Abstract
Background
Patients with advanced melanoma are faced with a poor prognosis and, until recently, limited treatment options. Ipilimumab, a novel immunotherapy that blocks cytotoxic T-lymphocyte-associated antigen-4, was the first agent to improve survival of patients with advanced melanoma in a randomised, controlled phase 3 trial. We used data from an expanded access programme (EAP) at Italian centres to evaluate the clinical activity and safety profile of ipilimumab 10 mg/kg in patients with advanced melanoma in a setting more similar to that of daily practice.
Methods
Data were collected from patients enrolled in an ipilimumab EAP across eight participating Italian centres. As per the EAP protocol, patients had life-threatening, unresectable stage III/IV melanoma, had failed or did not tolerate previous treatments and had no other therapeutic option available. Treatment comprised ipilimumab 10 mg/kg every 3 weeks for a total of four doses. If physicians believed patients would continue to derive benefit from ipilimumab treatment, maintenance therapy with ipilimumab 10 mg/kg was provided every 12 weeks. Tumour responses were assessed every 12 weeks using modified World Health Organization criteria and safety continuously monitored.
Results
Seventy-four pretreated patients with advanced melanoma were treated with ipilimumab 10 mg/kg. Of these, 9 (13.0%) had an objective response, comprising 3 patients with a complete response and 6 with a partial response. Median overall survival was 7.0 months (95% confidence interval, 5.3–8.7) and 16.6% of patients were alive after 3 years. Forty-five patients (60.8%) reported treatment-related adverse events of any grade, which were most commonly low-grade pruritus, pain, fever and diarrhoea. Grade 3 or 4 treatment-related AEs were reported in 8 patients (10.8%).
Conclusions
The clinical activity and safety profile of ipilimumab 10 mg/kg in the EAP was similar to that seen in previous clinical trials of ipilimumab in pretreated patient populations.
Similar content being viewed by others
Background
Until recently, patients with unresectable stage III and IV (advanced) melanoma faced a dismal prognosis and had limited treatment options [1–4]. However, advances in the understanding of cancer immunology and the molecular pathways involved in melanoma pathogenesis led to exciting developments and new treatments with the potential to provide patients with improved survival. In 2011, two new agents were approved for the treatment of patients with advanced melanoma; ipilimumab, a novel immunotherapy, and vemurafenib, a specific BRAF inhibitor for patients with BRAFV600-mutation positive melanoma. These agents have changed the treatment landscape for this difficult to treat disease, with other novel therapeutic approaches on the horizon [5, 6].
Ipilimumab is a fully-humanised monoclonal antibody that blocks cytotoxic T-lymphocyte-associated antigen-4, a negative regulator of T cells. This blockade potentiates T-cell activation, proliferation and infiltration into tumours [7]. The antitumour activity of ipilimumab in patients with advanced melanoma has been demonstrated in a number of phase 2 trials [8–12] and in two phase 3 trials; one as monotherapy in patients with pretreated metastatic melanoma at a dose of 3 mg/kg, and the second in combination with chemotherapy in patients with previously untreated metastatic melanoma at a dose of 10 mg/kg [13, 14]. Recent updates from clinical trials of pretreated and treatment-naïve patients treated with ipilimumab 10 mg/kg have shown long-term clinical benefit, with some patients surviving at least 5 years [15, 16]. Most adverse events (AEs) to ipilimumab reported in clinical trials are low-grade and immune-related and, in most cases, can be managed with appropriate medical therapy, treatment interruption or withdrawal [17–19].
Clinical trials, by virtue of design, include highly-selected patient populations and investigative agents are provided according to tightly-regulated protocols. The ipilimumab Expanded Access Programme (EAP; formerly known as the ipilimumab Compassionate Use Programme) was initiated to provide ipilimumab to patients who were not eligible to receive ipilimumab within clinical trials, therefore providing a real-world perspective on the efficacy, safety and general management of ipilimumab in a setting representative of daily clinical practice. Initially, ipilimumab was provided at a dose of 10 mg/kg; however, the protocol was later amended to allow the use of ipilimumab 3 mg/kg, in line with the approved European label.
Results from 27 patients treated with ipilimumab 10 mg/kg at a single centre in the EAP in Italy have previously been reported [20, 21]. Here, we provide long-term follow-up from all patients treated with ipilimumab 10 mg/kg within a participating Italian centre.
Methods
Patient population
This was a retrospective analysis of data from patients whose physician requested compassionate use of ipilimumab through the EAP. Patients older than 16 years of age with histologically confirmed unresectable stage III and stage IV skin, ocular or mucosal melanoma were eligible for inclusion in the EAP. Patients must have failed systemic therapy, been intolerant to ≥1 systemic therapy, or had no other therapeutic option available to them. An Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0, 1, or 2 was required, and an interval of at least 28 days since treatment with chemotherapy, biochemotherapy, surgery, radiation, or immunotherapy was recommended. Patients with asymptomatic brain metastases were allowed. Exclusion criteria included any other systemic therapy for melanoma, concomitant autoimmune diseases or other malignancies, and known HIV, hepatitis B, or hepatitis C infection.
Study design and data collection
Ipilimumab 10 mg/kg was administered intravenously over 90 minutes every 3 weeks for a total of four doses (induction phase). Eligible patients could receive maintenance therapy with ipilimumab 10 mg/kg every 12 weeks, if tolerated, for as long as the physician believed that benefit would be derived from treatment. Patients who progressed following either stable disease (SD) of ≥3 months’ duration or an initial objective response (partial [PR] or complete response [CR]) were offered retreatment with the same dosing schedule used during the induction phase.
Treatment was discontinued in cases of confirmed disease progression as determined using modified World Health Organization (mWHO) criteria, a related AE necessitating discontinuation of ipilimumab, clinical deterioration (as per protocol), withdrawal of consent, or pregnancy.
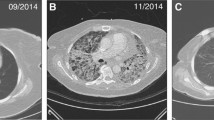
Tumour assessments utilising helical (spiral) computerised tomography scans of brain, chest, abdomen, and pelvis, were conducted at baseline and every 12 weeks thereafter. Clinical response was defined according to mWHO criteria as CR, PR, SD or progressive disease (PD). Disease control rate (DCR) was defined as the percentage of patients achieving CR, PR, or SD lasting at least 24 weeks from the first dose of ipilimumab.
Safety was continuously monitored and assessed in all patients who received ipilimumab in the EAP. AEs were graded according to the National Cancer Institute Common Terminology Criteria for AEs, version 3.0.
Statistical analysis
Data were analysed using descriptive statistics, such as median and range. Progression-free survival (PFS) and overall survival (OS) were estimated using Kaplan–Meier analysis and expressed as median values with corresponding two-sided 95% confidence intervals (CIs).
Results
Patients
As part of the EAP, 74 patients with advanced melanoma were treated with ipilimumab 10 mg/kg across eight participating Italian centres. Baseline characteristics of these patients are provided in Table 1. Of the 74 patients treated with ipilimumab, 43 (58.1%) received all four induction doses, 14 (18.9%) received three doses, 5 (6.8%) received two doses and 12 (16.2%) received only one dose. Reasons for discontinuation comprised disease progression (n = 13), death due to disease progression (n = 10), loss to follow-up (n = 5), study drug toxicity (n = 2), and unknown reasons (n = 1). The median number of doses received was four (range: 1–4). Twenty-six patients (35.1%) received maintenance treatment with ipilimumab, with a median number of cycles of two (range: 1–13). Reasons for not receiving maintenance therapy were disease progression (n = 30), death due to disease progression (n = 9), loss to follow up (n = 5) and toxicity (n = 2; 1 patient with grade 2 diarrhoea and nausea, and 1 with grade 1 diarrhoea). As of December 2012, 6 patients were still receiving maintenance therapy. Reasons for discontinuing maintenance therapy were disease progression (n = 14), death due to disease progression (n = 4) and physician decision (n = 1). Two patients with disease progression after four cycles of maintenance therapy were retreated with ipilimumab 10 mg/kg every 3 weeks for a total of four doses; an additional patient with disease progression who did not receive maintenance therapy was retreated with ipilimumab at 3 mg/kg every 3 weeks for a total of four doses.
Tumour response
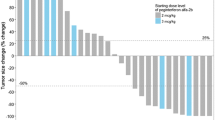
Of all treated patients, 69 were evaluable for tumour response (1 patient received three infusions but was not assessed by CT scan, 2 patients were lost to follow-up and 2 patients stopped treatment: 1 due to toxicity and the other for unspecified reasons). Tumour responses according to mWHO criteria are summarised in Table 2. At week 12, 6 patients (8.7%) had an objective response, including 1 patient (1.5%) with a CR and 5 (7.2%) with a PR. Responses to ipilimumab continued to improve beyond week 12: across all treatment phases, a total of 9 patients (13.0%) had an objective response, including 3 patients (4.3%) with a CR and 6 (8.7%) with a PR. Of the 3 patients with an improved response, 1 patient with disease progression improved to CR, and 2 with SD improved to a PR and CR, respectively. The median duration of response has not yet been reached. In total, 22 patients (31.9%) achieved disease control. The median duration of SD was 10 months. Of the 2 patients retreated with ipilimumab 10 mg/kg, 1 regained disease control. The patient retreated with ipilimumab 3 mg/kg also regained disease control, as previously described [21]. Among the 11 patients with brain metastases at baseline, 10 were evaluable for response. Best response to treatment was SD in 2 (20.0%) patients and PD in 8 patients (80.0%).
Survival
With a median follow-up of 44 months, median OS was 7.0 months (95% CI, 5.3–8.7) for all patients (Figure 1) and 4.0 months (95% CI, 2.4–5.6) for the 11 patients with brain metastases. Excluding patients with brain metastases did not impact median OS (7.0 months [95% CI, 6.2–9.8]). The 1, 2 and 3-year OS rates were 30.9%, 19.6% and 16.6%, respectively. Ten patients had long-term survival of at least 3 years. The median lactate dehydrohenase (LDH) level for these patients was 280 units/L, and their best response to induction therapy was a PR in 3 patients (30.0%), SD in 6 patients (60.0%) and PD in 1 patient (10.0%). With a median 13 cycles of ipilimumab maintenance therapy, there was an evolution in best response with 2 patients (20.0%) having a CR, and 4 patients (40.0%) each having a PR or SD. Median PFS was 3.0 months (95% CI, 2.3–3.7) for all patients (Figure 2), 3.0 months (95% CI, 2.4–3.6) for the 11 patients with brain metastases and 4.0 months (95% CI, 2.9–5.0) when patients with brain metastases were excluded. As with OS, there was a plateau in PFS after 2 years. Of the 10 patients who survived more than 3 years, only 2 (20.0%) subsequently progressed (after 45 and 57 months, respectively).
Safety
Of the 74 treated patients, 45 (60.8%) reported at least 1 AE that was considered related to ipilimumab treatment. These are detailed in Table 3. The most commonly reported AEs were pruritus, pain, fever and diarrhoea. Most AEs were grade 1 or 2, with only 8 grade 3 or 4 events reported (by 8 patients [10.8%] in total). Grade 3 or 4 events comprised 2 reports each of diarrhoea and pain; and 1 report each of fever, epigastric pain, elevated aspartate aminotransferase (AST) and pancytopenia. Time to onset of these events was 10–73 days for the diarrhoea, increased AST and pain, and 21 days for fever and pancytopenia. As previously described, grade 4 pancytopenia was successfully managed through the discontinuation of ipilimumab and use of supportive medications (growth factors, transfusions and antibiotics), immunoglobulins and immunosuppressive therapy (cyclosporin) [20].
Discussion
In clinical trials of patients with advanced melanoma, ipilimumab has been shown to provide long-term clinical benefit and have a manageable safety profile [8–14, 19]. To evaluate the efficacy and safety profile of ipilimumab in a setting more representative of daily clinical practice, we analysed data from 74 heavily pretreated patients who received ipilimumab 10 mg/kg as part of an EAP in Italy. With an estimated 44 months follow-up across all eight participating centres, median OS was 7.0 months and one-fifth patients had long-term survival of at least 2 years, with approximately 17% of patients alive at 3 years. These findings are consistent with data from clinical trials [22]. In a recent analysis, survival data was pooled from 4846 individual patients treated with ipilimumab within clinical studies or the US EAP. The analysis showed a plateau in OS beginning after approximately 3 years with follow-up of up to 10 years in some patients. Approximately 21% of patients were alive at three years, and survival outcomes did not appear to be impacted by prior therapy, dose or treatment regimen [22]. These data support the durability of long-term survival with ipilimumab and suggest that if patients respond to treatment and are still alive 2 or 3 years after treatment, they have a good chance of achieving long-term tumour control. Indeed, among the 24 patients treated at the University Hospital of Siena in the current analysis, 20% were alive at 4 years, further exemplifying the consistency in long-term survival outcomes [21].
At the first tumour assessment (week 12), 9% of patients in this analysis had achieved an objective response. This is also consistent with previous phase 2 and 3 trials of ipilimumab monotherapy in pretreated populations, with rates ranging from 4–11% with ipilimumab 3 mg/kg and 6–11% with ipilimumab 10 mg/kg [9, 12, 13].
In previous phase 2 clinical trials of pretreated patients who received ipilimumab 10 mg/kg, median OS has been shown to be approximately 10 months [9, 12]. As expected, the median OS reported in this analysis is shorter. Patients had a particularly poor prognosis and in most cases had failed to respond to one or more prior treatments for metastatic disease. Their disease was therefore very advanced. Indeed, most patients (72%) had M1c disease at the time of enrolment. Previous studies have highlighted poor PS, presence of visceral disease, brain metastases, elevated LDH and disease M-stage as statistically significant prognostic factors of poor OS [2, 23]. Interestingly, in this analysis, LDH at baseline was lower among patients who survived more than 3 years than for all treated patients: median 280 units/L (range: 59–913) vs 466 units/L (range: 139–4416). Considering the median OS, tumour responses, and 3-year survival rate of patients analysed here, the efficacy of ipilimumab in a real-world setting appears consistent with that observed in selected clinical trial populations.
Beyond week 12, the percentage of patients experiencing an objective response increased. This apparent evolution in responses may reflect the indirect, immune-mediated mechanism of action of ipilimumab. Because it can take time to build an immune response against a tumour, clinically measurable antitumour effects may occur over weeks to months and can be observed after the appearance of new lesions or an initial increase in tumour volume, or in the form of a delayed, slow, steady decline in total tumour volume [14, 24, 25]. In most cases, clinical benefit with ipilimumab comprised durable SD, which is often the predominant response of patients receiving ipilimumab. In clinical trials, survival outcomes among patients with SD are similar to those of patients with an objective response to ipilimumab [26], suggesting that SD is a meaningful clinical outcome for patients treated with this agent. This observation that most patients seem to obtain a state of tumour control rather than one of complete tumour eradication gives credence to the concept that ‘clinical cure’ or long-term cancer containment is possible, and requires induction of an antitumour immune response [27].
The AE profile seen in patients receiving ipilimumab in the EAP was consistent with that reported in previous clinical trials of ipilimumab 10 mg/kg, with most events being dermatological or gastrointestinal in nature [9, 11, 12]. Among 155 patients treated with ipilimumab 10 mg/kg in a phase 2 trial, 84% of patients had treatment-related AEs of any grade, with one treatment-related death resulting from liver failure [9]. In our analysis, 61% of patients had treatment-related AEs of any grade and there were no treatment-related deaths. Rates of grade ≥3 AEs appeared similar to those seen in previous clinical trials of ipilimumab 3 mg/kg, but were lower than has been observed in clinical trials of ipilimumab 10 mg/kg [8, 9, 11–13]. In a trial of ipilimumab 0.3, 3 and 10 mg/kg in pretreated patients with advanced melanoma, the rate and severity of AEs increased with increasing dose; therefore the lack of high-grade events in this analysis is perhaps surprising, although the retrospective nature of this analysis may have influenced the number of AEs reported. Prompt recognition of symptoms and appropriate management are essential to minimise life-threatening complications from ipilimumab. A decrease in the percentage of patients requiring intervention for bowel perforation from 0.9% to 0.5%, for example, was thought to be due to the introduction of management guidelines established over the course of ipilimumab’s clinical development [28]. It is possible, therefore, that an increased awareness of the specific AEs associated with ipilimumab, together with the implementation and consistent use of established treatment algorithms, may have contributed to the safety profile observed in this analysis. The availability of these treatment algorithms also means that, after thorough education on the appropriate management methods, ipilimumab 10 mg/kg can be administered by community-based clinicians, thus extending its use beyond specialised treatment centres.
Conclusions
Results from this analysis of heavily pretreated patients with advanced melanoma who received ipilimumab 10 mg/kg as part of an EAP in Italy suggest that the clinical activity and safety profile of ipilimumab in a real-world setting is similar to that observed in clinical trials. Importantly, long-term survival benefits were observed in patients who had a particularly poor prognosis and had failed to benefit from prior therapy, with some patients surviving at least 3 years from the start of ipilimumab treatment. Although further studies are needed to establish which dose of ipilimumab (3 or 10 mg/kg) will provide patients with the most appropriate risk to benefit ratio, this analysis shows that in the daily-clinical setting, ipilimumab 10 mg/kg has clinical activity in patients with advanced melanoma with manageable side effects.
References
Agarwala SS: Current systemic therapy for metastatic melanoma. Expert Rev Anticancer Ther. 2009, 9: 587-595. 10.1586/era.09.25.
Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG, Ding S, Eggermont AM, Flaherty KT, Gimotty PA, Kirkwood JM, McMasters KM, Mihm MC, Morton DL, Ross MI, Sober AJ, Sondak VK: Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009, 27: 6199-6206. 10.1200/JCO.2009.23.4799.
Tarhini AA, Agostara B: Cutaneous melanoma: available therapy for metastatic disease. Dermatol Ther. 2006, 19: 19-25. 10.1111/j.1529-8019.2005.00052.x.
Maio M, Ascierto P, Testori A, Ridolfi R, Bajetta E, Queirolo P, Guida M, Romanini A, Chiarion-Sileni V, Pigozzo J, Di Giacomo AM, Calandriello M, Didoni G, van Baardewijk M, Konto C, Lucioni C: The cost of unresectable stage III or stage IV melanoma in Italy. J Exp Clin Cancer Res. 2012, 31: 91-103. 10.1186/1756-9966-31-91.
Dummer R, Guggenheim M, Arnold AW, Braun R, von Moos R, Project Group Melanoma of the Swiss Group for Clinical Cancer Research: Updated swiss guidelines for the treatment and follow-up of cutaneous melanoma. Swiss Med Wkly. 2011, 141: w13320-
Finn L, Markovic SN, Joseph RW: Therapy for metastatic melanoma: the past, present, and future. BMC Med. 2012, 10: 23-10.1186/1741-7015-10-23.
Fong L, Small EJ: Anti-cytotoxic T-lymphocyte antigen-4 antibody: the first in an emerging class of immunomodulatory antibodies for cancer treatment. J Clin Oncol. 2008, 26: 5275-5283. 10.1200/JCO.2008.17.8954.
Hamid O, Schmidt H, Nissan A, Ridolfi L, Aamdal S, Hansson J, Guida M, Hyams DM, Gómez H, Bastholt L, Chasalow SD, Berman D: A prospective phase II trial exploring the association between tumor microenvironment biomarkers and clinical activity of ipilimumab in advanced melanoma. J Transl Med. 2011, 9: 204-10.1186/1479-5876-9-204.
O’Day SJ, Maio M, Chiarion-Sileni V, Gajewski TF, Pehamberger H, Bondarenko IN, Queirolo P, Lundgren L, Mikhailov S, Roman L, Verschraegen C, Humphrey R, Ibrahim R, de Pril V, Hoos A, Wolchok JD: Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann Oncol. 2010, 21: 1712-1717. 10.1093/annonc/mdq013.
Prieto PA, Yang JC, Sherry RM, Hughes MS, Kammula US, White DE, Levy CL, Rosenberg SA, Phan GQ: CTLA-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanoma. Clin Cancer Res. 2012, 18: 2039-2047. 10.1158/1078-0432.CCR-11-1823.
Weber J, Thompson JA, Hamid O, Minor D, Amin A, Ron I, Ridolfi R, Assi H, Maraveyas A, Berman D, Siegel J, O’Day SJ: A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res. 2009, 15: 5591-5598. 10.1158/1078-0432.CCR-09-1024.
Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, Waterfield W, Schadendorf D, Smylie M, Guthrie T, Grob JJ, Chesney J, Chin K, Chen K, Hoos A, O’Day SJ, Lebbé C: Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010, 11: 155-164. 10.1016/S1470-2045(09)70334-1.
Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbé C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ: Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010, 363: 711-723. 10.1056/NEJMoa1003466.
Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, Davidson N, Richards J, Maio M, Hauschild A, Miller WH, Gascon P, Lotem M, Harmankaya K, Ibrahim R, Francis S, Chen TT, Humphrey R, Hoos A, Wolchok JD: Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011, 364: 2517-2526. 10.1056/NEJMoa1104621.
Lebbe C, Weber JS, Maio M, Neyns B, Harmankaya K, Hamid O, O’Day S, Chin KM, Opatt McDowell D, Cykowski L, McHenry B, Wolchok JD, Lebbe C, Weber JS, Maio M, Neyns B, Harmankaya K, Hamid O, O’Day S, Chin KM, Opatt McDowell D, Cykowski L, McHenry B, Wolchok JD: Long-term survival in patients with metastatic melanoma who received ipilimumab in four phase II trials [abstract]. J Clin Oncol. 2013, 31: 561s-
Maio M, Bondarenko I, Robert C, Thomas L, Garbe C, Testori A, Francis S, Chin K, Wolchok J: Four-year survival update for metastatic melanoma patients treated with ipilimumab plus dacarbazine in phase 3 study CA184-024 [abstract]. Ann Oncol. 2012, 23: ix367-10.1093/annonc/mdr122.
Andrews S, Holden R: Characteristics and management of immunerelated adverse effects associated with ipilimumab, a new immunotherapy for metastatic melanoma. Cancer Manag Res. 2012, 4: 299-307.
Di Giacomo AM, Biagioli M, Maio M: The emerging toxicity profiles of anti-CTLA-4 antibodies across clinical indications. Semin Oncol. 2010, 37: 499-507. 10.1053/j.seminoncol.2010.09.007.
Weber JS, Kahler KC, Hauschild A: Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012, 30: 2691-2697. 10.1200/JCO.2012.41.6750.
Di Giacomo AM, Danielli R, Calabro L, Bertocci E, Nannicini C, Giannarelli D, Balestrazzi A, Vigni F, Riversi V, Miracco C, Biagioli M, Altomonte M, Maio M: Ipilimumab experience in heavily pretreated patients with melanoma in an expanded access program at the university hospital of Siena (Italy). Cancer Immunol Immunother. 2011, 60: 467-477. 10.1007/s00262-010-0958-2.
Di Giacomo AM, Calabrò L, Danielli R, Fonsatti E, Bertocci E, Pesce I, Fazio C, Cutaia O, Giannarelli D, Miracco C, Biagioli M, Altomonte M, Maio M: Long-term survival and immunological parameters in metastatic melanoma patients who responded to ipilimumab 10 mg/kg within an expanded access programme. Cancer Immunol Immunother. 2013, 62: 1021-1028. 10.1007/s00262-013-1418-6.
Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, Chen TT, Berman DM, Wolchok JD: Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in metastatic or locally advanced, unresectable melanoma. Eur J Cancer. 2013, 49 (2): 24LBA-
Korn EL, Liu PY, Lee SJ, Chapman JA, Niedzwiecki D, Suman VJ, Moon J, Sondak VK, Atkins MB, Eisenhauer EA, Parulekar W, Markovic SN, Saxman S, Kirkwood JM: Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol. 2008, 26: 527-534. 10.1200/JCO.2007.12.7837.
Hoos A, Eggermont AM, Janetzki S, Hodi FS, Ibrahim R, Anderson A, Humphrey R, Blumenstein B, Old L, Wolchok J: Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst. 2010, 102: 1388-1397. 10.1093/jnci/djq310.
Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbé C, Maio M, Binder M, Bohnsack O, Nichol G, Humphrey R, Hodi FS: Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009, 15: 7412-7420. 10.1158/1078-0432.CCR-09-1624.
Pennock GK, Waterfield W, Wolchok JD: Patient responses to ipilimumab, a novel immunopotentiator for metastatic melanoma: how different are these from conventional treatment responses?. Am J Clin Oncol. 2012, 35: 606-611. 10.1097/COC.0b013e318209cda9.
Eggermont AM, Kroemer G, Zitvogel L: Immunotherapy and the concept of a clinical cure. Eur J Cancer. 2013, 49: 2965-2967. 10.1016/j.ejca.2013.06.019.
Kahler KC, Hauschild A: Treatment and side effect management of CTLA-4 antibody therapy in metastatic melanoma. J Dtsch Dermatol Ges. 2011, 9: 277-286.
Acknowledgements
This work was supported in part by the Associazione Italiana per la Ricerca sul Cancro and Italian Ministry of Health, via the Ricerca Finalizzata 2010. The EAP was sponsored by Bristol-Myers Squibb (BMS). Editorial and writing assistance was provided by Fiona Bolland of StemScientific, funded by BMS. The authors would like to thank the patients and investigators who participated in the European EAP.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
Paolo Ascierto has served in a consultancy/ advisory role for Bristol-Myers Squibb (BMS), Merck Sharp & Dohme (MSD), Roche-Genentech, GlaxoSmithKline (GSK), Amgen and Celgene; has received research funding from BMS, and honoraria from BMS, MSD, Roche-Genentech and GSK. Paola Queirolo has served in a consultant or advisory role for BMS, GSK and Roche-Genentech. Riccardo Danielli has received honoraria from BMS for meeting presentations. Vanna Chiarion-Sileni has had an advisory role for BMS, GSK, MSD and Roche-Genentech. Pier Francesco Ferrucci received honoraria from BMS for participation in a speaker training programme. Michele Maio has had an advisory role and received funding for communication programs from BMS, Roche-Genentech and MSD and has received research funding from BMS. None of the other authors have any conflicts of interest to disclose.
Authors’ contributions
All authors made substantial contributions to the acquisition and interpretation of data. Statistical support was provided by Dr Giannarelli. All authors were involved in drafting the article or revising it critically for important intellectual content and provided final approval of the version to be published.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Altomonte, M., Di Giacomo, A.M., Queirolo, P. et al. Clinical experience with ipilimumab 10 mg/kg in patients with melanoma treated at Italian centres as part of a European expanded access programme. J Exp Clin Cancer Res 32, 82 (2013). https://doi.org/10.1186/1756-9966-32-82
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1756-9966-32-82