Abstract
Background
We examined differences in reproductive activities and intraspecific variations in advertisement calls of Duttaphrynus melanostictus (Schneider, 1799) that lives in a tropical region of central Vietnam. The snout-vent length (SVL) of sexually mature males ranged from 50.2 to 70.3 mm, while that of females ranged from 65.2 to 97.3 mm. Histological analyses of testes revealed that sperm was not present throughout the year, indicating discontinuous reproductive activity for adult males. Adult females were captured year-round, but reproductive females were observed only during months of the auxiliary rainy season (mainly April to July), indicating that females reproduce seasonally. We also estimated levels of within-male variation of each call property and the influences of the ambient temperature, humidity, and the SVL of calling males on acoustic features.
Results
The call rate and pulse rate showed intermediate levels of variation, whereas the dominant frequency and call duration were the most stereotyped properties. One-way analyses of variance for six acoustic properties showed that the call rate, pulse rate, and rise time significantly differed (p < 0.05) among localities. Advertisement calls were a series of groups of 56 to 244 (145 ± 54) pulses with an average call duration of 26.722 s, an average pulse rate of 11.69 pulses/s, and an average dominant frequency of 1.293 kHz. The results of the multiple regressions for possible effects of temperature, humidity, and SVL on the six acoustic properties indicated that the dominant frequency, pulse rate, call duration, and rise time were positively significant.
Conclusions
In Bach Ma National Park, when air temperature in the recording area decreased to <16.6°C, and advertisement calls of adult males virtually ceased in all three populations.
Similar content being viewed by others

Background
The Asian common toad Duttaphrynus melanostictus (formerly Bufo melanostictus) is a widely distributed species in South Asia. It inhabits Taiwan and southwestern and southern China, including Hainan Island, southward through Southeast Asia to Indonesia, and westward to India and Sri Lanka (Shieh 1993). Currently, numerous amphibian species exist in Vietnam. However, D. melanostictus represents the only species of its genus in Vietnam and is distributed throughout the entire country at different elevations, and in various temperature and humidity regimes (Nguyen et al. 2009). According to Pratihar and Kundu (2010), some adult females of this species grow to almost 20 cm long (from the tip of the snout to the tip of the fourth toe). This species is commonly distributed in villages, towns, and open areas, and only seen occasionally in primary forest. In winter, the species can hibernate in mud holes (Nguyen et al. 2005; Lin et al. 2011).
Most investigations of anuran reproduction are of species inhabiting the temperate zone where reproductive patterns reflect the annual climatic cycle (Jørgensen et al. 1986; Wells 2007). Females exhibit well-defined ovarian cycles characterized by vitellogenic growth of a complement of oocytes during summer and spawning of eggs after hibernation (Jørgensen et al. 1986; Duellman and Trueb 1994). The ovarian cycle thus follows the seasons, which act to synchronize reproduction within a population. Anurans inhabiting warmer climates may breed throughout the year, and ovarian cycles within a population are thus basically asynchronous (Jørgensen et al. 1978; Duellman and Trueb 1994; Wells 2007). Individual cycles may, however, remain well defined, as observed in a population of toads, Bufo viridis, from a Mediterranean warm temperate region (Jørgensen 1984). Little definite is known about patterns of ovarian function, including the duration of an ovarian cycle, in tropical animals and anurans (Jørgensen et al. 1986; Huang 2011; Lu 2011; Tumkiratiwong et al. 2012). Hence, studies of reproductive activities of tropical anuran species combined with male advertisement calls are necessary, because calls of males are not merely vocal signals for attracting males and females for congregation (Wells and Bard 1987; Reichert 2010) or vocal warnings in male-male competition and for territorial protection (Wells and Schwartz 1984; Wells 2007), but they are also an acoustic stimulus that enhances spermatogenic activities to accelerate sperm production for greater reproductive success (Lin et al. 2011).
Some previous studies showed that advertisement calls play a key role in the mating system of anuran species, in both attracting females and repelling rival males (Duellman and Trueb 1986; Gerhardt 1994). Concomitantly, males in many species, besides giving advertisement calls that attract females and play a role in male-male competition, also show that females prefer conspecific advertisement calls in most anuran species that were studied (Gerhardt 1994; Ryan 2009). The acoustic features of advertisement calls, including the call duration, dominant frequency, call rate, and pulse rate, were frequently used to delimit anuran species in taxonomic studies of complex frog groups (De la Riva et al. 1996; Channing et al. 2002; Angulo and Reichle 2008; Padial et al. 2008). On the other hand, in most anuran species, females use acoustic cues of conspecific males to discriminate between conspecifics and heterospecifics (Blair 1964 1968; Gerhardt and Huber 2002; Wells 2007) and locate mates (Arch et al. 2011). Differentiation of the acoustic features of calling corresponds to that of some other characteristics such as DNA sequences, karyotypes, histology, and morphology, which permit exact conclusions about taxonomy to be drawn (Schneider and Sinsch 2007; Fávero et al. 2011; Gómez and Kehr 2011; Liao and Chang 2011; Goldberg et al. 2012). In addition, delimiting anuran species is useful, and call variations among anuran species were studied and indicated that they do not necessarily have phylogenetic signals. Therefore, they might not accurately predict relationships of evolutionary processes among species (Cannatella et al. 1998; Giacoma and Castellano 2001; Littlejohn 2001; Wollenberg et al. 2007; Wang et al. 2011).
D. melanostictus is a bufonid toad common to Vietnam, which is important in ecosystems, agriculture, medicine, and scientific studies. However, numbers of individuals in populations are declining in recent years due to habitat loss (Nguyen et al. 2005). Most studies on this species in Vietnam focused on classification, description, morphology, and distribution (Vo et al. 2003; Le et al. 2004; Hoang et al. 2007; Hendrix et al. 2008). On the other hand, studies of reproductive ecology and behavior of animals in Vietnam are few (Kantor et al. 2012; Ngo et al. 2012). Behavioral studies of the reproductive activity and advertisement calls were therefore extended to local populations of D. melanostictus living in the tropical region of Bach Ma National Park, central Vietnam. This study attempted to document in greater detail its reproductive activity and to assess the functional status of the gonads in adult-sized male and female toads collected throughout the year. Moreover, we studied the body size at sexual maturation, the relationship between reproductive output and body size, and the reproductive activity in the life history of the species. Concomitantly, this study also attempted to describe in greater detail intraspecific variations of the species' advertisement calls among different localities, to address the influence of body size (snout-vent length, SVL), temperature, and humidity on the acoustic characteristics of the species.
Methods
Description of the study site
This study was conducted at Bach Ma National Park, Thua Thien-Hue Province, central Vietnam. A total core area covered approximately 37,487 ha, with geographic coordinates (WGS 84) of 15°59′ to 16°16′N and 107°37′ to 107°54′E (Figure 1). The study area is currently divided into three different ecological categories: primary forest (32.18% of the total area), ecological restoration forest (53.98%), and administrative management areas (13.84%) (Oikawa 2009, unpublished data). According to Nguyen et al. (2004), the climate is characterized by a tropical monsoon that dominates a montane rainforest (at 400 to 1,400 m in elevation) and cloud forests from 1,450 m in elevation to the summit (at 1,712 m in elevation), subtropical climates, no dry season, only one rainy season (months with monthly rainfall of ≥100 mm with the occurrence frequency of >75% in the observational sequence), and one small rainy season (Figure 2). The rainy season (May to December) was further divided into two periods: the auxiliary (in May to August) and main (in September to December) rainy seasons, respectively.
Monthly mean rainfall and temperature in the study area. Data correspond to the monthly mean precipitation (broken line and open circles, in millimeters) and air temperature (solid line and filled circles, in degrees Celsius) over the last 20 years. Data were recorded from statistics of the climatic-hydrology characters of Thua Thien-Hue Province (Nguyen et al. 2004).
Sexual maturity and reproductive activity
The individuals used in the reproductive study were captured by visual encounter during two nights of surveys (at 20:00 to 02:00) each month in January to December 2009. Sampling was standardized as two people for 6 h each night. In total, 169 individuals (53 adult males, 56 adult females, and 60 juveniles) were collected during 2009. Specimens were collected by hand and placed into individually labeled bags. The following variables were recorded for each collected individual: elevation, air temperature, humidity, and temperature of the water.
In the laboratory, toads were humanely euthanized with MS-222 the same day they were collected and kept frozen until they could be processed within 2 days after collection. After the carcass had thawed, we measured the SVL with electronic digital calipers (to the nearest 0.01 mm) and determined the mass of the carcass using an electronic balance (to ± 0.01 g). We dissected each specimen, determined the sex by directly examining the gonads, and removed the digestive tract; the reproductive tract was removed for analysis of sexual maturity and reproductive activity. Analytical results of productive activity and histology of the testes and ovaries were determined, and some of these D. melanostictus specimens were preserved in 70% ethanol and deposited in the herpetological collection of the Department of Biology, College of Education, Hue University, Vietnam. We recorded the longest and shortest diameters, and calculated the testicular and ovarian volumes using the formula for a prolate spheroid (Caldwell and Vitt 1999; Magnusson et al. 2003; Biavati et al. 2004):
To inspect for reproductive activity and document the SVL range for the onset of sexual maturity, the left and right testes were extracted, fixed in 70% ethanol, embedded in paraffin, sectioned at 6 μm with a microtome, and stained with hematoxylin-eosin (Liao and Chang 2011). The stage of spermatogenesis was determined by the following classification (Chan 2003): stage 1, primary spermatocytes; stage 2, secondary spermatocytes; and stage 3, spermatozoa.
Female sexual maturity and reproductive activity were confirmed by the presence of yolked follicles, oviductal eggs, and enlarged convoluted oviducts. We recorded the ovarian volume and diameters of yolked follicles or oviductal eggs. Reproductive activity data for each toad were employed to establish the percentage of adult males and females in each reproductive stage and over per month and season throughout the year. Ovarian egg data were used to estimate the mean clutch size, and numbers of eggs in both the left and right ovaries of 30 females were counted to determine the average number of eggs per clutch (Ngo and Ngo 2011). To detect significant intra- and intersexual variations in reproductive stages over time, we employed Chi-squared tests or G-tests. We also calculated a linear regression between the gonad volume and SVL of each individual. Reproductive data of each month were grouped into 2-month intervals (that corresponded to the three rainfall periods that occur during the year, see Figure 2).
Male advertisement call
Recording was conducted in two fieldwork trips in January and February 2011. Thirty-five calls were successfully recorded within three localities at the following coordinates: (1) 9 recordings at Truoi Lake (16°13′59.36″N, 107°48′48.48″E, at 240 m in elevation) and (2) 11 recordings at Voi Stream (16°14′19.49′N, 107°53′46.91″ E, at 126 m in elevation) in Phu Loc District; and (3) 15 recordings in Nam Dong District (16°05′37.49″N, 107°42′11.19″E, at 411 m in elevation) (Figure 1). Calling individuals were located during 19:00 to 03:00 using headlamps. All calls were recorded with a solid state recorder (Marantz Professional, PMD671, Kanagawa, Japan) with a memory card and an unidirectional Sony microphone (ECM-G7M; Sony, Kanagawa, Japan). Microphones were pointed at the focal male at a distance of approximately 100 cm (Marshall and Gerhardt 2010). Recordings proceeded for 5 to 10 min to register both silent periods and at least two entire call sequences of each male. After the recordings and behavioral observations were made, the males were captured to measure their SVL to the nearest 0.01 mm with digital calipers. Each adult male was marked with a combination of a visible implant elastomer and toe-clipping to prevent repeated recordings, and the temperature at the recording site was measured to the nearest 0.1°C and humidity (%) with a quick-reading thermometer (Wisewind 0912 or 5330, Centenary Materials Co., Ltd., Taipei, Taiwan).
The recorded files were digitized on Asus computers (Asus Computer Inc., Taipei, Taiwan), and a sound analysis was performed with the aid of Avisoft-SASLab Pro software (version 5.1, Berlin, Germany) at a 44.1-kHz sampling frequency and 16-bit resolution, and Sound Ruler software (version 0.9.6.0, Stockton, CA, USA), which were employed to quickly check the recordings and objective acoustic measurements of the recordings (Bee 2004Gridi-Papp 2007). The software was set up with the following spectrogram parameters: a Hamming window function, 1,024 points of fast Fourier transformation (FFT), a frame size of 100%, an overlap of 93.75%, a color of 0%, a gray.pal color table, and the power spectrum (logarithmic) of that call. All recordings were checked with a random set, the suitable calling recognition settings were concurrently adjusted, and the calls were accurately delineated in oscillograms and spectrograms at the same settings employed for all analyses. Following the call detection, oscillograms and spectrograms were visually inspected to reject incorrectly measured calls.
We recorded 35 adult males from three localities visited in Bach Ma National Park, central Vietnam. Males of D. melanostictus only emit a certain type of call, and the advertisement call was measured from ten calls emitted by a given male. We calculated the coefficients of variation (CV = [standard deviation (SD) / mean] × 100%) of the acoustic features of adult males to quantify within-individual variations of advertisement calls. We employed the criteria of Gerhardt (1991) and classified as static those acoustic properties with an average within-individual CV of <5% and dynamic those with a CV of >12%, while a CV of 5% to 12% was considered an intermediate level of within-individual variation.
An adult male's SVL and the ambient temperature and humidity often influence some acoustic properties of the calls of anuran species (Ryan and Wilczynski 1991; Gerhardt 1994; Castellano et al. 2002). Therefore, we analyzed six acoustic parameters for each adult male's call, with SVL, temperature, and humidity as covariates of each acoustic parameter: call duration (measured at 0 amplitude on the oscillogram); call rate [(total number of calls – 1) / the time from the beginning of the first call to the beginning of the last call]; rise time (time from call onset to the peak amplitude in the oscillogram); dominant frequency (frequency of the maximum energy on the spectrogram); frequency modulation (frequency range between the onset and offset of the dominant harmonic in the call spectrogram); and pulse rate [(total number of pulses − 1) / time from the beginning of the first pulse to the beginning of the last pulse] (Cocroft and Ryan 1995; Tárano 2001; Ryan and Rand 2003; Briggs 2010; Rodríguez et al. 2010).
All statistical analyses of advertisement calls were performed with Statistica software (version 6.0, StatSoft Inc., Tulsa, OK, USA). In addition, the spectral characteristics of advertisement calls between the three localities and reproductive activities among seasons of D. melanostictus were tested by a one-way analysis of variance (ANOVA). Statistical differences in reproductive activity among seasons were assessed using an analysis of covariance (ANCOVA) of gonadal volumes with body size (SVL) as a covariate using SPSS version. 14.0 (IBM, Armonk, NY, USA). The possible effects of climatic factors on reproductive activity were tested with multiple linear regressions of the monthly scores of rainfall (in mm) and temperature (°C) with gonadal volumes. Data are presented as the mean ± 1 SD, and p < 0.05 was considered statistically significant.
Results
Reproductive activity
In total, 169 specimens were collected from Bach Ma National Park, Thua Thien-Hue Province, central Vietnam. Of these 169 specimens, 53 were males, 56 were females, and 60 were juveniles. Males were 50.2 to 70.3 mm in SVL (mean ± SD = 60.73 ± 6.22 mm, n = 53), females were 65.2 to 97.3 mm in SVL (mean ± SD = 81.34 ± 9.22 mm, n = 56), and juveniles were 26.1 to 55.4 mm in SVL (mean ± SD = 39.23 ± 7.78 mm, n = 60) (Figure 3). The number of collected males, females, and juveniles did not significantly differ (X 2 2 = 2.879, p > 0.05, n = 169) showing equal capture for both sexes and juveniles during all seasons. Mature males were not captured throughout the year: X 2 2 = 5.182, p < 0.05, n = 53; similarly, the occurrence of reproductive mature females varied significantly in different months: X 2 2 = 21.481, p < 0.001, n = 56 (Figure 3). This population exhibited strong sexual dimorphism in body size (SVL, t 107 = −7.67, p = 0.001) (Figure 4).
Distribution of SVL (mm) and sex of individuals collected from populations of D. melanostictus . Reproductive adult males, filled circles; nonreproductive males, open circles; reproductive adult females, filled triangles; nonreproductive adult females, open triangles; juvenile males, filled squares; juvenile females, open squares; juveniles of undetermined sex, rhombuses. Arrows indicate the minimum size at sexual maturity for each sex.
Distribution of SVL (mm) for individuals in different categories considered in populations of D. melanostictus . Note the restricted size distribution of adult males compared to the wide range of sizes and larger SVLs attained by adult females. Juveniles of undetermined sex, gray bars; juvenile males, vertical lines bars; juvenile females, horizontal lines bars; adult males, black bars; adult females, white bars.
Reproductive males were not observed throughout the year. Spermatogenesis was observed to be polarized in each testis: only primary spermatocytes were present in the anterior portion of the testis, and only spermatozoa were found in the posterior portion. The occurrence of males with testes in stage 3 (with spermatozoa) significantly differed over time (G 10 = 19.417, p < 0.0001). The testicular volume of adult males significantly differed among seasons (ANOVA, F 2,50 = 9.69, p < 0.0001). The relationship between SVL and testicular volume of adult males was significant (ANCOVA, F 2,49 = 12.09, p < 0.0001, Figure 5A). When testis volume was compared among months, it was possible to observe a dramatic increase from March to August, when adult females had the largest follicular sizes and were preovulatory, whereas testis volumes fell from September to December of the year when most of the females had oviposited (Figure 6A). Almost all males (48 males, 91%) producing sperm had visible secondary sexual characteristics such as nuptial spines, and hypertrophied forearms and callosities on the forelegs. Only 9% of adult males had sperm in their testes but no discernible callosities.
Monthly changes in the mean gonadal volume in males and females of D. melanostictus and its relationship with mean rainfall values. (A) Testicular volume (solid line, filled circles) and rainfall values (broken line, open circles). (B) Ovarian volume (solid line, filled squares) and rainfall values (broken line, open circles).
Reproductive females were only collected between the end of March and August (in the auxiliary rainy season). The relationship between the SVL and ovarian volume of adult females was significant (ANCOVA, F 2,52 = 20.82, p < 0.0001, Figure 5B). There were also significant differences in ovarian volume among seasons (ANOVA, F 2,53 = 17.58, p < 0.0001). Between March and August, adult females had yolked follicles, and ovaries had their largest volumes (mean ± SD = 25.73 ± 10.24 cm3, n = 32; follicle diameter 1.5 to 1.8 mm, for 7,125 to 9,547 yolked follicles, mean ± SD = 8,447 ± 928, n = 30). There was a positive correlation between body size and fecundity (number of yolked follicles, r = 0.906, p < 0.0001). The maximum ovarian size was attained from May to July.
The first post-reproductive females (with distended oviducts) were found at the end of March; in September to February, females had ovaries of small volumes (mean ± SD = 9.43 ± 6.04 cm3, n = 20, with follicle diameter of ≤0.5 mm). Therefore, ovulation and oviposition occurred between the end of the little rainy season and the end of the auxiliary rainy season of the year (March to August, but mainly April to July, Figure 6B). Results of the multiple regressions indicated that both ovarian and testicular volumes were positively associated with rainfall [ovarian volume: beta temperature (°C) = 0.348, beta rainfall (mm) = −0.721, r 2 = 0.812, F 2,52 = 17.39, p < 0.0001; testicular volume: beta temperature (°C) = 0.259, beta rainfall (mm) = −0.478, r 2 = 0.635, F 2,49 = 11.21, p < 0.0001].
Male advertisement call
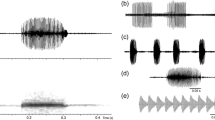
Descriptive statistics of the call acoustic properties are shown in Table 1. The average SVL of calling males was 64.38 mm (range of 60.1 to 69.3 mm, n = 35). During recordings, the mean air temperature was 24°C (range of 20.9 ~ 26.4°C, n = 35), and the mean air humidity was 95% (range of 90% to 100%, n = 35). The advertisement call of D. melanostictus is a long train of pulses (Figure 7A) repeated at a regular rate, with an average dominant frequency of 1.293 kHz (Figure 7B); each pulse (Figure 8A) is composed by five to eight sub-pulses (Figure 8B), all of similar duration, with the exception of the last sub-pulse, which is longer (Figure 8B).
The rise time (mean CV = 25.41%) and frequency modulation (mean CV = 15.17%) were highly variable within individuals; the call rate (mean CV = 9.14%) and pulse rate (mean CV = 10.21%) exhibited an intermediate level of within-individual variation, whereas the dominant frequency (mean CV = 0.86%) and call duration (mean CV = 3.47%) were the most stereotyped properties. The criteria of Gerhardt (1991) suggested that the dominant frequency and call duration in some anuran species can be considered as static properties, whereas frequency modulation and the rise time can be considered as dynamic properties among individuals.
The one-way ANOVA and least significant difference (LSD) post hoc tests showed statistically significant differences among localities in call rate (F 2,32 = 3.76, p = 0.034), pulse rate (F 2,32 = 11.86, p < 0.0001), rise time (F 2,32 = 4.46, p = 0.021), and dominant frequency (F 2,32 = 35.26, p < 0.0001, with highly significant differences between Truoi Lake (TL) and Voi Stream (VS), and between VS and Nam Dong (ND) District, whereas neither call duration (F 2,32 = 1.81, p = 0.181) nor frequency modulation (F 2,32 = 2.42, p = 0.105) significantly differed among populations.
Multiple regression results for possible effects of temperature, humidity, and SVL on six acoustic parameters of advertisement calls of D. melanostictus in the populations were positively significant in dominant frequency (r 2 = 0.361, F 3,31 = 5.816, p = 0.003); pulse rate (r 2 = 0.225, F 3,31 = 3.003, p = 0.045); call duration (r 2 = 0.402, F 3,31 = 6.961, p = 0.001); and rise time (r 2 = 0.234, F 3,31 = 3.152, p = 0.039). Conversely, frequency modulation (r 2 = 0.046, F 3,31 = 0.498, p = 0.687) and call rate (r 2 = 0.042, F 3,31 = 0.453, p = 0.717) were not statistically significant. When the air temperature in the recording area decreased below 16.6°C, advertisement calls of adult males virtually ceased, while the air humidity had barely any effect.
Discussion
Reproductive activity
Females of D. melanostictus mature at larger body sizes and have larger maximum sizes than males. This female-biased sexual size dimorphism is common in anurans (Shine 1979); it tends to be more pronounced in larger species (Salthe and Duellman 1973) and is usually explained as the effect of different selective pressures acting on male and female body sizes. In fact, since in females' body size is strongly and positively associated with fecundity (Salthe and Duellman 1973; Elmberg 1991), directional selection favoring a large body size is supposed to be stronger in females than males. The Asian common toad D. melanostictus conforms to the rule because female fecundity increases with an increasing body size (Wake and Dickie 1998). Female-biased sexual size dimorphism may be due to faster growth rates of females rather than to lower survival rates of males (Turner 1960; Wells 2007). According to an alternative hypothesis, however, differences in size may reflect sexual differences in age structure: to grow larger, females prolong pre-adult growth and reach sexual maturity later and at a larger size than males (Monnet and Cherry 2002; Huang 2011; Tumkiratiwong et al. 2012).
The reproductive activity of D. melanostictus is seasonal and is related to the rainfall regime. Ovarian follicles begin vitellogenesis at the end of the little rainy season and reach their maximal diameter from May to July, months when the little rainy season abruptly ceases. Oviposition takes place during the auxiliary rainy season, and egg development occurs during the auxiliary rainy season and the first main rainy season of the year. Seasonality in female reproductive activity seems to be common in anurans from Bach Ma National Park. In fact, it was suggested that in tropical anuran species living in areas of pronounced difference between the little and main rainy seasons, oviposition is concentrated at the end of the little rainy season, and hatching occurs during the auxiliary rainy season and early in the main rainy season (Ngo et al. 2009).
During the reproductive season of D. melanostictus, adult nonreproductive females were not found, suggesting that all mature females produce only one clutch annually. Results of the analysis of D. melanostictus females collected from different localities of the Bangalore agglomeration showed a smaller oocyte volume and fewer oocytes than our results (Jørgensen et al. 1986). These differences may have been due to different allocation strategies of female reproductive resources among localities. Although females of D. melanostictus reproduce seasonally, the presence of developed secondary sexual characteristics (nuptial spines and hypertrophied forearms) and histological observations of the testes and vas deferens showed that there is no continuous spermiogenesis or spermiation, suggesting that males of this population are not capable of courtship and mating throughout the year. Some amphibian species show continuous mating activity and spermatogenetic cycles, such as Dendrotriton bromeliacius and Pseudoeurycea goebeli (Chan 2003).
According to Houck (1977), continuous spermatogenesis may represent an evolutionarily stable strategy for amphibians if the cost of maintaining and producing mature sperm is low. However, using a detailed histological examination of the testis, Chan (2003) identified subtle levels of change and cryptic temporal patterns in testicular activity, but could not detect the presence of secondary sexual characteristics. In male D. melanostictus, testicular volume significantly differed among seasons. Maximal testicular volume was observed in April to July, when females are periovulatory, and testicular volume fell during the following months, perhaps in response to the increased mating opportunities. Because there was no correlation among precipitation, temperature, and testicular volume, the observed changes in testicular volume are more likely triggered by mating activity than by climatic factors.
When the air temperature decreased below 16.6°C, advertisement calls of adult males virtually ceased, while the air humidity had almost no effect. However, in the study area, this species does not hibernate, as evidenced by sampling twice a month during 2009, when animal specimens, including juveniles, and adult males and females, were collected in all months. Conversely, the study of the male reproductive cycle of D. melanostictus in Taiwan, with a longer and colder winter compared to Vietnam, showed that the number of sperm bundles and free sperm were lowest in October to January; in the same period, adult toads were difficult to find in the field. Thus, this period was considered the torpid period; the number of sperm bundles in the seminiferous tubules, the number of free sperm, and plasma androgen levels from February to April were all higher than during the other three periods. In Taiwan, this period corresponds to the breeding season, while from May to June (post-breeding period), the number of sperm bundles, free sperm, and adult individuals of D. melanostictus were lower (Huang et al. 1997). Synthetic chorusing stimuli performed during spring in Taiwan enhanced the breeding status of males of D. melanostictus. The average number of sperm bundles of the test group that received daily stimulation with male choruses was 56% higher than that of the control group that did not receive the chorus stimulation. Otherwise, there were no significant differences in food intake, body condition, or testicular size between the two groups after the experiment (Lin et al. 2011).
Although advertisement calls of D. melanostictus were collected from the end of January at Bach Ma National Park, the first reproductive females were not found until the end of March. In Taiwan, male spectacled toads (D. melanostictus) began gathering at the water's edge and emitting advertisement calls in early spring. The male chorus continued for nearly a month prior to exhibiting amplexus behavior in March (Lin et al. 2011). Such a long period of premating male chorusing cannot simply be for female attraction or territory protection (Duellman and Trueb 1994Tobias et al. 2004). Male calls are known to be an energetically expensive activity in ectothermic vertebrates (Taigen and Wells 1985; Pough et al. 1992; Emerson and Hess 2001) and may expose males to predation risks. Therefore, besides attraction, recognition, or competition, the long period of male chorusing before the arrival of females might have some other biological significance. Lin et al. (2011) suggested that the male chorus may play an important role in the male reproductive cycle as an acoustic stimulus that enhances spermatogenic activities by accelerating sperm production.
Male advertisement calls
Male's acoustic communication plays an important role in the reproductive activity of most anuran amphibians (Arch et al. 2011). Female mate choice is an important mechanism of sexual selection; in many species, females take into account multiple characteristics of potential mates (Burke and Murphy 2007), and the Asian common toad D. melanostictus is no exception. Our results showed that adult males have advertisement calls with a maximum amplitude (dB) at a frequency of 1.309 to 1.321 kHz. Playback experiments on calling males showed that in many anurans, call amplitude plays an important role in male spacing within a chorus (Burke and Murphy 2005). Previous studies indicated that the spacing between the speaker and the nearest male in playback experiments was greater for speakers broadcasting calls at a higher amplitude, but this effect occurred only in some anurans with low-density choruses (Murphy and Floyd 2005; Wells 2007). This suggests that the immediate benefit of producing high-amplitude signals may differ at different chorus densities.
In this study, air temperature negatively influenced advertisement calls of D. melanostictus among localities. When the air temperature of the study areas decreased below 16.6°C, advertisement calls of adult males virtually stopped. Acoustic features derived from active muscular contractions, such as the call rate, call duration, and aggressive calls, are highly temperature dependent in anuran species (Gayou 1984; Castellano et al. 2002; Wells 2007). Our observations of adult male's calls showed that the production of each advertisement call involves contraction of the trunk muscles. Hence, metabolic constraints could explain the positive correlation found between temperature and the call rate. Additionally, for many frogs, energetic costs of advertisement calls increase with the pulse and call rates (Bevier 1997; Wells 2007). The call duration and dominant frequency were the most stereotyped properties of D. melanostictus calls. Previous studies suggested that the dominant frequency and call duration can be used to estimate species limits and divergence processes in anuran amphibians (Márquez and Bosch 1995; Gergus et al. 2004; Gerhardt 1991; Gerhardt and Huber 2002); those and our findings could be relevant for studies of the genus Duttaphrynus in the near future.
The advertisement call of D. melanostictus was a series of groups of 56 to 244 pulses (mean ± SD = 145 ± 54 pulses) (Figure 7A) with an average call duration of 26,722 ms, an average dominant frequency of around 1,293 Hz (Table 1; Figure 7B), and an average pulse rate of 11.69 pulses/s. Conversely, at Nusa Dua (Bali, Indonesia), the advertisement call of D. melanostictus was a series of groups of 2 ~ 14 pulses with an average call duration of 456 ms, an average dominant frequency of around 1,059 Hz, and an average pulse rate of 11.9 pulses/s (Márquez and Eekhout 2006). These data were obtained at temperatures of 28 ~ 29°C, from males with an average SVL of 64.2 mm. Does call data indicate that this widespread species has geographic variations in its call features, or that it may be more than one species? We also support the hypothesis that geographic variations and body sizes of adult males are related to calling parameters. The results of multiple regressions between the covariates (SVL, temperature, and humidity) and six acoustic parameters showed that the call duration, dominant frequency, rise time, and pulse rate were positively significant. In particular, dominant frequencies were highly variable among populations studied (1.0 to 1.7 kHz in Bangkok, Thailand; 3.0 kHz in Burma, Myanmar; 1.6 kHz in Coorg, India; and 1.35 to 1.51 kHz in Lishui, China; Márquez and Eekhout 2006; Wei et al. 2012); whereas in this study, the dominant frequency of toads was approximately 1.24 to 1.32 kHz.
In the advertisement call of D. melanostictus, the pulse rate and dominant frequency appear to have high potential for conveying information about an individual male's status in a sexual selection context. In the Nusa Dua population, the dominant frequency appeared to have a relatively high between-individual variation, while two call characteristics (dominant frequency and pulse rate) had coefficients of variation of <10% (Márquez and Eekhout 2006), indicating some potential for species recognition. However, calls emitted in a dense chorus may also have additional potential for sexual selection, although the sample size was too limited (n = 5) to confirm this point. In addition, the advertisement call of D. melanostictus from Coorg, India was previously described by Hampson and Bennet (2002). They reported that the average duration call of the specimens of India was 40 s, and the average dominant frequency was 1.6 kHz. Compared to our recordings, the dominant frequency was very similar, but the duration call extremely differed. In any event, comparisons of call characteristics do not suggest clear differences among the populations recorded. This species is usually found closely related to anthropogenic and highly disturbed habitats throughout its distribution (Iskandar 1998). However, we found some adult males living in primary forest in Vietnam, expanding the range of habitats where this species is present.
Conclusions
In toads, female preferences for longer calls and faster call rates were demonstrated in some species (Bufo valliceps; W Wagner and B Sullivan, personal communication, unpublished data). This pattern of preference provides alternative and mutually exclusive pathways to increased attractiveness to females. Given that the total acoustic output is limited (Taigen and Wells 1985), males of a given species can package their acoustic output into longer calls at lower rates or into shorter calls at higher rates. The mutually exclusive nature of these alternative pathways might lead to divergence in signaling strategies among lineages in which female preferences exist. Such a pattern appears to be present within D. melanostictus, Bufo cognatus (Anaxyrus cognatus), and Bufo compactilis (Anaxyrus compactilis). These three species exhibit the longest calls and the lowest call rates of any species in this comparison, while Bufo speciosus (Anaxyrus speciosus) exhibits the shortest calls and the highest call rate, although the basic pulse structure was conserved (Cocroft and Ryan 1995).
References
Angulo A, Reichle S: Acoustic signals, species diagnosis, and species concepts: the case of a new cryptic species of Leptodactylus (Amphibia, Anura, Leptodactylidae) from the Chapare region, Bolivia. Zool J Linn Soc 2008, 152: 59–77. 10.1111/j.1096-3642.2007.00338.x
Arch VS, Richards-Zawaki CL, Feng AS: Acoustic communication in the Kihansi spray toad ( Nectophrynoides asperginis ): insights from a captive population. J Herpetol 2011, 45: 45–49. 10.1670/10-084.1
Bee MA: Equipment review: sound ruler acoustical analysis: a free, open code, multi-platform sound analysis and graphing package. Bioacoustics 2004, 14: 171–178. 10.1080/09524622.2004.9753520
Bevier CR: Utilization of energy substrates during calling activity in tropical frogs. Behav Ecol Sociobiol 1997, 41: 343–352. 10.1007/s002650050394
Biavati GM, Wiederhecker HC, Colli GR: Diet of Epipedobates flavopictus (Anura: Dendrobatidae) in a Neotropical Savanna. J Herpetol 2004, 38: 510–518. 10.1670/30-04A
Blair WF: Isolating mechanisms and interspecific interactions in anuran amphibians. Q Rev Biol 1964, 39: 334–344. 10.1086/404324
Blair WF: Amphibians and reptiles. In Animal communication. Edited by: Seboek TA. Indiana University Press, Bloomington; 1968:289–310.
Briggs VS: Call trait variation in Morelett's tree frog, Agalychnis moreletii , of Belize. Herpetologica 2010, 66: 241–249. 10.1655/HERPETOLOGICA-D-09-00011.1
Burke JE, Murphy GC: The effect of call amplitude on male spacing in choruses of barking treefrogs, Hyla gratiosa . Anim Behav 2005, 69: 419–426. 10.1016/j.anbehav.2004.03.016
Burke JE, Murphy GC: How female barking treefrogs, Hyla gratiosa , use multiple call characteristics to select a mate. Anim Behav 2007, 74: 1463–1472. 10.1016/j.anbehav.2007.02.017
Caldwell JP, Vitt LJ: Dietary asymmetry in leaf litter frogs and lizards in a transitional northern Amazonian rain forest. Oikos 1999, 84: 383–397. 10.2307/3546419
Cannatella DC, Hillis DM, Chippindale PT, Weigt L, Rand AS, Ryan MJ: Phylogeny of frogs of the Physalaemus pustulosus species group, with an examination of data incongruence. Syst Biol 1998, 47: 311–335. 10.1080/106351598260932
Castellano S, Cuatto B, Rinella R, Rosso A, Giacoma C: The advertisement call of the European treefrogs ( Hyla arborea ): a multilevel study of variation. Ethology 2002, 108: 75–89. 10.1046/j.1439-0310.2002.00761.x
Chan LM: Seasonality, microhabitat and cryptic variation in tropical salamander reproductive cycles. Biol J Linnean Soc 2003, 78: 489–496. 10.1046/j.0024-4066.2002.00157.x
Channing A, Moyer D, Burger M: Cryptic species of sharp-nosed reed frogs in the Hyperolius nasutus complex: advertisement call differences. Afr Zool 2002, 37: 91–99.
Cocroft RB, Ryan MJ: Patterns of advertisement call evolution in toads and chorus frogs. Anim Behav 1995, 49: 283–303. 10.1006/anbe.1995.0043
De la Riva I, Márquez R, Bosch J: Advertisement calls of four microhylid frogs from Bolivia (Amphibia, Anura). Am Midl Nat 1996, 136: 418–422. 10.2307/2426746
Duellman WE, Trueb L: Biology of amphibians. McGraw-Hill, New York; 1986.
Duellman WE, Trueb L: Biology of amphibians. Johns Hopkins University Press, Baltimore and London; 1994.
Elmberg J: Factors affecting male yearly mating success in the common frog, Rana temporaria . Behav Ecol Sociobiol 1991, 28: 125–131.
Emerson SB, Hess DL: Androgens, testis mass, and the energetics of vocalization in breeding male frogs. Horm Behav 2001, 39: 59–69. 10.1006/hbeh.2000.1635
Fávero ER, Veiga-Menoncello ACP, Rossa-Feres DC, Strüssmann C, Giaretta AA, Andrade GV, et al.: Intrageneric karyotypic variation in Pseudopaludicola (Anura: Leiuperidae) and its taxonomic relatedness. Zool Stud 2011, 50: 826–836.
Gayou DC: Effects of temperature on the mating call of Hyla versicolor . Copeia 1984, 1984: 733–738. 10.2307/1445157
Gergus EWA, Reeder TW, Sullivan BK: Geographic variation in Hyla wrightorum : advertisement calls, allozymes, mtDNA, and morphology. Copeia 2004, 2004: 758–769. 10.1643/CG-04-061R
Gerhardt HC: Female mate choice in treefrogs: static and dynamic acoustic criteria. Anim Behav 1991, 42: 615–635. 10.1016/S0003-3472(05)80245-3
Gerhardt HC: The evolution of vocalization in frogs and toads. Annu Rev Ecol Syst 1994, 25: 193–324.
Gerhardt CH, Huber F: Acoustic communication in insect and anurans. University of Chicago Press, Chicago; 2002.
Giacoma C, Castellano S: Advertisement call variation and speciation in the Bufo viridis complex. In Anuran communication. Edited by: Ryan MJ. Smithsonian Institution Press, Washington, DC; 2001:205–219.
Goldberg T, Nevo E, Degani G: Phenotypic plasticity in larval development of six amphibian species in stressful natural environments. Zool Stud 2012, 51: 345–361.
Gómez VI, Kehr AI: Morphological and developmental responses of anuran larvae ( Physalaemus albonotatus ) to chemical cues from the predators Moenkhausia dichoroura (Characiformes: Characidae) and Belostoma elongatum (Hemiptera: Belostomatidae). Zool Stud 2011, 50: 203–210.
Gridi-Papp M: Sound Ruler: Acoustic Analysis and Graphing Sourceforge (version 0.9.6.0). 2007. Available at . Accessed 15 Apr 2011 http://soundruler.sourceforge.net/main
Hampson K, Bennet D: Advertisement calls of amphibians at Lackunda Estate, Coorg, Karnataka. In Frogs of Coorg, Karnataka. Edited by: Bennet D. India. Viper Press, Glossop; 2002:115–136.
Hendrix R, Nguyen QT, Böhme W, Ziegler T: New anuran records from Phong Nha-Ke Bang National Park, Truong Son, central Vietnam. Herpetol Notes 2008, 1: 23–31.
Hoang XQ, Hoang NT, Ho AT: Amphibians and reptiles addition to the Central North Region recorded at Bach Ma National Park. In The problems of basic research in life sciences. Edited by: Nguyen VS, Nguyen VK. Science and Technology Publishing House, Hanoi; 2007:139–142. (in Vietnamese)
Houck LD: Life history patterns and reproductive biology of Neotropical salamanders. In The reproductive biology of amphibians. Edited by: Taylor DH, Guttman SJ. Plenum, New York; 1977:43–72.
Huang WS: Ecology and reproductive patterns of the littoral skink Emoia atrocostata on an East Asian tropical rainforest island. Zool Stud 2011, 50: 506–512.
Huang WS, Lin JY, Yu JYL: Male reproductive cycle of the toad Bufo melanostictus in Taiwan. Zool Sci 1997, 14: 497–503. 10.2108/zsj.14.497
Iskandar DT: The amphibians of Java and Bali. Research and Development Centre for Biology-LIPI. Bogor, Indonesia; 1998.
Jørgensen BC: Ovarian functional patterns in Baltic and Mediterranean populations of a temperate zone anuran, the toad Bufo viridis . Oikos 1984, 43: 309–321. 10.2307/3544148
Jørgensen BC, Hede KE, Larsen LO: Environmental control of annual ovarian cycle in the toad, Bufo bufo bufo L.: role of temperature. In Environmental endocrinology. Edited by: Assenmacher I, Farner DS. Springer, Berlin; 1978:28–36.
Jørgensen C, Barker KS, Vijayakumar S: Body size, reproduction and growth in a tropical toad, Bufo melanostictus , with a comparison of ovarian cycles in tropical and temperate zone anurans. Oikos 1986, 46: 379–389. 10.2307/3565838
Kantor YI, Fedosov AE, Marin IN: An unusually high abundance and diversity of the Terebridae (Gastropoda: Conoidea) in Nha Trang Bay. Vietnam Zool Stud 2012, 51: 663–670.
Le VK, Vo VP, Ngo DC, Le TS: Animal biodiversity of Bach Ma National Park. Thuan Hoa Publishing House, Hue, Vietnam; 2004. (in Vietnamese)
Liao YY, Chang YH: Reproductive biology of the needlefish Tylosurus acus melanotus in waters around Hsiao-Liu-Chiu Island, southwestern Taiwan. Zool Stud 2011, 50: 296–308.
Lin CF, Yeh TC, Lue KY: Enhancement of spermatogenesis from spring male chorus for the spectacled toad Duttaphrynus melanostictus (Schneider, 1799). Taiwan J Biodivers 2011, 13: 29–35.
Littlejohn MJ: Patterns of differentiation in temporal properties of acoustic signals of anurans. In Anuran communication. Edited by: Ryan MJ. Smithsonian Institution Press, Washington, DC; 2001:102–120.
Lu X: Reproductive ecology of three Tibetan waterbird species, with special reference to life-history alterations along elevational gradients. Zool Stud 2011, 50: 192–202.
Magnusson WE, Lima AP, Alves W, da Silva M, de Araújo C: Use of geometric forms to estimate volume of invertebrates in ecological studies of dietary overlap. Copeia 2003, 2003: 13–19. 10.1643/0045-8511(2003)003[0013:UOGFTE]2.0.CO;2
Márquez R, Bosch J: Advertisement calls of the midwife toads Alytes (Amphibia, Anura, Discoglossidae) in continental Spain. J Zool Syst Evol Res 1995, 33: 185–192.
Márquez R, Eekhout RX: Advertisement calls of six species of anurans from Bali, Republic of Indonesia. J Nat Hist 2006, 40: 571–588. 10.1080/00222930600712129
Marshall VT, Gerhardt HC: A precedence effect underlies preferences for calls with leading pulses in the grey treefrog, Hyla versicolor . Anim Behav 2010, 80: 139–145. 10.1016/j.anbehav.2010.04.014
Monnet JM, Cherry MI: Sexual size dimorphism in anurans. Proc R Soc Lond B 2002, 269: 2301–2307. 10.1098/rspb.2002.2170
Murphy CG, Floyd SB: The effect of call amplitude on male spacing in choruses of barking treefrogs, Hyla gratiosa . Anim Behav 2005, 69: 419–426. 10.1016/j.anbehav.2004.03.016
Ngo VB, Ngo DC: Morphological characters, sexual ratio, testis and egg development of Quasipaa verrucospinosa (Bourret, 1937) (Amphibia: Anura: Dicroglossidae) from Thua Thien-Hue Province, central Vietnam. Russ J Herpetol 2011, 18: 157–164.
Ngo VB, Tran TN, Tran CT: Nutritional and reproductive characters of three species ( Quasipaa verrucospinosa, Hylarana guentheri , and Fejervarya limnocharis ) in Thua Thien-Hue. In Proceedings of the 1st National Scientific Workshop of Amphibians and Reptiles. Edited by: Ngo CD, Ta TH, Le NN, Hoang QX, Vo PV, Nguyen SV. University of Hue Press, Hue, Vietnam; 2009:179–187. in Vietnamese
Ngo VB, Ngo CD, Nguyen XT, Hou PC, Tran NT: Advertisement calls and reproductive activity of Hylarana guentheri (Boulenger, 1882) from Bach Ma National Park. Russ J Herpetol 2012, 19: 239–250.
Nguyen V, Truong DH, Hoang TL, Nguyen VH, Phung DV, Ha HK, et al.: The climatic-hydrology characters of Thua Thien-Hue Province. Thuan Hoa, Hue, Vietnam; 2004. (in Vietnamese)
Nguyen VS, Ho TC, Nguyen QT: Checklist of amphibians and reptiles. Agricultural Publishing House, Hanoi, Vietnam; 2005. (in Vietnamese)
Nguyen VS, Ho TC, Nguyen QT: Herpetofauna of Vietnam, Edition Chimaira. Frankfurt am Main, Germany; 2009.
Padial JM, Köhler J, Munöz A, De la Riva I: Assessing the taxonomic status of tropical frogs through bioacoustics: geographical variation in the advertisement calls in the Eleutherodactylus discoidalis species group (Anura). Zool J Linnean Soc 2008, 152: 353–365. 10.1111/j.1096-3642.2007.00341.x
Pough FH, Magnusson W, Ryan M, Wells K, Taigen T: Behavioral energetics. In Environmental physiology of the amphibians. Edited by: Feder M, Burggren W. University of Chicago Press, Chicago; 1992:395–436.
Pratihar S, Kundu JK: Hematological and immunological mechanisms of adaptation to hibernation in common Indian toad Duttaphrynus melanostictus . Russ J Herpetol 2010, 17: 97–100.
Reichert MS: Aggressive thresholds in Dendropsophus ebraccatus : habituation and sensitization to different call types. Behav Ecol Sociobiol 2010, 64: 529–539. 10.1007/s00265-009-0868-5
Rodríguez A, de la Nuez D, Alonso R: Intraspecific variation in the advertisement call of the cloud-forest frog Eleutherodactylus glamyrus (Anura: Eleutherodactylidae). J Herpetol 2010, 44: 457–466. 10.1670/09-038.1
Ryan MJ: Communication in frogs and toads. In Encyclopedia of neuroscience. Edited by: Squire LR. Academic, Oxford; 2009:1159–1166.
Ryan MJ, Rand AS: Sexual selection in female perceptual space: how female túngara frogs perceive and respond to complex population variation in acoustic mating signals. Evolution 2003, 57: 2608–2618.
Ryan MJ, Wilczynski W: Evolution of intraspecific variation in the advertisement call of a cricket frog ( Acris crepitans , Hylidae). Biol J Linn Soc 1991, 44: 249–271. 10.1111/j.1095-8312.1991.tb00619.x
Salthe SN, Duellman WE: Quantitative constrains associated with reproductive mode in anurans. In Evolutionary biology of the anurans. Edited by: Vial JL. University of Missouri Press, Columbia; 1973:229–249.
Schneider H, Sinsch U: Contributions of bioacoustics to the taxonomy of Anura. Surrey Beatty & Sons. In Phylogeny and systematics, amphibian biology, vol 7. Edited by: Heatwole H. Chipping Norton, Australia; 2007:2892–2931.
Shieh JN: The breeding ecology of Bufo melanostitus. Tunghai University, Taichung, Taiwan, Thesis; 1993. in Chinese
Shine R: Sexual selection and sexual dimorphism in the Amphibia. Copeia 1979, 1979: 297–306. 10.2307/1443418
Taigen TL, Wells KD: Energetics of vocalization by an anuran amphibian ( Hyla versicolor ). J Comp Physiol B 1985, 155: 163–170. 10.1007/BF00685209
Tárano Z: Variation in male advertisement calls in the Neotropical frog Physalaemus enesefae . Copeia 2001, 2001: 1064–1072. 10.1643/0045-8511(2001)001[1064:VIMACI]2.0.CO;2
Tobias ML, Barnard C, Hagan RO, Horng SH, Rand M, Kelley DB: Vocal communication between male Xenopus laevis . Anim Behav 2004, 67: 353–365. 10.1016/j.anbehav.2003.03.016
Tumkiratiwong P, Meesuk W, Chanhome L, Aowphol A: Reproductive patterns of captive male and female monocled cobra, Naja kaouthia (Lesson, 1831). Zool Stud 2012, 51: 692–700.
Turner FB: Population structure and dynamics of the western spotted frog, Rana p. pretiosa Baird & Girard, in Yellowstone Park. Wyoming Ecol Monogr 1960, 30: 251–278. 10.2307/1943562
Vo VP, Le TS, Le VK, Ngo DC: Animal biodiversity of Bach Ma National Park. In The problems of basic research in life sciences. Edited by: Nguyen VS, Nguyen VK, Le VC. Science and Technology Publishing House, Hanoi, Vietnam; 2003:205–209. (in Vietnamese)
Wake MH, Dickie R: Oviduct structure and function and reproductive modes in amphibians. J Exp Zool 1998, 282: 477–506. 10.1002/(SICI)1097-010X(199811/12)282:4/5<477::AID-JEZ6>3.0.CO;2-#
Wang YL, Zhang J, Li XQ, Ren BZ: Acoustic and molecular differentiation between macropters and brachypters of Eobiana engelhardti engelhardti (Orthoptera: Tettigonioidea). Zool Stud 2011, 50: 636–644.
Wei L, Zhao L, Ma X, Fan X, Ma X, Lin Z: Advertisement call variability in the black-spined toad Bufo melanostictus (Anura: Bufonidae) during the breeding season in Lishui, Zhejiang. China Asian Herpetol Res 2012, 3: 157–162.
Wells KD: The ecology and behavior of amphibians. University of Chicago Press, Chicago; 2007.
Wells KD, Bard KM: Vocal communication in a neotropical treefrog, Hyla ebraccata : responses of females to advertisement and aggressive calls. Behaviour 1987, 101: 200–210. 10.1163/156853987X00431
Wells KD, Schwartz JJ: Vocal communication in a Neotropical treefrog, Hyla ebraccata : aggressive calls. Behaviour 1984, 91: 128–145. 10.1163/156853984X00254
Wollenberg KC, Glaw F, Meyer A, Vences M: Molecular phylogeny of Malagasy reed frogs, Heterixalus , and the relative performance of bioacoustics and color-patterns for resolving their systematics. Mol Phylogenet Evol 2007, 45: 14–22. 10.1016/j.ympev.2007.06.024
Acknowledgements
We wish to thank the heads of the Department of Biology, College of Education, Hue University, Vietnam and of the Department of Life Sciences, National Cheng Kung University, Taiwan for their support of this study. We would like to thank two anonymous reviewers for critically reading the manuscript, the management board of Bach Ma National Park, and staff of the A Luoi and A Pat Forest Stations, border stations 629 and 633 for their help during fieldwork in Nam Dong and Phu Loc Districts. Lastly, we heartily thank TX Nguyen, H Ngo, CA Ho, OV Ho, PV Ho, BV Ho, BV Ho, and SV Ho for their support during fieldwork in 2009 to 2011.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
Both authors declare that they have no competing interests.
Authors’ contributions
BVN carried out the reproductive and call studies, directly participated in the sampled collection and data analysis, and wrote the manuscript. CDN originally formulated the idea, carried out the scientific guide and critically reading the manuscript. Both authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Ngo, B.V., Ngo, C.D. Reproductive activity and advertisement calls of the Asian common toad Duttaphrynus melanostictus (Amphibia, Anura, Bufonidae) from Bach Ma National Park, Vietnam. Zool. Stud. 52, 12 (2013). https://doi.org/10.1186/1810-522X-52-12
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1810-522X-52-12