Abstract
Background
Wnt inhibitory factor-1(WIF-1) acts as a Wnt-antagonists and tumor suppressor, but hypermethylation of WIF-1 gene promoter and low expression activate Wnt signaling aberrantly and induce the development of various human tumors. With this work we intended to investigate the expression and promoter methylation status of WIF-1 gene in human astrocytomas.
Methods
The tissue samples consisted of 53 astrocytomas and 6 normal brain tissues. The expression levels of WIF-1 were determined by immunohistochemistry and semiquantitative RT-PCR. The results were analyzed in correlation with clinicopathological data. Methylation status of WIF-1 gene promoter was investigated using methylation specific PCR. The relationship between methylation and expression of the genes was analyzed.
Results
The average expression levels of WIF-1 protein and mRNA in astrocytomas were decreased significantly compared with normal control tissues. The protein and mRNA expression of WIF-1 gene in astrocytomas was decreased with the increase of pathological grade. Furthermore, WIF-1 promoter methylation was observed by MS-PCR in astrocytomas which showed significant reduction of WIF-1 expression. The WIF-1 promoter hypermethylation was associated with reduced expression of WIF-1 expression.
Conclusion
Our results demonstrate that the WIF-1 gene is frequently down-regulated or silenced in astrocytomas by aberrant promoter methylation. This may be an important mechanism in astrocytoma carcinogenesis.
Similar content being viewed by others
Background
Astrocytomas are the most common primary tumors of the central nervous system. Despite recent advances in diagnosis and therapies such as surgery, radiation, and chemotherapy, the prognosis and survival times of high-grade astrocytomas(WHO grade III, IV)remains poor. The median survival is only 12 to 15 months for patients with glioblastoma(WHO grade IV)and 2 to 5 years for patients with anaplastic astrocytoma(WHO grade III)[1].
The Wnt/β-catenin signaling pathway plays a significant role in various processes of early development and the pathogenesis of human diseases, including human malignancies. Recently, there are several reports which evident the involvement of Wnt/β-catenin signaling in astrocytomas [2–5]. Wnt inhibitory factor-1 (WIF-1) is identified as one of the secreted antagonists that can directly bind to Wnt proteins to inhibit Wnt/β-catenin signaling[6]. Down-regulation and promoter hypermethylation of WIF-1 gene have been reported in human hepatocellular, nasopharyngeal, pulmonary, urocystic and gastrointestinal malignancies [7–11]. Yet little is known regarding the expression and promoter methylation of WIF-1 in astrocytomas. In this study, we describe for the first time that the expression of WIF-1 was frequently downregulated by its promoter hypermethylation in astrocytomas compared with normal tissue samples, which might contribute to the upregulation of Wnt/β-catenin signaling in astrocytoma carcinogenesis.
Materials and methods
Patients and tissue samples
53 fresh astrocytoma samples (T1-T53)were collected after informed consent from patients who underwent brain operations for astrocytoma at Xiangya Hospital (Hunan, China). Immediately after surgical resection, portions of the tumors were frozen and stored at -80°C for RNA and DNA extraction, and the remanets were fixed in 10% formalin. Tumors were graded and classified according to the World Health Organization (2007)[12], including gradeI(1), grade II(22), grade III(12), and grade IV(18). In all cases of astrocytomas, there were 32 (60.38%) males and 21 (39.62%) females with the median age of 38.5 years (range: 5~66 years). For comparison, 6 normal human tissues (N1-N6) from patients with contusion and laceration of brain were obtained at the time of decompressive operation.
Immunohistochemistry
WIF-1 protein expression was determined by using immunohistochemical staining (IHC) on formalin-fixed paraffin-embedded tissue sections. Briefly, 5 μm thick sections were deparaffinized, rehydrated using xylene and alcohol, incubated with 0.3% H2O2 to block endogenous peroxidase activity, and incubated with normal goat serum to block nonspecific antibody binding. Before immunostaining, antigen retrieval was done by immersing sections in a 10 mM concentration of citrate buffer (pH6.0) and boiling in a pressure cooker for 2 minutes. The sections were incubated at 4°C overnight with a 8 μg/ml monoclonal antibody against human WIF-1 protein (R&D, Minneapolis, USA). The sections were then incubated with biotinylated goat anti-mouse IgG antibody (Zymed, San Francisco, CA, USA) for 30 min. The antigen-antibody complexes were visualized using streptavidin-horseradish peroxidase conjugate (Zymed, San Francisco, CA, USA) and diaminobenzidine (DAB)as a chromogen.
The slides were counterstained with hematoxylin. For WIF-1 protein expression, nuclear staining was considered to be negative, whereas cytoplasmic and membranous expression was analyzed according to the intensity and proportion of positive cells to all cells[10]. IPP6.0 (Media Cybernetics, Bethesda, MD, USA)was applied to semiquantify immunohistochemical results. Staining was scored for intensity [0 (negative), 1+ (weak), and 2+ (strong)] and percentage of postive staining in malignant cells [0 (0-4%), 1 (5-24%),2 (25-49%), 3 (50-74%), or 4 (75-100%)]. The multiplication of intensity and percentage counts was used as the final immunohistochemistry scores [13]. For heterogenous staining patterns, each component was scored independently and the results were summed. For example, a specimen containing 25% tumor cells with strong intensity (1 × 2 + = 2), 25% tumor cells with weak intensity (1 × 1 + = 1), and 50% tumor cells without immnoreactivity received a score of 2 + 1 + 0 = 3. Cytoplasmic and membranous staining in normal brain tissue served as internal positive controls. Negative controls were included in the IHC analyses by omitting the primary antibody.
RNA extraction and Semiquantitative RT-PCR
Total RNA from tumor tissues and normal tissues were isolated using a TRIzol procedure(Invitrogen, Carlsbuel, CA, USA). An equal amount of RNA from each sample was added to 25 μl of reaction mixture and cDNA was synthesized by First Strand cDNA Synthesis kit (Fermentas, Burlington, Canada). Primers for semiquantitative RT-PCR were obtained from Takaro (Dalian, China). Primer sequences for the human WIF-1 cDNA were 5'-CCGAAATGGAGGCTTTTGTA-3' (forward) and 5'-TGGTTGAGCAGTTTGCTTTG-3' (reverse)[8]. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control. Primer sequences for GAPDH were 5'-CAATGACCCCTTCATTGACC-3' (forward) and 5'-TGGAAGATGGTGATGGGATT-3' (reverse). The cycle was defined at 95°C for 5 min, followed by 32 cycles of denaturing at 95°C for 30 sec, annealing at 56°C for 40 sec and extension at 72°C for 40 sec. This was followed by the final extension at 72°C for 10 min. The PCR products were electrophoresed in 2% agarose gels. Relative WIF-1 mRNA levels were evaluated by UVP software (UVP Inc., Upland, CA, USA) and were expressed as the fold-difference relative to GAPDH mRNA levels.
Genomic DNA Extraction and Methylation-specific PCR (MS-PCR)
DNA was extracted from astrocytoma tissues by standard proteinase K digestion, phenol chloroform and ethanol precipitation proceeded. Bisulfite modification of genomic DNA was carried out by using a EZ DNA methylation kit (Zymo, CA, USA), according to the manufacturer's protocol. WIF-1 promoter region has been identified and described previously [14]. Bisulfite-treated genomic DNA was amplified using either a methylation-specific or an unmethylation-specific primer set. GC Rich DNA polymerase (Qiagen, Hilden, Germany) was used in the experiments. Sequences of the methylation-specific primers were 5'-GGGCGTTTTATTGGGCGTAT-3' (forward) and 5'-AAACCAACAATCAACGAAC-3' (reverse). Sequences of the unmethylation-specific primers were 5'-GGGTGTTTTATTGGGTGTAT-3' (forward) and 5'-AAACCAACAATCAACAAAAC-3' (reverse) corresponding to the WIF-1 promoter region sequences -488 to -468 and -310 to -290, respectively. The PCR was carried out in a Techne TC-412 Thermal Cycler(Keison, Essex, UK) under the following conditions: one cycle of 95°C for 10 min, followed by 35 cycles of denaturing at 94°C for 1 min, annealing at 60°C for 50 sec and extension at 72°C for 50 sec. This was followed by the final extension at 72°C for 10 min. The PCR products were analysed by electrophoresis on 2% agarose gel and samples were evaluated. Normal human lymphocyte DNA was either treated directly with sodium bisulfite or after in vitro methylation by SssI methyltransferase(New England Biolabs, Ipswich, MA) to serve as unmethylated and methylated controls, respectively.
Statistical analysis
Statistical analyses were performed using SPSS software version 13.0(SPSS, Chicago, USA). Data were presented as mean ± SD. Differences of the variables between groups were tested by Student's t test. P < 0.05 was regarded as statistically significant for all the tests.
Results
Expression of WIF-1 protein
To detect the expression level of WIF-1, immunohistochemistry was performed in 6 normal brain tissues and in 53 astrocytoma tissues (Tab. 1 and Fig. 1). Reactivity was generally cytoplasmic and membranous. The average values of WIF-1 expression were 7.33 ± 0.52 and 2.94 ± 2.19 respectively in normal brain tissues and astrocytomas. Statistical analysis indicated that the level of WIF-1 expression was significantly lower in tumors than that in normal brain tissues (P < 0.001), and it was decreased as the pathological grade increased (P = 0.002) (Tab. 2). No significant correlation was found between WIF-1 protein expression and age(P = 0.53)or sex(P = 0.69)respectively.
Selected results of immunohistochemical analysis for anti-human WIF-1 antibodies. Paraffin-embedded sections of representative astrocytomas and normal brain tissues were stained with the antibodies against human WIF-1. The products of WIF-1 expression (brown) located in cytoplasm and membrane. The photographs of A and B are normal brain tissues and pilocytic astrocytoma (WHO grade I)which showed strong staining for WIF-1, respectively. In contrast, the anaplastic astrocytoma(WHO grade III) and glioblastomas(WHO grade IV)that have weak or negative expression levels of WIF-1 were shown in C and D, respectively. Pathological malignancy grade of astrocytoma correlated with IHC score of WIF-1.
Expression of WIF-1 mRNA transcript
Semiquantitative RT-PCR assay was also performed to analyze the expression of WIF-1 at transcription level. WIF-1 mRNA was examined in 6 normal brain tissues as well as in 53 resected astrocytoma tissues [Tab. 1 and Fig. 2(A)]. The results showed that WIF-1 expression in tumor samples (0.35 ± 0.29)was significantly lower compared with normal brain tissues (0.90 ± 0.06, P < 0.001). Significant association was found between WIF-1 mRNA downregulation and the pathological grade(P= 0.001). However, WIF-1 gene expression was not correlated with age(P = 0.23)or sex(P = 0.50) in tumor samples (Tab. 2).
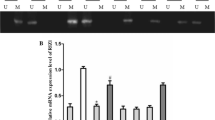
Selected results of mRNA expression and promoter methylation of WIF-1. A, RT-PCR results of the WIF-1 gene in normal brain tissue (N1-N2) and astrocytoma (T1-T8) is shown. GAPDH is shown as a control. The fragments of amplified human WIF-1 and GAPDH cDNA are188 and135 bp, respectively. B, Representative methylation status of the WIF-1 promoter in 10 matched pairs of normal brain tissue (N1-N2) and astrocytomas(T1-T8).T1,3,5:WHO grade II;T2,4,6:WHO grade III;T7,8: WHO grade IV. U, unmethylated control;M, methylated control;NTC, no template control.
Relationship between promoter methylation and expression of WIF-1
To examine whether the methylation status of promoter correlates with the expression of WIF-1, MS-PCR was carried out [Tab. 1 and Fig. 2(B)]. No hypermethylation was obseved in all normal brain tissues. In contrast, aberrant methylation was observed in 29(54.72%) of 53 tumor samples. Especially, 22 (73.33%) of 30 high-grade astrocytomas(WHO grade III, IV) showed promoter hypermethylation.
Unmethylation-specific PCR band was detected in 9 of 29(31.03%) methylated samples, probably due to unavoidable contamination of non-tumor cells, or partial methylation of the gene. The promoter methylated tumors showed low WIF-1 protein and mRNA expression, whereas the promoter unmethylated tumors displayed high protein and mRNA expression levels (Fig. 3). Thus, these data indicated a significant correlation (both P < 0.001) between hypermethylation and decreased expression of WIF-1 in astrocytomas.
Discussion
WNT/β-catenin signaling pathway is important in tumorigenesis and embryogenesis [15, 16]. The signaling pathway mediated by Wnt proteins currently includes two classes - canonical and noncanonical - on the basis of the activity of Wnt proteins in cell lines or in vivo assays. The canonical pathway, in which β-Catenin plays a crucial role, is the most studied Wnt pathway in cancers. The activation of canonical pathway allows β-catenin to accumulate in the cytosol and enter the nucleus and induces expression of Wnt target genes like c-Myc, N-Myc, and cyclin D1 [17–19], many of which have been implicaticated in human cancers. In astrocytoma, the level of Wnt-2, Wnt-5a and β-catenin protein is strikingly increased compared with normal brain tissue[2, 3, 5]. Knockdown of Wnt and its key mediator β-catenin in the canonical Wnt pathway by siRNA in human astrocytoma cells inhibited cell proliferation and invasive ability and induced apoptotic cell death, and reduced tumorigenicity in vivo. The above findings suggest Wnt signaling in astrocytoma is constitutively activated and of critical importance in the astrocytoma genesis.
WIF-1 is an endogenous Wnt antagonist. Downregulation of WIF-1 may release the inhibitory effect exerted by WIF-1 on the WNT/β-catenin signaling[20]. This then enhances the accumulation of β-catenin and promotes tumorigenesis. Although it is known that WIF-1 is strongly expressed in embryonic mouse brain [21], its expression in brain tumors has not yet been a matter of investigation. In this study, we analysed the protein and mRNA level of WIF-1 in astrocytomas using immunohistochemistry and RT-PCR. The level of protein and mRNA expression in astrocytomas was significantly lower than that in normal tissues. As the pathological grade increased, the protein and mRNA expression of WIF-1 gene in astrocytoma were decreased. These results indicated that WIF-1 was frequently and significantly downregulated in astrocytomas, especially in high-grade astrocytomas, which might contribute to the upregulation of Wnt/β-catenin signaling in astrocytoma carcinogenesis.
Aberrant methylation of promoter regions that silences transcription of the genes has been recognized as a mechanism for inactivating tumor suppressor genes in human cancer [22, 23]. It occurs at cytosine bases located 5' to a guanosine and so-called CpG dinucleotide short regions of CpG dinucleotides known as CpG islands are found in the proximal promoter region of over half of human genes [23]. The methylation of these gene promoters is generally not detected in normal tissues but in the hypermethylation of CpG islands resulting in a loss of gene function, which is a common feature in many tumor types. Now, many other genes such as LHX9, MGMT, CDKN2A, PTEN, and P15 have been shown to be methylated in astrocytomas [24–28]. WIF-1 silencing may be an early epigenetically carcinogenic event and plays a role in tumor development and progression[29]. In this study, we demonstrated that WIF-1 downregulation or silencing was associated with aberrant methylation of promoter region in malignant astrocytoma tissue samples. This finding reveals an important epigenetic event during the development of astrocytoma, suggesting that WIF-1 may be a key antagonist of Wnt signaling in astrocytoma.
In summary, we provide evidence that WIF-1 is not only frequently hypermethylated in astrocytomas but this epigenetic alteration of the WIF-1 gene is associated with reduced expression. This study reveals a novel epigenetic event in the pathogenesis of astrocytoma, which may shed light on developing new approaches for this fatal disease. The reversibility of methylation silencing may allow restoration of WIF-1 function and regulation of Wnt signaling. This could be important in the development of new and effective strategy in astrocytoma treatment.
References
Wen PY, Kesari S: Malignant astrocytomas in adults. N Engl J Med. 2008, 359: 492-507. 10.1056/NEJMra0708126.
Pu P, Zhang Z, Kang C, Jiang R, Jia Z, Wang G, Jiang H: Downregulation of Wnt2 and beta-catenin by siRNA suppresses malignant astrocytoma cell growth. Cancer Gene Ther. 2009, 16: 351-361. 10.1038/cgt.2008.78.
Yu JM, Jun ES, Jung JS, Suh SY, Han JY, Kim JY, Kim KW, Jung JS: Role of Wnt5a in the proliferation of human glioblastoma cells. Cancer Lett. 2007, 257: 172-181. 10.1016/j.canlet.2007.07.011.
Sareddy GR, Challa S, Panigrahi M, Babu PP: Wnt/beta-catenin/Tcf signaling pathway activation in malignant progression of rat astrocytomas induced by transplacental N-ethyl-N-nitrosourea exposure. Neurochem Res. 2009, 34: 1278-188. 10.1007/s11064-008-9906-3.
Sareddy GR, Panigrahi M, Challa S, Mahadevan A, Babu PP: Activation of Wnt/beta-catenin/Tcf signaling pathway in human astrocytomas. Neurochem Int. 2009, 55: 307-317. 10.1016/j.neuint.2009.03.016.
Hsieh JC, Kodjabachian L, Rebbert ML, Rattner A, Smallwood PM, Samos CH, Nusse R, Dawid IB, Nathans J: A new secreted protein that binds to Wnt proteins and inhibits their activities. Nature. 1999, 398: 431-436. 10.1038/18899.
Ding Z, Qian YB, Zhu LX, Xiong QR: Promoter methylation and mRNA expression of DKK-3 and WIF-1 in hepatocellular carcinoma. World J Gastroenterol. 2009, 15: 2595-2601. 10.3748/wjg.15.2595.
Lin YC, You L, Xu Z, He B, Mikami I, Thung E, Chou J, Kuchenbecker K, Kim J, Raz D, Yang CT, Chen JK, Jablons DM: Wnt signaling activation and WIF-1 silencing in nasopharyngeal cancer cell lines. Biochem Biophys Res Commun. 2006, 341: 635-640. 10.1016/j.bbrc.2005.12.220.
Mazieres J, He B, You L, Xu Z, Lee AY, Mikami I, Reguart N, Rosell R, McCormick F, Jablons DM: Wnt inhibitory factor-1 is silenced by promoter hypermethylation in human lung cancer. Cancer Res. 2004, 64: 4717-4720. 10.1158/0008-5472.CAN-04-1389.
Urakami S, Shiina H, Enokida H, Kawakami T, Tokizane T, Ogishima T, Tanaka Y, Li LC, Ribeiro-Filho LA, Terashima M, Kikuno N, Adachi H, Yoneda T, Kishi H, Shigeno K, Konety BR, Igawa M, Dahiya R: Epigenetic inactivation of Wnt inhibitory factor-1 plays an important role in bladder cancer through aberrant canonical Wnt/beta-catenin signaling pathway. Clin Cancer Res. 2006, 12: 383-391. 10.1158/1078-0432.CCR-05-1344.
Taniguchi H, Yamamoto H, Hirata T, Miyamoto N, Oki M, Nosho K, Adachi Y, Endo T, mai K, Shinomura Y: Frequent epigenetic inactivation of Wnt inhibitory factor-1 in human gastrointestinal cancers. Oncogene. 2005, 24: 7946-7952. 10.1038/sj.onc.1208910.
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P: The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007, 114: 97-109. 10.1007/s00401-007-0243-4.
Joki T, Heese O, Nikas DC, Bello L, Zhang J, Kraeft SK, Seyfried NT, Abe T, Chen LB, Carroll RS, Black PM: Expression of cyclooxygenase 2 (COX-2) in human glioma and in vitro inhibition by a specific COX-2 inhibitor, NS-398. Cancer Res. 2000, 60: 4926-4931.
Reguart N, He B, Xu Z, You L, Lee AY, Mazieres J, Mikami I, Batra S, Rosell R, McCormick F, Jablons DM: Cloning and characterization of the promoter of human Wnt inhibitory factor-1. Biochem Biophys Res Commun. 2004, 323: 229-234. 10.1016/j.bbrc.2004.08.075.
Turashvili G, Bouchal J, Burkadze G, Kolar Z: Wnt signaling pathway in mammary gland development and carcinogenesis. Pathobiology. 2006, 73: 213-223. 10.1159/000098207.
Fodde R, Brabletz T: Wnt/beta-catenin signaling in cancer stemness and malignant behavior. Curr Opin Cell Biol. 2007, 19: 150-158. 10.1016/j.ceb.2007.02.007.
Shiina H, Igawa M, Breault J, Ribeiro-Filho L, Pookot D, Urakami S, Terashima M, Deguchi M, Yamanaka M, Shirai M, Kaneuchi M, Kane CJ, Dahiya R: The human T-cell factor-4 gene splicing isoforms, Wnt signal pathway, and apoptosis in renal cell carcinoma. Clin Cancer Res. 2003, 9: 2121-2132.
He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW: Identification of c-MYC as a target of the APC pathway. Science. 1998, 281: 1509-1512. 10.1126/science.281.5382.1509.
Tetsu O, McCormick F: Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999, 398: 422-426. 10.1038/18884.
Kawano Y, Kypta R: Secreted antagonists of the Wnt signalling pathway. J Cell Sci. 2003, 116: 2627-34. 10.1242/jcs.00623.
Hu YA, Gu X, Liu J, Yang Y, Yan Y, Zhao C: Expression pattern of Wnt inhibitor factor 1(Wif1) during the development in mouse CNS. Gene Expr Patterns. 2008, 8: 515-522. 10.1016/j.gep.2008.06.001.
Jones PA, Baylin SB: The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002, 3: 415-428. 10.1038/nrg962.
Herman JG, Baylin SB: Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med. 2003, 349: 2042-2054. 10.1056/NEJMra023075.
Vladimirova V, Mikeska T, Waha A, Soerensen N, Xu J, Reynolds PC, Pietsch T: Aberrant methylation and reduced expression of LHX9 in malignant astrocytomas of childhood. Neoplasia. 2009, 11: 700-711.
Blanc JL, Wager M, Guilhot J, Kusy S, Bataille B, Chantereau T, Lapierre F, Larsen CJ, Karayan-Tapon L: Correlation of clinical features and methylation status of MGMT gene promoter in glioblastomas. J Neurooncol. 2004, 68: 275-283. 10.1023/B:NEON.0000033385.37098.85.
Schmidt EE, Ichimura K, Messerle KR, Goike HM, Collins VP: Infrequent methylation of CDKN2A(MTS1/p16) and rare mutation of both CDKN2A and CDKN2B(MTS2/p15) in primary astrocytic tumours. Br J Cancer. 1997, 75: 2-8.
Wiencke JK, Zheng S, Jelluma N, Tihan T, Vandenberg S, Tamguney T, Baumber R, Parsons R, Lamborn KR, Berger MS, Wrensch MR, Haas-Kogan DA, Stokoe D: Methylation of the PTEN promoter defines low-grade astrocytomas and secondary glioblastoma. Neuro Oncol. 2007, 9: 271-279. 10.1215/15228517-2007-003.
Wemmert S, Bettscheider M, Alt S, Ketter R, Kammers K, Feiden W, Steudel WI, Rahnenfuhrer J, Urbschat S: p15 promoter methylation - a novel prognostic marker in glioblastoma patients. Int J Oncol. 2009, 34: 1743-1748.
Wissmann C, Wild PJ, Kaiser S, Roepcke S, Stoehr R, Woenckhaus M, Kristiansen G, Hsieh JC, Hofstaedter F, Hartmann A, Knuechel R, Rosenthal A, Pilarsky C: WIF1, a component of the Wnt pathway, is down-regulated in prostate, breast, lung, and bladder cancer. J Pathol. 2003, 201: 204-212. 10.1002/path.1449.
Acknowledgements
The work was supported by National Natural Science Foundation of China Grants 30600636(to YJW)and Innovation Foundation of Central South University For Postgraduate(to YZY).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
YZY and YW carried out the experiment of this manuscript and drafted the manuscript. YZY and JSF participated in the design of the study and organized the whole study process. FHC, JFL and JW participated the experiment and revised the manuscript. YZY and YJW conceived the study project, provided financial support. All authors read and approved the final manuscript.
Zhuanyi Yang, Ying Wang contributed equally to this work.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Yang, Z., Wang, Y., Fang, J. et al. Expression and aberrant promoter methylation of Wnt inhibitory factor-1 in human astrocytomas. J Exp Clin Cancer Res 29, 26 (2010). https://doi.org/10.1186/1756-9966-29-26
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1756-9966-29-26