Abstract
Background
To evaluate the expression of EGFR and COX-2 and their correlation with prognosis in NSCLC
Methods
The paraffin embedded tumor samples of 50 NSCLC patients receiving radical resection were analyzed immunohistochemically for EGFR and COX-2 expression and their prognostic values were explored.
Results
The positive rate of EGFR protein in NSCLC tumor cells was 46%, which was significantly higher than its expression in normal lung (p = 0.0234) and paracancerous tissues (p = 0.020). EGFR expression was significantly higher in nodal positive than in nodal negative patients (p = 0.04). The mean survival time for EGFR positive patients (31 months) was significantly lower than that for patients with EGFR negative expression (48 months) (p = 0.008,). In patients receiving post-operation thoracic irradiation, the mean survival time for EGFR positive patients was significantly lower than that for patients without EGFR positive expression (25 vs. 48 months, P = 0.004). The positive rate of COX-2 protein expression in NSCLC tumor cells was 90%, which was significantly higher than that in normal tissue(p = 0.00) and paracancerous tissue (p = 0.00). There was no correlation between COX-2 expression and patient survival, and no correlation between COX-2 and EGFR protein expression (P = 0.555).
Conclusions
COX-2 and EGFR are over-expressed in NSCLC. EGFR is an independent prognostic factor and a predictive factor for radiotherapy response in NSCLC.
Similar content being viewed by others
Background
Lung cancer is the leading cause of death world wide. The non-small cell lung cancer (NSCLC) accounts for 75-85% among all lung cancers. The conventional treatment e.g. surgery, radiotherapy and chemotherapy yields a dismal overall 5-year survival of 14% which necessitates the development of new treatment options [1]. With advances in cytogenetic and molecular biology, the detection and analysis of tumor suppressor gene and oncogene may provide predictive values for prognosis and treatment choice for NSCLC. Among these molecular markers, the epidermal growth factor receptor (EGFR) and cyclooxygenase-2 (COX-2) over expression are common in NSCLC [2–9].
EGFR (HER1, ErbB) is a transmembrane glycoprotein with three functional domains: an extracellular domain containing two EGF binding sites; a hydrophobic transmembrane domain and a cytoplasmic domain (tyrosine kinase (TK) and a carboxyl autophosphorylation region) [10, 11]. EGFR is abnormally upregulated and activated in a variety of tumors [12]. Deregulation of receptor tyrosine kinases as a result of overexpression or activating mutations leads to the promotion of cell proliferation or migration, inhibition of cell death, or the induction of angiogenesis [13, 14].
The expression and activity of EGFR are determinants of response to target therapy and radiosensitivity in several tumour types [15]. EGFR overexpression in non-small cell lung cancer (NSCLC) is variable ranging from 19% to 89% and its prognostic value remains controversial [16, 17]. COX-2 over expression is also found in many tumor types [18]. The carcinogenic effect of COX-2 mainly exerted through the increase of prostaglandin levels (PGE2, PGF2a, PGD2, TXA2, PGI2 and PGJ2). In lung cancer, COX-2 expression has been reported to inhibit apoptosis [19], promote angiogenesis [20] and metastasis [2]. It has been reported in a recent meta-analysis that COX-2 might be an independent prognostic factor for NSCLC [21]. COX-2 inhibitor has been investigated in both pre-clinical and clinical study, and has shown synergistic effects with radiation and chemtoxic drugs on tumor [3, 22]. COX-2 catalyzes the conversion of arachidonic acid into prostanoids including prostaglandin E2, which is often associated with oncogenesis of lung tumors. The oncogenic signals are transducted through the MAPK/Erk pathway [23] which therefore closely correlates EGFR with COX-2. A number of in vitro studies have postulated a link between EGFR activation and subsequent COX-2 upregulation. The relationship between these factors has not been established in patients with NSCLC.
In order to evaluate the EGFR and COX-2 expression and their impact on prognosis of NSCLC patients receiving post-operative adjuvant therapy, the paraffin embedded tumor samples from 50 NSCLC were analyzed immunohistochemically for EGFR and COX-2 expression and their prognostic values were explored.
Methods
Tumor specimen
Paraffin-embedded tissue sections from 50 histopathologically proven NSCLC patients who received radical resection during June 2001 and March 2004 were collected.
Patient data
All patients were histopathologically diagnosed NSCLC and had not received preoperative chemotherapy nor radiotherapy. Among them there were 31 males and 19 females, aged 36-76 (mean 58) years. According to WHO classification (2000), there were 21 squamous, 26 adenomatous and 3 adenosquamous carcinomas, with 40 moderate and well differentiated (G1-G2) and 10 low differentiated (G3). 15 cases were staged I-II and 35 III-IV based on the revised AJC staging for lung cancer (1997). Thirty-nine cases had intra-thoracic lymph node metastasis (N1-N2), and 11 were negative lymph node metastasis. The paracancerous tissues (defined as more than 5 cm away from the carcinoma tissue) taken from 7 cases and the normal tissues from 6 cases were used as controls. All patients received 4 cycles of adjuvant platinum based two drug chemotherapy. Among them, 28 patients received post-operative combined chemotherapy and thoracic radiotherapy and 22 patients had chemotherapy alone.
Immunohistochemistry (IHC)
The paraffin embedded tumor specimens were cut into 4-um sections for IHC staining against EGFR and COX-2 according to the manufacturer's instructions. In brief, after deparaffinization and rehydration, the samples were treated with sodium citrate buffer and microwave for epitope retrieval, block non-specificity antigen with normal goat serum incubating 10 minutes; After a washing procedure with distilled water, tissue sections were covered for 5 min with 3% H2O2 to block endogenous peroxidase, followed by an additional washing procedure with the supplied buffer. Slides were then placed in a 37°C water bath and incubated for 30 min with the primary mouse anti-EGFR MAb (Chemicon International, Inc.) diluted 1:200 and anti-COX-2 MAb (Beijing Zhongsan Biological Company) diluted 1:100. After two rinses in buffer the slides were incubated with the detection system for 30 min. Tissue staining was visualized with a DAB substrate chromogen solution. Slides were counterstained with hematoxylin, dehydrated, and mounted. To validate each staining, the EGFR positive colon cancer section provided with the EGFR kit was used as positive control in each staining run. For COX-2 staining, the positive control used the sample itself (internal control). The negative control for both EGFR and COX-2 used PBS to substitute the primary antibody.
Scoring method
The EGFR positive cell is defined as having clearly shown brownish yellow granules within cytoplasm and cell membrane; the COX-2 positive cell having clearly shown brown granules in cytoplasm; with clear background. Slide evaluation was independently performed by two investigators blinded to all subject characteristics. The slides were first observed for staining status under low power microscope, and then randomly selected 5 fields under high power (200×) light microscope. For assessment of staining positivity, the number of positive cells out of 200 tumor cells in each field was counted. The positive cell counts from all 5 fields were averaged and then divided by the total cell number of 5 fields to get the positivity ratio. Staining positivity was defined if the ratio ≥ 10% (+), and negative if ration < 10% (-). As EGFR and COX-2 were not expressed in normal tissues, any observed positivity of EGFR and COX-2 was thus considered as over expression [4].
Statistical analysis
The data were analyzed using SPSS 13.0 software package. The correlation of EGFR expression with different clinical characteristics was analyzed with chi-square test. COX proportional-hazards model was used to analyze the correlation of survival with various clinical characteristics and EGFR protein expression. The Kaplan-Meier method and Log-rank test were used to analyze the correlation of patient survival with EGFR expression. A significance level of P < 0.05 was used.
Results
EGFR protein expression
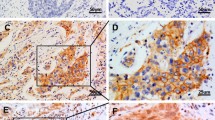
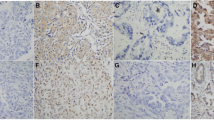
The positive rate of EGFR protein in NSCLC tumor cells were 46%, which was significantly higher than its expression in normal lung (p = 0.0234) and paracancerous (p = 0.020)(Figures 1A & 1B, Tables 1 & 2).
Correlation between EGFR expression and clinical features
The expression of EGFR in different subgroups were compared and summarized in Table 3. It shows that the difference of EGFR expression was only significant between the nodal positive and negative subgroups (56.4% vs.10%, p = 0.04). There is no significant difference between age (60 vs. under 60 ys), gender, adeno- vs. non-adenocarcinoma, the differentiation of tumor, and staging.
EGFR expression and overall survival
Cox proportional hazards analysis showed that EGFR protein positive expression independently predicted patient survival, with RR of 2.311, p = 0.038, and 95% confidence interval (CI) of 1.049 - 5.095. The mean survival time for EGFR positive patients was 31 months, whereas the survival time was 48 months for patients with EGFR negative expression, with the latter significantly longer than the former (p = 0.008, Log Rank (Mantel-Cox))(Figure 2).
EGFR expression and outcome of radiotherapy
In patients receiving post-operation thoracic irradiation, the mean survival time for EGFR positive patients (n = 15)was 25 months which was significantly shorter than that (48 months)for patients (n = 13)with EGFR negative expression (P = 0.004)(Figure 3).
COX-2 expression
The positive rate of COX-2 protein expression in NSCLC tumor cells was 90%, which was significantly higher than that in normal tissue(p = 0.00) and paracancerous tissue (p = 0.00)(Figure 4, Tables 4 and 5).
The COX-2 expression was 100% in adenocarcinoma and significantly higher than that in squamous carcinoma (76.2%) of the lung. No correlation was found between COX-2 expression and patient survival (Figures 4, Table 6).
EGFR and COX-2 expression on chemotherapy outcome
Based on COX proportional hazards analysis which also takes account of other clinical characteristics, there was no correlation of EGFR and COX-2 expression with overall survival in 22 patients receiving chemotherapy alone (P > 0.05).
Correlation of EGFR and COX-2 expression
As shown in Table 7, no correlation was found between COX-2 and EGFR protein expression (Χ2 = 0.112, P = 0.555).
Discussion
EGFR and COX-2 are molecular targets which have shown importance for NSCLC. Previous studies reported that the levels of EGFR and COX-2 expression might correlate with poor disease prognosis and reduced survival [20, 24]. In this study the prognostic values of EGFR and COX-2 were evaluated with immunohistochemical assay.
Activation of the EGFR results in activation of downstream signaling pathways, including the Ras-Raf-MKK-extracellular signal-regulated kinase (ERK) and lipid kinase phosphatidylinositol 3-kinase/Akt pathways. Dysregulation of these pathways can result in oncogenesis and cancer progression [4, 25–27]. Similarly, our results implied that EGFR over-expression participated in lung cancer development. EGFR expression was negative in paracancerous and normal tissues, which was significantly lower than that in lung cancer tissue (46%)(P < 0.05). It was similarly reported in studies with the utilization of the immunohistochemical assay that EGFR expression was very low in normal tissue but often over-expressed in lung cancer tissue. In normal tissue, EGFR expression was limited to the basal layer of the epithelium where proliferation occured. EGFR expression was significantly increased in dysplastic cells, indicating that EGFR pathway involves in lung cancer development [28]. Therefore, the detection of EGFR expression in tissue sample before surgery might be helpful in diagnosis of NSCLC.
In our study EGFR expression in NSCLC was not significantly correlated with patients' age, gender, histopathologic type, cell differentiation, tumor size and TNM stages (P > 0.05). However, EGFR over-expression was correlated with lymph node metastasis, the probability of lymph node metastasis was significantly greater in patients with EGFR over-expression than in EGFR negative group (P = 0.006). This might indicate that EGFR was not only involved in cancer genesis but also played an important role in cancer progression. Though EGFR was most commonly found in squamous cell (70%) followed by adenocarcinoma (50%) [29], and large cell carcinomas [28], in our study, EGFR positivity rates were similar between squamous carcinoma (40%) and adenocarcinorma (50%). This discrepancy might be explained by the small sample size of our study which could limit the power of detection.
Our results showed that EGFR positive expression was an independent prognostic factor for NSCLC, among various factors including patient's age, gender, histopathology, tumor differentiation, tumor size, TNM staging and chemotherapy/radiotherapy. Based on the COX proportional hazard analysis adjusting for other significant variables, the mortality of patients with positive tumor EGFR expression was 2.31 times that of the EGFR negative NSCLC (P < 0.05). Nicholson et al[30] reported a meta-analysis based on 200 studies published in Medline between 1985 and 2000, which showed that EGFR over-expression was correlated with patient's prognosis in 10 tumor types. But only 30% of the studies considered EGFR to be associated with NSCLC prognosis. However, it might not be conclusive since some of the studies in the meta-analysis did not include treatment for multivariate analysis, which might have an impact on survival.
A recent study reported that EGFR positive expression assessed by IHC in NSCLC was associated with better survival in patients receiving EGFR TKI [31], which was contrasted to our study that EGFR positivity predicted for worse survival in patients treated with radiotherapy. In our study, for patients receiving radiotherapy, the mean survival for EGFR positive patients (25 months) was significantly lower than that for EGFR non-positive patients (48 months) (p = 0.004). It suggested that EGFR positivity might relate to resistance to radiotherapy, which agreed with the finding from head and neck study that EGFR expression was correlated with radiation resistance [32]. However, no such findings have been reported in NSCLC, and further prospective studies with larger sample size are needed to validate the role of EGFR in NSCLC response to radiation. To better evaluate the prognostic value of EGFR in NSCLC, the detection of activated EGFR (e.g. EGFR phosphorylation) or combined detection with other molecular markers should be used [33].
In our study the positive rate of COX-2 protein expression was 90% for NSCLC tumors and was significantly higher than that for normal lung (0%) and paracancerous tissue (14.3%). Therefore, it suggested that COX-2 might participate in oncogenesis of NSCLC. Similar COX-2 positivity rates ranging from 54 to 100% have been reported for NSCLC tumors as measured by immunohistochemistry [34].
In our study it was found that COX-2 protein expression in adenocarcinoma was significantly higher than that in squamous carcinoma (p = 0.022), which was consistent to previous findings of other study [21]. This might provide basis for applying COX-2 inhibitor in adenocarinoma patients receiving tyrosine kinase inhibitor (TKIs), as COX-2 inhibitor offered synergistic antitumor effects with TKI [21].
Although COX-2 expression was also found higher in female patients, patients with ages≤60 years, non-smokers, moderate and well differentiated tumors, nodal metastasis, and in stages III-IV, the difference had no statistical significance. Studies examining the relationship between COX-2 tumor expression and survival among lung cancer patients were inconsistent, with reports of an inverse relationship with survival [35], no association [36], or a direct association with survival [37]. In our study, there was no correlation between COX-2 expression and patient's overall survival. However, unlike some previously reported studies which showed that COX-2 expression was most consistently associated with poorer survival among stage I and II NSCLC patients [38, 39], our study neither showed the correlation of COX-2 expression with patient's survival nor prognostic value in early stage adenocarcinma [21]. This might be due to the small sample size in our study.
No correlation was found between EGFR expression and COX-2 in our study, though both EGFR and COX-2 involve in the carcinogenesis and progression of NSCLC both individually and, as recently suggested, synergistically [40]. A number of in vitro studies postulated a link between EGFR activation and subsequent COX-2 up-regulation. EGFR activation could induce COX-2 expression via the ras/raf MAPK pathway [3]. On the other hand, COX-2 could induce the activation and expression of EGFR. The lack of correlation of EGFR and COX-2 expression in our study implied that the expression of these 2 proteins might be controlled by independent mechanisms. As suggested by a recent study that examined the expression of p-EGFR, EGFR, and COX-2 by immunohistochemistry in surgically-resected stage I/II NSCLC, pathways other than EGFR activation may influence COX-2 overexpression[38]. Our results were similar: both EGFR and COX-2 are overexpressed in NSCLC; the predominant patterns of COX-2 and EGFR staining were cytoplasmic. However, in our study, the positivity of COX-2 in tumor was as high as 90%, and the number of cases was too small to analyze survival with further stratification between COX-2 and EGFR positive patients. It might be possible that the dual positive expression of COX-2 and EGFR could exert synergistic prognostic and predictive effect on NSCLC survival [31]. Besides, as TKI is becoming the treatment of choice in EGFR gene mutated advanced NSCLC patients, the role of COX-2 positivity on patient's response to TKI might be worthy of further investigation with larger samples. However, it was reported in recently published clinical trials that combined therapy with COX-2 inhibitors and the EGFR inhibitors had no additional benefit in patients who were not responsive to platinum therapy or who were chemotherapy-naive when compared to efficacy reported in previous studies with treatment of EGFR inhibitors alone [41, 42]. Though there was no correlation between EGFR and COX-2 in NSCLC, they might remain as potential, though independent targets for treatment.
Conclusions
In conclusion, this preliminary study illustrated that COX-2 and EGFR are both over-expressed in NSCLC. EGFR not only is an independent prognostic factor for overall survival but also a predictive factor for NSCLC receiving radiotherapy. The prognostic value of EGFR and COX-2 co-expression needs further study.
References
Spira A, Ettinger DS: Multidisciplinary management of lung cancer. N Engl J Med. 2004, 350: 379-392. 10.1056/NEJMra035536. 2004
Dohadwala M, Luo J, Zhu L, Lin Y, Dougherty GJ, Sharma S, Huang M, Põld M, Batra RK, Dubinett : Non-small cell lung cancer cyclooxygenase-2-dependent invasion is mediated by CD44. J Biol Chem. 2001, 276: 20809-20812. 10.1074/jbc.C100140200.
McKay MM, Morrison DK: Integrating signals from RTKs to ERK/MAPK. Oncogene. 2007, 26: 3113-3121. 10.1038/sj.onc.1210394.
Schlessinger J: Cell signalling by receptor tyrosine kinases. Cell. 2000, 103: 211-225. 10.1016/S0092-8674(00)00114-8.
Pold M, Zhu LX, Sharma S, et al.: Cyclooxygenase-2-dependent expression of angiogenic cxc chemokines ena-78/cxc ligand (cxcl) 5 and interleukin-8/cxcl8 in human non-small cell lung cancer. Cancer Res. 2004, 64: 1850-1860.
Choe MS, Zhang X, Shin HJC, Shin DM, Chen Z: Interaction between epidermal growth factor receptor-and cyclooxygenase 2-mediated pathways and its implications for the chemoprevention of head and neck cancer. Mol Cancer Ther. 2005, 4 (9): 1448-55. 10.1158/1535-7163.MCT-04-0251. (Georgia)
Sahin M, Sahin E, Gümüslü S: Cyclooxygenase-2 in cancer and angiogenesis. Angiology. 2009, 60 (2): 242-253.
Lee HJ, Xu X, Choe G, Chung DH, Seo JW, Lee JH, Lee CT, Jheon S, Sung SW, Chung JH: Protein overexpression and gene amplification of epidermal growth factor receptor in nonsmall cell lung carcinomas: Comparison of four commercially available antibodies by immunohistochemistry and fluorescence in situ hybridization study. Lung Cancer. 2009,
Lee JM, Yanagawa J, Peebles KA, Sharma S, Mao JT, Dubinett SM: Inflammation in lung carcinogenesis: new targets for lung cancer chemoprevention and treatment. Crit Rev Oncol Hematol. 2008, 66 (3): 208-17. 10.1016/j.critrevonc.2008.01.004.
Sueoka N, Sato A, Eguchi H, Komiya K, Sakuragi T, Mitsuoka M, Satoh T, Hayashi S, Nakachi K, Sueoka E: Mutation profile of EGFR gene detected by denaturing high-performance liquid chromatography in Japanese lung cancer patients. J Cancer Res Clin Oncol. 2007, 133: 93-102. 10.1007/s00432-006-0144-2.
Yin XW, Jiang XT, Yuan YT, Du YP: Influence of mutations in epidermal growth factor receptor gene on growth, metastasis and survival rate of non-small cell lung carcinoma. Zhonghua Yi Xue Za Zhi. 2010, 90: 1808-12.
Al-haddad S, Zhang Z, Leygue E, Snell L, Huang A, Niu Y, Hiller-Hitchcock T, Baselga J, Arteaga CL: Critical update and emerging trends in epidermal growth factor receptor targeting in cancer. J Clin Oncol. 2005, 23: 2445-2459. 10.1200/JCO.2005.11.890.
Gschwind A, Fischer OM, Ullrich A: The discovery of receptor tyrosine kinases: targets for cancer therapy. Nat Rev Cancer. 2004, 4: 361-370. 10.1038/nrc1360.
Ko JC, Hong JH, Wang LH, Cheng CM, Ciou SC, Lin ST, Jheng MY, Lin YW: Role of repair protein Rad51 in regulating the response to gefitinib in human non-small cell lung cancer cells. Mol Cancer Ther. 2008, 7 (11): 3632-3641. 10.1158/1535-7163.MCT-08-0578.
Akashi Y, Okamoto I, Iwasa T, Yoshida T, Suzuki M, Hatashita E, Yamada Y, Satoh T, Fukuoka M, Ono K, Nakagawa K: Enhancement of the antitumor activity of ionising radiation by nimotuzumab, a humanised monoclonal antibody to the epidermal growth factor receptor, in non-small cell lung cancer cell lines of differing epidermal growth factor receptor status. Br J Cancer. 2008, 98 (4): 749-55. 10.1038/sj.bjc.6604222.
Meert AP, Martin B, Delmotte P, Berghmans T, Lafitte JJ, Mascaux C, Paesmans M, Steels E, Verdebout JM, Sculier JP: The role of EGF-R expression on patient survival in lung cancer: a systematic review with meta-analysis. Eur Respir J. 2002, 20: 975-981. 10.1183/09031936.02.00296502.
Lee HJX: The potential predictive value of cyclooxygenase-2 expression and increased risk of gastrointestinal hemorrhage in advanced non-small cell lung cancer patients treated with erlotinib and celecoxib. Clin Cancer Res. 2008, 14 (7): 2088-2094. 10.1158/1078-0432.CCR-07-4013.
Soslow RA, Dannenberg AJ, Rush D, Woerner BM, Khan KN, Masferrer J, Koki AT: COX-2 is expressed in human pulmonary, colonic, and mammary tumors. Cancer. 2000, 89: 2637-2645. 10.1002/1097-0142(20001215)89:12<2637::AID-CNCR17>3.0.CO;2-B.
Põld M, Zhu LX, Sharma S, Burdick MD, Lin Y, Lee PP, Põld A, Luo J, Krysan K, Dohadwala M, Mao JT, Batra RK, Strieter RM, Dubinett SM: Cyclooxygenase-2-dependent expression of angiogenic cxc chemokines ena-78/cxc ligand (cxcl) 5 and interleukin-8/cxcl8 in human non-small cell lung cancer. Cancer Res. 2004, 64: 1853-1860.
Leahy KM, Koki AT, Masferrer JL: Role of cyclooxygenases in angiogenesis. Curr Med Chem. 2000, 7: 1163-1170.
Khuri FR, Wu H, Lee JJ, Kemp BL, Lotan R, Lippman SM, Feng L, Hong WK, Xu XC: Cyclooxygenase-2 Overexpression is a Marker of Poor Prognosis in Stage I Non-Small Cell Lung Cancer. Clinical Cancer Research. 2001, 7: 861-867.
Kim BM, Won J, Maeng KA, Han YS, Yun YS, Hong SH: Nimesulide: A selective COX-2 inhibitor, acts synergistically with ionizing radiation against A549 human lung cancer cells through the activation of caspase-8 and caspase-3. Int J Oncol. 2009, 34 (5): 1467-1473.
Mutter R, Lu B, Carbone DP, Csiki I, Moretti L, Johnson DH, Morrow JD, Sandler AB, Shyr Y, Ye F, Choy H: A phase II study of celecoxib in combination with paclitaxel, carboplatin, and radiotherapy for patients with inoperable stage IIIA/B non-small cell lung cancer. Clin Cancer Res. 2009, 15 (6): 2158-2165. 10.1158/1078-0432.CCR-08-0629.
Shepherd FA, Tsao MS: Unraveling the mystery of prognostic and predictive factors in epidermal growth factor receptor therapy. J Clin Oncol. 2006, 24: 1219-1223. 10.1200/JCO.2005.04.4420.
Yarden Y, Sliwkowski MX: Untangling the ErbB signaling network. Nature Rev Mol Cell Biol. 2001, 2: 127-137. 10.1038/35052073.
Jorissen RN, Walker F, Pouliot N, Garrett TP, Ward CW, Burgess AW: Epidermal growth factor receptor: mechanisms of activation and signalling. Exp Cell Res. 2003, 284: 31-53. 10.1016/S0014-4827(02)00098-8.
Chou YT, Lin HH, Lien YC, et al.: EGFR promotes lung tumorigenesis by activating miR-7 through a Ras/ERK/Myc pathway that targets the Ets2 transcriptional repressor ERF. Cancer Res. 2010, 70: 8822-31. 10.1158/0008-5472.CAN-10-0638.
Scagliotti GV, Selvaggi G, Novello S: The biology of epidermal growth factor receptor in lung cancer. Clin Cancer Res. 2004, 10: 4227s-4232s. 10.1158/1078-0432.CCR-040007.
Veale D, Kerr N, Gibson GJ, Kelly PJ, Harris AL: The relationship of quantitative epidermal growth factor receptor expression in non-small cell lung cancer to long term survival. Br J Cancer. 1993, 68: 162-165. 10.1038/bjc.1993.306.
Nicholson RI, Gee JMW, Haper ME: EGFR and cancer prognosis. European Journal of Cancer. 2001, 37 (4): 9-15. 10.1016/S0959-8049(01)00231-3.
Van Dyke AL, Cote ML, Prysak GM, Claeys GB, Wenzlaff AS, Murphy VC, Lonardo F, Schwartz AG: COX-2/EGFR expression and survival among women with adenocarcinoma of the lung. Carcinogenesis. 2008, 29 (9): 1781-1787. 10.1093/carcin/bgn107.
Ang KK, Berkey BA, Tu X, Zhang HZ, Katz R, Hammond EH, Fu KK, Milas L: Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 2002, 62: 7350-7356.
Hirsch FR, Varella-Garcia M, Bunn PA, Di Maria MV, Veve R, Bremmes RM, Barón AE, Zeng C, Franklin WA: Epidermal growth factor receptor in non-small-cell lung carcinomas: correlation between gene copy number and protein expression and impact on prognosis. J Clin Oncol. 2003, 21: 3798-3807. 10.1200/JCO.2003.11.069.
Hida T, Yatabe Y, Achiwa H, Muramatsu H, Kozaki K, Nakamura S, Ogawa M, Mitsudomi T, Sugiura T, Takahashi T: Increased expression of cyclooxygenase 2 occurs frequently in human lung cancers, specifically in adenocarcinomas. Cancer Res. 1998, 58: 3761-3764.
Laga AC, Zander DS, Cagle PT: Prognostic significance of cyclooxygenase 2 expression in 259 cases on non-small cell lung cancer. Arch Pathol Lab Med. 2005, 129: 1113-1117.
Hosomi Y, Yokose T, Hirose Y, Nakajima R, Nagai K, Nishiwaki Y, Ochiai A: Increased cyclooxygenase 2 (COX-2) expression occurs frequently in precursor lesions of human adenocarcinoma of the lung. Lung Cancer. 2000, 30: 73-81. 10.1016/S0169-5002(00)00132-X.
Yamaguchi NH, Lichtenfels AJ, Demarchi LM, da Silva AP, Garippo AL, Alves VF, Michelin C, Azevedo PM, Moya T, Takagaki T, Saldiva PH, Vollmer RT, Capelozzi VL: COX-2, MMP-9, and Noguchi classification provide additional prognostic information about adenocarcinoma of the lung. A study of 117 patients from Brazil. Am J Clin Pathol. 2004, 121: 78-86. 10.1309/P2CDFGU1U7CLV5YR.
Kim SJ, Rabbani ZN, Dong F, Vollmer RT, Schreiber EG, Dewhirst MW, Vujaskovic Z, Kelley MJ: Phosphorylated epidermal growth factor receptor and cyclooxygenase-2 expression in localized non-small cell lung cancer. Med Oncol. 2009,
Richardson CM, Richardson D, Swinson DE, Swain WA, Cox G, O'Byrne KJ: Cyclooxygenase-2 protein levels are independent of epidermal growth factor receptor expression or activation in operable non-small cell lung cancer. Lung Cancer. 2005, 48 (1): 47-57. 10.1016/j.lungcan.2004.09.007.
Liu M, Yang SC, Sharma S, Luo J, Cui X, Peebles KA, Huang M, Sato M, Ramirez RD, Shay JW, Minna JD, Dubinett SM: EGFR signaling is required for TGF-b1-mediated COX-2 induction in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2007, 37: 578-588. 10.1165/rcmb.2007-0100OC.
O'Byrne KJ, Danson S, Dunlop D, Botwood N, Taguchi F, Carbone D, Ranson M: Combination therapy with gefitinib and rofecoxib in patients with platinum-pretreated relapsed non-small-cell lung cancer. J Clin Oncol. 2007, 25: 3266-3273.
Gadgeel SM, Ruckdeschel JC, Heath EI, Heilbrun LK, Venkatramanamoorthy R, Wozniak A: Phase II study of gefitinib, an epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI), and celecoxib, a cyclooxygenase-2 (COX-2) inhibitor, in patients with platinum refractory non-small cell lung cancer (NSCLC). J Thorac Oncol. 2007, 2: 299-305. 10.1097/01.JTO.0000263712.61697.69.
Acknowledgements
The authors would like to acknowledge the generous financial support from the Science and Technology Key Project of Sichuan Province, PR. China (Project 03SG022-008 to J.W. and 04SG022-007 to F.X.).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the enclosed manuscript.
Authors' contributions
FL carries out the design of the study and drafting the manuscript; HC is responsible for the control of pathological observation; YL worked on the analysis; DL and YS participated in the immunohistochemical process; FX and JW contribute equally to the conception of this study and the final approval of the version to be published. All authors read and approved the final manuscript.
Feng Xu and Jin Wang contributed equally to this work.
An erratum to this article is available at http://dx.doi.org/10.1186/1756-9966-30-32.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Li, F., Liu, Y., Chen, H. et al. EGFR and COX-2 protein expression in non-small cell lung cancer and the correlation with clinical features. J Exp Clin Cancer Res 30, 27 (2011). https://doi.org/10.1186/1756-9966-30-27
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1756-9966-30-27