Abstract
Background and purpose of the study
The hypotensive activity of the essential oil of Mentha x villosa and its main constituent, the monoterpene rotundifolone, have been reported. Therefore, our objective was to evaluate the vasorelaxant effect of monoterpenes found in medicinal plants and establish the structure-activity relationship of rotundifolone and its structural analogues on the rat superior mesenteric artery.
Methods
Contractions of the vessels were induced with 10 μM of phenylephine (Phe) in rings with endothelium. During the tonic phase of the contraction, the monoterpenes (10-8 - 10-3, cumulatively) were added to the organ bath. The extent of relaxation was expressed as the percentage of Phe-induced contraction.
Results
The results from the present study showed that both oxygenated terpenes (rotundifolone, (+)-limonene epoxide, pulegone epoxide, carvone epoxide, and (+)-pulegone) and non-oxygenated terpene ((+)-limonene) exhibit relaxation activity. The absence of an oxygenated molecular structure was not a critical requirement for the molecule to be bioactive. Also it was found that the position of ketone and epoxide groups in the monoterpene structures influence the vasorelaxant potency and efficacy.
Major conclusion
The results suggest that the presence of functional groups in the chemical structure of rotundifolone is not essential for its vasorelaxant activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The essential oils are natural products extracted from species of aromatic plants and exhibit a variety of biological properties [1–6]. These effects are attributed mainly to the terpenes, which are the major chemical components of these oils. The cardiovascular activity of essential oils has been reported [7–10]. Some studies showed that the main chemical components of these oils are bioactive [7, 10–12].
The antihypertensive activity of some plants also has been related to the presence of terpenes. Extracts and diterpenoids of Salvia species have shown cardiovascular activity, such as ferruginol and 7-oxo-abieta-9,12,14-triene [13]. In another work, in normotensive anaesthetised rats, the cardiovascular effect of the essential oil of Mentha x villosa and its main constituent, rotundifolone, suggests that hypotensive activity may result from its vasodilatory effects directly upon vascular smooth muscle [11]. Additional studies showed that the hypotensive activity of the monoterpene rotundifolone is related to decrease in heart rate and peripheral vascular resistance, probably via non-selective muscarinic receptor stimulation [14].
For these reasons, it appeared possible that terpenes found in essential oils could also have cardiovascular activity. Therefore, our objective was to verify the potential vasorelaxant effect of representative monoterpenes present in medicinal plants and to determine the relationship between the chemical structure of rotundifolone and its vasorelaxant activity to understand the influence of the functional groups present in this monoterpene. Therefore, we assessed p-menthane monoterpenes containing ketone groups and/or epoxy in different positions in the chemical structure. In addition, a monoterpene that does not contain these functional groups was also evaluated.
Materials and methods
Chemicals and solutions
(+)-Limonene epoxide was prepared via oxidation of (+)-limonene with m-chloroperoxybenzoic acid [15]. Pulegone epoxide [16] and carvone epoxide [17] were prepared via oxidation of (+)-pulegone and (−)-carvone, respectively, using hydrogen peroxide in alkaline medium as previously described. (+)-Pulegone was purchased from Aldrich. (+)-Limonene was purchased from company Dierberger Óleos Essenciais S.A., Barra Bonita, Brazil. Rotundifolone was isolated from the essential oil of Mentha x villosa using a previously described procedure [18]. The purity of test compounds was higher than 95%. L-Phenylephrine chloride (Phe) and acetylcholine chloride (Ach) were purchase from Sigma (Sigma Chemical Co., USA). In the preparation of the stock solutions, the monoterpenes were diluted in Tyrode/cremophor (0.15% v/v) solution. Phe and Ach were diluted or in Tyrode solutions only. All stock solutions were maintained at 0°C and diluted to the desired concentration, when necessary. Cremophor (Sigma Chemical Co., USA) in used concentrations showed no effect on control experiments (data not shown).
(Composition of Tyrode’s solution in mM: NaCl 158.3, KCl 4.0, CaCl2 2.0, NaHCO3 10.0, C6H12O6 5.6, MgCl2 1.05 and NaH2PO4 0.42).
Animals
Male Wistar normotensive rats (200 – 300 g) were obtained from colonies maintained in the Department of Physiology, Federal University of Sergipe, Sergipe, Brazil. They were maintained in a large cage under controlled conditions of temperature and lighting (lights on: 06:00–18:00 h), fed with rodent diet and tap water ad libtum. All procedures were approved by the Animal Research Ethics Committee of the Universidade Federal de Sergipe, Brazil (Protocol number 15/2009) and were in compliance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication 85–23, revised 1996).
Pharmacological assays
The tissue preparation was performed as described in Menezes et al. [19]. Rats were sacrificed by exsanguination under ether aesthesia and superior mesenteric artery was removed, cleaned from connective and fat tissues and sectioned in rings (1 – 2 mm). These rings were suspended in organ baths containing 10 mL of Tyrode’s solution, gassed with carbogen and maintained at 37°C under a resting tension of 0.75 g for 60 min (stabilization period). The isometric tension was recorded through a force transducer (Letica, Model TRI210, Italy) coupled to an amplifier-recorder (AVS, Brazil). Endothelium functionality of the rings was assessed by the ability of Ach (10 μM) to induce more than 70% relaxation of Phe (10 μM) tonus.
After verify the functionality of vascular endothelium, contractions of the vessels were induced with 10 μM Phe in rings with endothelium. During the tonic phase of the contraction, the monoterpenes rotundifolone, (+)-limonene epoxide, pulegone epoxide, carvone epoxide, (+)-pulegone and (+)-limonene (10-8 - 10-3, cumulatively) were added to the organ bath. The extent of relaxation was expressed as the percentage of Phe-induced contraction.
Statistic analysis
Values were expressed as the mean ± SEM. The results were analyzed with one-way ANOVA followed by Bonferroni post-test. The potency was expressed as pD2 value (negative logarithm of molar concentration producing the half maximum effect - Emax) of in vitro experiments were obtained by non-linear regression. All procedures were performed by using Graph Pad Prism 3.02™.
Results and discussion
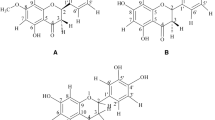
Several studies have reported pharmacological effects of essential oils on the rat cardiovascular system [9–11, 20, 21]. However, there are few studies on bioactive compounds that contribute to the pharmacological activity of these oils. We report in this comparative study the findings on the vasorelaxant activity of six monoterpenes (Figure1): rotundifolone (having α,β-unsaturated ketone and endocyclic epoxide groups), limonene epoxide (having only an epoxide group), pulegone epoxide (having ketone and exocyclic epoxide groups), carvone epoxide (having ketone and endocyclic epoxide groups), (+)-pulegone (having an α,β-unsaturated ketone), and (+)-limonene (non-oxygenated terpene).
The investigation of the influence of the position of ketone and epoxide groups in the molecular structure was performed by comparing the rotundifolone (1) with the pulegone epoxide (2) and carvone epoxide (3). The rotundifolone showed a vasorelaxant effect in rat mesenteric artery more potent than carvone epoxide (p < 0.01), suggesting that the position of the ketone group influences this spasmolytic property. In addition, the pharmacological activity of rotundifolone was lower than the pulegone epoxide (p < 0.05). This result demonstrates that the position of epoxide group in the p-menthane structure alters the pharmacological activity. The experimental data also showed that the pulegone epoxide exhibit more significant vasorelaxant activity in comparison to carvone epoxide (p < 0.001), indicating that the exocyclic epoxide group contributes more to the pharmacological effect than the endocyclic epoxide group. These results confirm that the position of epoxide and ketone functional groups in molecules change the vasorelaxant activity. Comparing rotundifolone with (+)-limonene (4), these compounds showed equipotent vasorelaxant activity (p > 0.05). The spasmolytic activity of (+)-limonene shown in this study demonstrates that non-oxygenated terpenes present in cardioactive essential oils may contribute to this activity. (+)-Limonene epoxide (5) and (+)-pulegone (6) presented vasorelaxant activity below 50%, so it was not allowed to calculate their pD2 (Table1).
Similarly, the effectiveness of the tested compounds was investigated by comparing their maximum effects. The rotundifolone showed a higher pharmacological effect than (+)-pulegone (p <0.05). While (+)-limonene was more effective than (+)-pulegone (p <0.01) and limonene epoxide (p <0.01). Carvone epoxide exhibited a maximum effect of relaxation greater than pulegone epoxide (p <0.01), (+)-pulegone (p <0.001), and limonene epoxide (p <0.001).
It is described in the literature that the monoterpene terpinen-4-ol produces hypotensive effect in rats. Terpinen-4-ol is the main constituent of the essential oil of Alpinia zerumbet (Pers.) B. L. Burtt. & R. M. Sm. (Zingiberaceae). Lahlou et al.[12] proved that the hypotensive effects of the essential oil of Alpinia zerumbet are partially attributed to the actions of terpinen-4-ol, which was more potent than this oil. In another study, Barbosa-Filho et al. [22] showed that the essential oils from stem and root of the plant Ocotea duckei have hypotensive activity. The main chemical constituents of these oils are the terpene β-eudesmol and elemol, respectively. These reports, together with our findings, demonstrate the importance of this natural chemical class as good candidates for antihypertensive drugs.
Furthermore, studies have demonstrated that some of these terpenes exhibit depressant properties in other organic systems. Sadraei et al. [4] and Camara et al. [5] showed that both, (+)-α- and (−)-β-pinene, relax rat and guinea-pig ileum, respectively. Other studies have shown that (±)-linalool promotes spasmolytic effect in guinea-pig ileum mediated by cAMP [23], and recently Tanida et al. [24] demonstrated that the inhalation of (±)-linalool reduces the blood pressure mediated by the central nervous system in rats. Therefore, the hypotensive activity could originate from vasodilatory effects induced by these compounds. This possible mechanism of action was studied in citronellol, which lowers blood pressure by a direct effect on the vascular smooth muscle leading to vasodilation [25].
The biotransformation of some compounds tested is known. For example, (+)-limonene is biotransformed to several metabolites, such as (+)-limonene epoxide, carvone, and perillyl alcohol [26, 27]. (+)-Limonene epoxide is one of the bioactive compounds of the present study. Carvone has ketone group which is present in the chemical structure of carvone epoxide, another evaluated compound. The monoterpene perillyl alcohol has hypotensive activity [28]. Pulegone also produces bioactive metabolites, such as menthol [29] which has vasorelaxant activity [30]. Therefore, the metabolites of (+)-limonene, pulegone, and other tested compounds should contribute to their vasorelaxant activity. In addition, the tested compounds must act by different mechanisms of action. In fact, monoterpene alcohols, aldehydes, ethers, and hydrocarbons show effects on the cardiovascular system via vasorelaxation, decreased heart rate or hypotension, among others [31]. The mechanisms of action of these compounds should be related to their metabolites.
Conclusion
The results from the present study showed that both oxygenated terpenes and hydrocarbon terpene exhibit spasmolytic activity. The absence of an oxygenated molecular structure was not a critical requirement for the molecule to be bioactive. The position of ketone and epoxide groups in the p-menthane structure influences the vasorelaxant potency and efficacy. Therefore, these functional groups contribute to the vasorelaxant activity of rotundifolone.
References
Mohamed AEH, El-Sayed MA, Hegazy ME, Helaly SE, Esmail AM, Mohamed NS: Chemical constituents and biological activities of Artemisia herba-alba. Rec Nat Prod. 2010, 4 (1): 1-25.
De Almeida RN, Araújo DAM, Gonçalves JCR, Montenegro FC, De Sousa DP, Leite JR, Mattei R, Benedito MAC, Carvalho JGB, Cruz JS, Maia JGS: Rosewood oil induces sedation and inhibits compound action potential in rodents. J Ethnopharmacol. 2009, 124: 440-443. 10.1016/j.jep.2009.05.044.
Arruda TA, Antunes RMP, Catão RMR, Lima EO, De Sousa DP, Nunes XP, Pereira MSV, Barbosa-Filho JM, Cunha EVL: Preliminary study of the antimicrobial activity of Mentha x villosa Hudson essential oil, rotundifolone and its analogues. Rev Bras Farmacogn. 2006, 16: 307-311. 10.1590/S0102-695X2006000300005.
Sadraei H, Asghari GR, Hajhashemi V, Kolagar A, Ebrahimi M: Spasmolytic activity of essential oil and various extracts of Ferula gummosa Boiss. on ileum contractions. Phytomedicine. 2001, 8: 370-376. 10.1078/0944-7113-00052.
Camara CC, Nascimento NR, Macedo-Filho CL, Almeida FB, Fonteles MC: Antispasmodic effect of the essential oil of Plectranthus barbatus and some major constituents on the guinea-pig ileum. Planta Med. 2003, 69: 1080-1085.
Compagnone RS, Chavez K, Mateu E, Orsini G, Arvelo F, Suárez AI: Composition and cytotoxic activity of essential oils from Croton matourensis and Croton micans from Venezuela. Rec Nat Prod. 2010, 4: 101-108.
Interaminense LF, Leal-Cardoso JH, Magalhães PJ, Duarte GP, Lahlou S: Enhanced hypotensive effects of the essential oil of Ocimum gratissimum leaves and its main constituent, eugenol, in DOCA-salt hypertensive conscious rats. Planta Med. 2005, 71: 376-378. 10.1055/s-2005-864109.
Lahlou S, Figueiredo AF, Magalhães PJ, Leal-Cardoso JH: Cardiovascular effects of 1,8-cineole, a terpenoid oxide present in many plant essential oils, in normotensive rats. Can J Physiol Pharmacol. 2002, 80: 1125-1131. 10.1139/y02-142.
Cunha RM, Farias SRQ, Duarte JC, Santos MRV, Ribeiro EAN, Medeiros IA: Cardiovascular effects induced by the essential oil of Ocotea duckei Vattimo (Lauraceae). Biol Geral Exper. 2004, 5: 12-18.
De Siqueira RJ, Magalhães PJ, Leal-Cardoso JH, Duarte GP, Lahlou S: Cardiovascular effects of the essential oil of Croton zehntneri leaves and its main constituents, anethole and estragole, in normotensive conscious rats. Life Sci. 2006, 78: 2365-2372. 10.1016/j.lfs.2005.09.042.
Lahlou S, Carneiro-Leao RF, Leal-Cardoso JH, Toscano CF: Cardiovascular effects of the essential oil of Mentha x villosa and its main constituent, piperitenone oxide, in normotensive anaesthetised rats: role of the autonomic nervous system. Planta Med. 2001, 67: 638-643. 10.1055/s-2001-17352.
Lahlou S, Interaminense LF, Leal-Cardoso JH, Duarte GP: Antihypertensive effects of the essential oil of Alpinia zerumbet and its main constituent, terpinen-4-ol, in DOCA-salt hypertensive conscious rats. Fundam Clin Pharmacol. 2003, 17: 323-330. 10.1046/j.1472-8206.2003.00150.x.
Ulubelen A: Cardioactive and antibacterial terpenoids from some Salvia species. Phytochemistry. 2003, 64: 395-399. 10.1016/S0031-9422(03)00225-5.
Guedes DN, Silva DF, Barbosa-Filho JM, Medeiros IA: Muscarinic agonist properties involved in the hypotensive and vasorelaxant responses of rotundifolone in rats. Planta Med. 2002, 86: 700-704.
Thomas AF, Bessière Y: Limonene. Nat Prod Rep. 1989, 6: 291-309. 10.1039/np9890600291.
Katsuhara J: Absolute configuration of pulegone oxide and piperitenone dioxide. J Org Chem. 1967, 32: 797-799. 10.1021/jo01278a062.
Santos RB, Brocksom TJ, Brocksom U: A convenient deoxygenation of α, β epoxy ketones to enones. Tetrahedron Lett. 1997, 38: 745-748. 10.1016/S0040-4039(96)02451-3.
Almeida RN, Hiruma CA, Barbosa-Filho JM: Analgesic effect of rotundifolone in rodents. Fitoterapia. 1996, 67: 334-338.
Menezes IAC, Moreira IJA, Carvalho AA, Antoniolli AR, Santos MRV: Cardiovascular effects of the aqueous extract from Caesalpinia ferrea: involvement of ATP-sensitive potassium channels. Vasc Pharmacol. 2007, 47: 41-47. 10.1016/j.vph.2007.03.005.
Lahlou S, Leal-Cardoso JH, Magalhães PJ: Essential oil of Croton nepetaefolius decreases blood pressure through an action upon vascular smooth muscle: studies in DOCA-salt hypertensive rats. Planta Med. 2000, 66: 138-143. 10.1055/s-2000-11133.
Lahlou S, Leal-Cardoso JH, Magalhães PJ, Coelho-de-Souza AN, Duarte GP: Cardiovascular effects of the essential oil of Croton nepetaefolius in rats: role of the autonomic nervous system. Planta Med. 1999, 65: 553-557. 10.1055/s-1999-14025.
Barbosa-Filho JM, Cunha RM, Dias CS, Athayde-Filho PF, Silva MS, Cunha EVL, Machado MIL, Craveiro AA, Medeiros IA: GC-MS analysis and cardiovascular activity of the essential oil of Ocotea duckei. Rev Bras Farmacogn. 2008, 18: 37-41.
Lis-Balchin M, Hart S: Studies on the mode of action of the essential oil of lavender (Lavandula angustifolia P. Miller). Phytother Res. 1999, 13: 540-542. 10.1002/(SICI)1099-1573(199909)13:6<540::AID-PTR523>3.0.CO;2-I.
Tanida M, Niijima A, Shen J, Nakamura T, Nagai K: Olfactory stimulation with scent of lavender oil affects autonomic neurotransmission and blood pressure in rats. Neurosci Lett. 2006, 398: 155-160. 10.1016/j.neulet.2005.12.076.
Bastos JFA, Moreira IJA, Ribeiro TP, Medeiros IA, Antoniolli AR, De Sousa DP, Santos MRV: Hypotensive and vasorelaxant effects of citronellol, a monoterpene alcohol, in rats. Basic Clin Pharmacol Toxicol. 2010, 106: 331-337.
Miyazawa M, Shindo M, Shimada T: Metabolism of (+)- and (−)-limonenes to respective carveols and perillyl alcohols by CYP2C9 and CYP2C19 in human liver microsomes. Drug Metab Dispos. 2002, 30: 602-607. 10.1124/dmd.30.5.602.
Shimada T, Shindo M, Miyazawa M: Species differences in the metabolism of R-(−)- and S-(+)-limonenes and their metabolites, carveols and carvones, by cytochrome P450 enzymes in liver microsomes of mice, rats, guinea pigs, rabbits, dogs, monkeys, and humans. Drug Metab Pharmacokinet. 2002, 17: 507-515. 10.2133/dmpk.17.507.
Saito K, Okabe T, Inamori Y, Tsujibo H, Miyake Y, Hiraoka K, Ishida N: The biological properties of monoterpenes: hypotensive effects on rats and antifungal activities on plant pathogenic fungi of monoterpenes. Mokuzai Gakkaishi. 1996, 42: 677-680.
Engel W: In vivo studies on the metabolism of the monoterpene pulegone in humans using the metabolism of ingestion-correlated amounts (MICA) approach: explanation for the toxicity differences between (S)-(−)- and (R)-(+)-pulegone. J Agric Food Chem. 2003, 51 (22): 6589-97. 10.1021/jf034702u.
Johnson CD, Melanaphy D, Purse A, Stokesberry SA, Dickson P, Zholos AV: Transient receptor potential melastatin 8 channel involvement in the regulation of vascular tone. Am J Physiol Heart Circ Physiol. 2009, 296 (6): H1868-H1877. 10.1152/ajpheart.01112.2008.
Santos MRV, Moreira FV, Fraga BP, De Sousa DP, Bonjardim LR, Quintans-Junior LJ: Cardiovascular effects of monoterpenes: a review. Rev Bras Farmacogn. 2011, 21 (4): 764-771. 10.1590/S0102-695X2011005000119.
Acknowledgements
We thank CNPq, CAPES and FAPITEC-SE for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
TCL was responsible for the preparation of monoterpenes and wrote the chemical content of the manuscript; MMM evaluated the pharmacological activity of monoterpenes; JMB-F isolated and identified the rotundifolone; MRVDS interpreted and wrote the pharmacological data of the manuscript; DPDS analyzed, interpreted and corrected the structure-activity relationships data of monoterpenes. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Cardoso Lima, T., Mota, M.M., Barbosa-Filho, J.M. et al. Structural relationships and vasorelaxant activity of monoterpenes. DARU J Pharm Sci 20, 23 (2012). https://doi.org/10.1186/2008-2231-20-23
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2008-2231-20-23