity in hippocampus of patients with AD [33, 42, 43]. Recent studies have work on dual inhibitors of AChE and BuChE [42, 43]. In these studies synthesized chemical compounds have shown promising inhibitory effect on both AChE and BuChE [42–44]. However, the present study was limited to the screening of AChE inhibitory properties of selected plants. Thus, further studies should focus on BuChE inhibitory activity of herbal products of the plants used in this study and other potent plants.
Conclusions
A primary screening process was run to investigate AChE inhibitory properties of medicinal plants of Iran. The primary findings of this study suggest that all herbs used in this study exhibited some degree of AChE inhibitory properties. Among the selected plants of this report C. sinensis had the most active components with inhibitory properties on AChE. Further researches should investigate more on the chemical composition and mechanism of actions of these herbal extract including in vitro and in vivo studies.
Abbreviations
- AChE:
-
Acetylcholinesterase
- AD:
-
Alzheimer’s disease.
- BuChE:
-
Butyrylcholinesterase.
References
Thies W, Bleiler L: Alzheimer’s disease facts and figures. Alzheimer’s and Dementia. 2012, 8: 131-168.
Zarotsky V, Sramek JJ, Culter NR: Galanthamine hydrobromide: an agent for Alzheimer’s disease. Am J Health- System Pharmacist. 2003, 60: 446-452.
Schneider JA, Arvanitakis Z, Bang W, Bennett DA: Mixed brain. Pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007, 69: 2197-2204. 10.1212/01.wnl.0000271090.28148.24.
World Alzheimer Report 2010: The Global Economic Impact of Dementia. Available at http://www.alz.co.uk/research/files/WorldAlzheimerReport2010.pdf Accessed January 2014
Tricco AC, Vandervaart S, Soobiah C, Lillie E, Perrier L, Chen MH, Hemmelgarn B, Majumdar SR, Straus SE: Efficacy of cognitive enhancers for Alzheimer’s disease: protocol for a systematic review and network meta-analysis. Syst Rev. 2012, 28: 1-31.
Perry N, Court G, Bidet N, Court J, Perry E: European herbs with cholinergic activities: Potential in dementia therapy. Int J Geriatr Psychiatry. 1996, 11: 1063-1069. 10.1002/(SICI)1099-1166(199612)11:12<1063::AID-GPS532>3.0.CO;2-1.
Hebert LE, Scherr PA, Beckeff LA: Age-specific incidence of Alzheimer’s disease in a community population. JAMA. 1995, 273: 1354-1359. 10.1001/jama.1995.03520410048025.
Arnold SE, Kumar A: Reversible dementias. Med Clin Nort Am. 1993, 77: 215-225.
Adams RL, Crai PL, Parsons OA: Neuropsychology of dementia. Neurol Clin. 1984, 4: 387-405.
Adams M, Gmünder F, Hamburger M: Plants traditionally used in age related brain disorders - A survey of ethnobotanical literature. J Ethnopharmacology. 2007, 113: 363-381. 10.1016/j.jep.2007.07.016.
Anon: FDA-approved treatments for Alzheimer’s. 2012, Available at http://www.alz.org/national/documents/topicsheet_treatments.pdf
Aisen PS, Davis KL: The search for disease-modifying treatment for Alzheimer’s Disease. Neurology. 1997, 48: 35-41. 10.1212/WNL.48.5_Suppl_6.35S.
Schneider LS: Treatment of Alzheimer’s disease with cholinesterase inhibitors. Clin Geriatric Med. 2001, 17: 337-358. 10.1016/S0749-0690(05)70072-0.
Aazza S, Lyoussi B, Miguel MG: Antioxidant and antiacetylcholinesterase activities of some commercial essential oils and their major compounds. Molecules. 2011, 16: 7672-7690. 10.3390/molecules16097672.
Houghton PJ, Ren Y, Howes MJ: Acetylcholinesterase inhibitors from plants and fungi. NatProd Rep. 2006, 23: 181-199.
Lahiri DK, Farlow MR, Greig NH, Sambamurti K: Current drug targets for Alzheimer’s disease treatment. Drug Dev Res. 2002, 56: 267-281. 10.1002/ddr.10081.
Darvesh S, Walsh R, Kumar R, Caines A, Roberts S, Magee D: Inhibition of human cholinesterases by drugs used to treat Alzheimer’s disease. Alzheimer Dis Assoc Disord. 2003, 17: 117-126. 10.1097/00002093-200304000-00011.
Adhami HR, Farsam H, Krenn L: Screening of medicinal plants from Iranian traditional medicine for acetylcholinesterase inhibition. Phytother Res. 2011, 25: 1148-1152. 10.1002/ptr.3409.
Adsersen A, Gauguin B, Gudiksen L, Jager AK: Screening of plants used in Danish folk medicine to treat memory dysfunction for acetylcholinesterase inhibitory activity. J Ethnopharmacology. 2005, 104: 118-122.
Akhondzadeh S, Noroozian M, Mohammadi M, Ohadinia S, Jamshidi AH, Khani M: Salvia officinalis extract in the treatment of patients with mild to moderate Alzheimer’s disease: a double blind, randomized and placebo-controlled trial. J Clin Pharm Ther. 2003, 28: 53-59. 10.1046/j.1365-2710.2003.00463.x.
Akhondzadeh S, Abbasi SH: Herbal medicine in the treatment of Alzheimer’s disease. Am J Alzheimers Dis Other Demen. 2006, 21: 113-118. 10.1177/153331750602100211.
Benamar H, Rached W, Derdour A, Marouf A: Screening of Algerian medicinal plants for acetylcholinesterase inhibitory activity. J Bio Sci. 2010, 10: 1-9.
Howes MR, Perry NSL, Houghton PJ: Plants with traditional uses and activities, relevant to the management of Alzheimer’s disease and other cognitive disorders. Phytother Res. 2003, 17: 1-18. 10.1002/ptr.1280.
Howes MR, Houghton PJ: Plants used in Chinese and Indian traditional medicine for improvement of memory and cognitive function. Pharmacol Biochem Behav. 2003, 75: 513-527. 10.1016/S0091-3057(03)00128-X.
Ferreira A, Proença C, Serralheiro ML, Araújo ME: The in vitro screening for acetylcholinesterase inhibition and antioxidant activity of medicinal plants from Portugal. J Ethnopharmacol. 2006, 108: 31-37. 10.1016/j.jep.2006.04.010.
Gholamhoseinian A, Moradi MN, Sharifi-Far F: Screening the methanol extracts of some Iranian plants for acetylcholinesterase inhibitory activity. Res Pharm Sci. 2009, 4: 105-112.
Ellman GL, Courtney KD, Andres V, Featherstone RM: A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961, 7: 88-95. 10.1016/0006-2952(61)90145-9.
Nadri H, Pirali-Hamedani M, Shekarchi M, Abdollahi M, Sheibani V, Amanlou M: Design, synthesis and anticholinesterase activity of a novel series of 1-benzyl-4-((6-alkoxy-3-oxobenzofuran-2(3H)-ylidene) methyl) pyridinium derivatives. Bioorg Med Chem. 2010, 18: 6360-6366. 10.1016/j.bmc.2010.07.012.
Bakthira H, Awadh Ali NA, Arnold N, Teichert A, Wessjohann L: Anticholinesterase activity of endemic plant extracts from soqotra. Afr J Tradit Complement Altern Med. 2011, 8: 296-299.
Kwak JH, Jeong CH, Kim JH, Choi GN, Shin Y, Lee SC: Acetylcholinesterase inhibitory effect of green tea extracts according to storage condition. Korean J Food Sci Technol. 2009, 41: 435-440.
Lee EN, Song JH, Lee JS: Screening of a potent antidementia acetylcholinesterase inhibitor-containing fruits and optimal extraction conditions. Korean J Food Nutr. 2010, 23: 318-323.
Chaiyana W, Okonogi S: Inhibition of cholinesterase by essential oil from food plant. Phytomedicine. 2012, 19: 836-839. 10.1016/j.phymed.2012.03.010.
Orhan I, Sener B, Choudhary MI, Khalid A: Acetylcholinesterase and butyrylcholinesterase inhibitory activity of some Turkish medicinal plants. J Ethnopharmacol. 2004, 91: 57-60. 10.1016/j.jep.2003.11.016.
Mahajan RT, Chopda MZ: Phyto-Pharmacology of Ziziphus jujuba Mill, A plant review. Phcog Rev. 2009, 3: 320-329.
Gomes NG, Campos MG, Orfão JM, Ribeiro CA: Plants with neurobiological activity as potential targets for drug discovery. Prog Neuropsychopharmacol Biol Psychiatry. 2009, 33: 1372-1389. 10.1016/j.pnpbp.2009.07.033.
Mantle D, Pickering AT, Perry EK: Medicinal plant extracts for the treatment of dementia: A review of their pharmacology, efficacy and tolerability. CNS Drugs. 2000, 13: 201-213. 10.2165/00023210-200013030-00006.
Martinez A, Castro A: Novel cholinesterase inhibitors as future effective drugs for the treatment of Alzheimer’s disease. Expert Opin Invest Drugs. 2006, 15: 1-12. 10.1517/13543784.15.1.1.
Mukherjee PK, Kumar V, Mal M, Houghton PJ: Acetylcholinesterase inhibitors from plants. Phytomedicine. 2007, 14: 289-300. 10.1016/j.phymed.2007.02.002.
Oh MH, Houghton PJ, Whang WK, Cho JH: Screening of Korean herbal medicines used to improve cognitive function for anti-cholinesterase activity. Phytomedicine. 2004, 11: 544-548. 10.1016/j.phymed.2004.03.001.
Sarris J: Herbal medicines in the treatment of psychiatric disorders: a systematic review. Phytother Res. 2007, 21: 703-716. 10.1002/ptr.2187.
Schultes RE: Plants in treating senile dementia in the Northwest Amazon. J Ethnopharmacology. 1993, 38: 129-135. 10.1016/0378-8741(93)90008-S.
Nadri H, Pirali-Hamedani M, Moradi A, Sakhteman A, Vahidi A, Sheibani V: 5,6-Dimethoxybenzofuran-3-one derivatives: a novel series of dual Acetylcholinesterase/Butyrylcholinesterase inhibitors bearing benzyl pyridinium moiety. Daru. 2013, 21: 15-10.1186/2008-2231-21-15.
Gholivand K, Abdollahi M, Mojahed F, Alizadehgan AM, Dehghan G: Acetylcholinesterase/butyrylcholinesterase inhibition activity of some new carbacylamidophosphate derivatives. J Enzyme Inhib Med Chem. 2009, 24: 566-576. 10.1080/14756360802316971.
Gholivand K, Alizadehgan AM, Mojahed F, Dehghan G, Mohammadirad A, Abdollahi M: Some new carbacylamidophosphates as inhibitors of acetylcholinesterase and butyrylcholinesterase. Z Naturforsch C. 2008, 63: 241-250.
Acknowledgements
This research was supported by a grant from the Research Council of Tehran University of Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
All authors contributed to the concept and design, making and analysis of data, drafting, revising and final approval. MA and PP are responsible for the study registration. SBJ, AA and NG carried out plant extraction and enzymatic tests and drafted manuscript. SBJ, PP and MA participated in collection and/or assembly of data, data analysis, interpretation and manuscript writing. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Jazayeri, S.B., Amanlou, A., Ghanadian, N. et al. A preliminary investigation of anticholinesterase activity of some Iranian medicinal plants commonly used in traditional medicine. DARU J Pharm Sci 22, 17 (2014). https://doi.org/10.1186/2008-2231-22-17
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2008-2231-22-17