Abstract
Background
The diagnosis of growth hormone (GH) deficiency (GHD) in short children seems unquestionable when both GH peak in stimulating tests (GHST) and IGF-I concentration are decreased. However, the discrepancies between the results of GHST and IGF-I secretion are observed. It seems purposeful to determine the significance of GHST and IGF-I assessment in diagnosing GHD. The relationship between GH secretion and thyroid function, as well as GH influence on the peripheral thyroxine (T4) to triiodothyronine (T3) deiodination, mediated by IGF-I, were identified. Thus, clear differences in thyroid function between GH-deficient and non-GH-deficient subjects should exist.
Methods
Analysis comprised 800 children (541 boys), age 11.6 ± 3.1 years (mean ± SD), with short stature, in whom two (2) standard GHST (with clonidine and with glucagon) were performed and IGF-I, free T4 (FT4), free T3 (FT3) and TSH serum concentrations were assessed. The patients were qualified to the following groups: GHD - decreased GH peak in GHST and IGF-I SDS (n = 81), ISS - normal GH peak and IGF-I SDS (n = 347), low GH - normal IGF-I SDS, and decreased GH peak (n = 212), low IGF - decreased IGF-I SDS, and normal GH peak (n = 160). The relationships among the results of particular tests were evaluated.
Results
In the groups with decreased IGF-I concentrations (GHD Group and low IGF Group), the more severe deficit of height was observed, together with higher TSH and FT4 but lower FT3 levels than in groups with normal IGF-I concentrations (ISS Group and low GH Group), independently of the results of GHST. TSH, FT4 and FT3 concentrations were - respectively - similar in two groups with decreased IGF-I secretion, as well as in two groups with normal IGF-I levels. Significant correlations were found between patients' height SDS and IGF-I SDS, between FT3 and IGF-I SDS (positive), and between FT4 and IGF-I SDS (negative), with no correlation between GH peak and any of the parameters analyzed.
Conclusion
The assessment of thyroid function in children with short stature provides the evidence that measurement of IGF-I concentration may be a procedure reliable at least to the some degree in diagnosing GHD as the results of GHST.
Similar content being viewed by others
Background
The growth hormone (GH) secretion is routinely evaluated in children with short stature, on the ground of the results of GH stimulating tests (GHST) with different pharmacological stimuli [1, 2]. Insulin-like growth factor-I (IGF-I) is the main peripheral mediator of GH activity. The assessment of IGF-I secretion is an important diagnostic tool, as its synthesis is GH-dependent, whereas serum concentration - on the contrary to GH levels - relatively stable [3]. The diagnosis of GH deficiency (GHD) seems unquestionable when both GH peak in GHST and IGF-I concentration are decreased. Consistently, GHD may be excluded in the patients, in whom both GH and IGF-I secretion are normal. In such cases, the diagnosis of idiopathic short stature (ISS) seems to be the most adequate after careful exclusion of other possible causes of short stature (including genetically-determined syndromes, chronic diseases, malnutrition, etc.). However, in clinical practice, the discrepancies either between the results of GHST and IGF-I secretion or between the results of particular GHST are - quite frequently - observed. According to current recommendations, two (2) different GHST are required in every case and their results should be interpreted together [1, 2]. The problem of the poor reproducibility of GHST was raised - among others - by Rosenfeld et al. [4] and Price et al. [5]. Next, the possibility of obtaining normal results of GHST in children, previously diagnosed as GH-deficient (i.e. in children with subnormal GH response to pharmacological stimulation in past) was documented in several studies [5–8]. The relationships between GH secretion, more presently - between the results of GHST and IGF-I levels were assessed in numerous studies. Unfortunately, their results are incoherent, either confirming [9–12] or denying [13–16] the correlation between GH peak in GHST and IGF-I secretion. In 2005, Cianfarani et al. [3] stated that IGF-I concentrations were reliable indicators of daily GH secretion, due to their GH dependency and relative stability in circulation. Similar were the results of much earlier observations of Rosenfeld et al. [17] and Shalet et al. [18]. In one of our previous studies [19], the stability of IGF-I concentration was also proved, despite divergent results of the repeated GHST.
Taking into account all the doubts concerning diagnosing GHD in children, it seems purposeful to search for other indices that could be helpful in the evaluation of GH secretory status. Particularly, further studies on determining the significance of both GHST and IGF-I assessment among the diagnostic procedures seem promising.
The relationships between GH secretion and thyroid function, as well as GH influence on the peripheral thyroxine (T4) to triiodothyronine (T3) deiodination were identified during the observations of patients with GHD, either untreated or subjected to GH therapy. The first studies, carried out about 30 years ago, revealed an increase of T4 to T3 conversion, leading to an increase of extrathyroidal T3 concentration, together with a decrease of T4 concentration during GH administration [20, 21]. These observations were also confirmed by the results of subsequent studies. In 1989, Jørgensen et al. [22] confirmed the observation that GH replacement induced an enhancement of T4 to T3 deiodination. In 1994, the same research group [23] demonstrated that in the adult patients with GHD the levels of T3 remained decreased, even in the course of T4 substitution, while normalizing during GH replacement therapy (parallel to the decrease of TSH levels). The key role of IGF-I in stimulating the process of T4 to T3 deiodination was also suggested by Jørgensen et al. [24].
On the other hand, the influence of thyroid function disorders on GH secretion, as well as on IGF system, was also described. Thus, it was shown that hypothyroidism (HypoT) - even in its subclinical form - might affect IGF-I secretion [25]. Moreover, it was previously supposed that the effects of thyroid hormone on IGF-I secretion could be independent from GH mediation [26, 27]. In particular, a significant decrease of IGF-I and IGF binding protein-3 (IGFBP-3) concentrations was documented in the patients with HypoT, both in adults [28] and in children [29]. Furthermore, it was shown that T4 replacement therapy improved the previously decreased GH and IGF-I secretion, however, with no complete recovery [30].
As it was mentioned before, a decrease of T4 to T3 deiodination, leading to the decrease of T3 concentration with reference to T4 level, should be observed in GH-deficient patients. Ceratinly, the individual variances of the thyroid function or/and in the activity of peripheral deiodinases must also be taken into account. Nevertheless, it seems that in the study including large groups of patients, the clear differences between GH-deficient and non-GH-deficient subjects should be identified.
The aim of current study was to compare the selected indices of thyroid function in children with short stature, diagnosed towards GHD, with reference to both the results of GHST and IGF-I secretion. In fact, we expected to reveal the differences between the groups with either normal or subnormal results of GHST and/or between those with either normal or decreased IGF-I levels, as since such observations could contribute to optimizing the assessment of GH secretory status in children with short stature.
Methods
The retrospective analysis comprised 800 children (541 boys, 259 girls), age 11.6 ± 3.1 years (mean ± SD), with short stature (i.e., patient's height below 3rd centile for age and sex), diagnosed at our Department (2001-2010). All the children underwent the following diagnostic procedures:
-
patients' height was measured on admission to the hospital and was expressed as height SDS (hSDS) for age and sex for Polish children [31]; taking into account the normal distribution of heights in the population of children, an exact value -1.88 was assumed as hSDS for 3rd centile;
-
two (2) GHST were performed in children admitted to the hospital, remaining fasting, in the morning hours. For stimulation, clonidine in a dose of 0.15 mg/m2 p.o. (1st test), and glucagon in a dose of 30 μg/kg i.m., not exceeding 1 mg (2nd test) were used. Blood samples for GH estimation were collected every 30 min from 0 to 120 min in the tests with clonidine and at 0, 90, 120, 150 and 180 min in the test with glucagon;
-
serum IGF-I, free T4 (FT4), free T3 (FT3) and TSH concentrations were measured in single blood samples, obtained in 0 min of 1st stimulating test, just before clonidine administration.
The main assumption of the study was to include the patients either with ISS or with isolated non-acquired GHD only. In order to avoid the possible influence of disorders of thyroid function on GH and/or IGF-I secretion and action, only the children with values of TSH, FT4, and FT3 concentrations within the reference range were included into the analysis. The exclusion criteria encompassed any chronic diseases, other hormonal deficiencies, acquired GHD, Turner syndrome in girls, other genetically determined syndromes, malnutrition and obesity (assessed as body mass index either below or over the reference range respectively for age and sex [31]).
The concentrations of GH were measured by the two-site chemiluminescent enzyme immunometric assay (hGH IMMULITE, DPC) for the quantitative measurement of human GH, calibrated to WHO IRP 80/505 standard. The analytical sensitivity of the assay was up to 0.01 ng/ml, the calibration range up to 40 ng/ml, the sensitivity of 0.01 ng/ml, the intra-assay coefficient of variation (CV) of 5.3-6.5% and the inter-assay CV of 5.5-6.2%. The cut-off value for normal and subnormal GH peak in response to stimulation was 10.0 ng/ml according to current recommendations [1, 2].
Serum IGF-I concentrations were assessed by Immulite, DPC assays; WHO NIBSC 1st IRR 87/518 standard was applied, with analytical sensitivity 20 ng/ml, calibration range up to 1600 ng/ml, intra-assay CV - 3.1-4.3% and inter-assay CV - 5.8-8.4%. For comparison among children of different age and sex, IGF-I concentrations were expressed as IGF-I SDS, according to DPC reference data. As it was stated before, IGF-I values higher than -1.0 SD reflected a normal GH secretion [3]. Thus, we decided to select just that threshold value as the cut-off level for normal and decreased IGF-I secretion in our study.
Serum TSH, FT4 and FT3 concentrations were measured by the electroimmunochemiluminescent method (ECLIA), Roche, Elecsys®Systems 1010/2010/modular analytics E170. For TSH, the analytical sensitivity was 0.005 μIU/ml, range - up to 100 μIU/ml, intra-assay coefficient of variance (CV) - 1.5-8.6%, accuracy - 1.1-3.0%. The analytical range for FT4 was 0.023-7.77 ng/ml, intra-assay CV - 1.4-2.9%, accuracy - 2.7-6.6%. For FT3, the analytical range was 0.26-32.55 pg/ml, intra-assay CV - 3.7-9.5%, accuracy - 3.8-11.2%. For the assessment of FT3/FT4 molar ratio, the concentrations of FT4 and FT3 were expressed as molar ones.
According to the results of GHST (normal or subnormal) and to IGF-I secretion (normal or decreased), the patients were qualified to the following groups:
-
GHD - the patients with decreased both GH peak in GHST and IGF-I SDS (n = 81);
-
ISS - the patients with normal both the results of GHST and IGF-I SDS (n = 347);
-
low GH - the patients with normal IGF-I SDS, despite decreased GH peak in GHST (n = 212);
-
low IGF - the patients with decreased IGF-I SDS, despite normal GH peak in GHST (n = 160).
The analysis of the relationships between the components of somatotrophic axis (the results of GHST, IGF-I concentration) and the components of the pituitary - thyroid axis (TSH, FT4 and FT3 concentrations) were assessed. Statistical analysis included Kruskal-Wallis' nonparametric test for independent samples, as well as the assessment of correlations between patient's height and the results of particular hormonal tests.
Results
The deficit of height (expressed as hSDS for age and sex) proved to be the most expressed in low IGF Group (i.e. in the patients with low IGF-I and normal GH peak in GHST), being significantly lower than that in both ISS Group (p < 0.001) and low GH Group (p < 0.001), as well as than that in GHD Group (p = 0.031). Interestingly, both the mean value and the distribution of hSDS values in ISS Group and in low GH Group (i.e. in the groups of patients with normal IGF-I secretion) were similar, despite completely different results of GHST in them. Moreover, hSDS values in these groups were also higher than those in the groups with decreased IGF-I, i.e. GHD Group and low IGF Group (also independently of the results of GHST).
The levels of TSH were also very similar in ISS Group and in low GH Group, being - at the same time - lower than both in GHD Group (the differences insignificant - NS) and than in low IGF Group (NS and p = 0.032, respectively), while the difference between GHD Group and low IGF Group was insignificant.
Moreover, FT4 concentrations turned out to be very similar in ISS Group and in low GH Group, being also significantly lower than both in GHD Group (p < 0.001 and p = 0.002, respectively) and in low IGF Group (p < 0.001 for both pairs compared).
Interestingly, there was no significant difference in FT3 concentrations between ISS Group and low GH Group, while in these two groups, FT3 concentrations were significantly higher than those in both GHD Group (p = 0.012 and p = 0.010, respectively) and low IGF Group (p = 0.001 for both pairs compared). The difference between GHD Group and low IGF Group did not reach the border of significance.
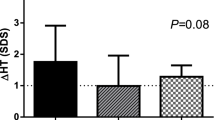
Finally, FT3/FT4 molar ratio was similar in ISS Group and low GH Group, being - in these two groups - significantly higher (p < 0.0001) than those in GHD Group and in low IGF Group, while the difference between the latter two groups was also insignificant. More detailed data, are presented Table 1 and Figures 1, 2, 3, 4 and 5.
Summing up, in groups of patients with decreased IGF-I concentrations (i.e. in GHD Group and in low IGF Group), the more severe deficit of height was observed, together with higher TSH and FT4 levels but lower FT3 concentrations than those in groups with normal IGF-I concentrations (i.e. in ISS Group and in low GH Group). All the assessed parameters of thyroid function presented similar in the two groups with decreased IGF-I secretion (i.e. GHD Group and low IGF Group). There were no important differences between groups with normal IGF-I levels (i.e. ISS Group and low GH Group).
As a matter of fact, there were no strong correlations between the assessed indices of thyroid function (TSH, free thyroid hormones) and the parameters of somatotrophic axis (GH peak in GHST, IGF-I). However, a significant, positive correlation was found between patients' hSDS and IGF-I SDS (r = 0.25, p < 0.05), while there was no correlation between hSDS and GH peak in GHST (r = 0.03, NS). A positive correlation was also observed between IGF-I SDS and FT3 concentration (r = 0.24, p < 0.05), together with a negative one between IGF-I SDS and FT4 (r = -0.20, p < 0.05) and with no correlation between IGF-I SDS and TSH level (r = -0.06, NS). Thus, the observed correlations seem to be independent from TSH level, while an increase of IGF-I concentration may be related to the increase of FT3 and decrease of FT4. Moreover, there was no correlation between GH peak and any of the parameters analyzed. Detailed data are shown in Table 2.
Discussion
In our study, groups with decreased IGF-I levels (i.e., GHD Group and low IGF-I Group) presented with the relatively higher TSH and FT4 but lower FT3 concentrations than those with normal IGF-I secretion, independently from either subnormal or normal GH peak in GHST. It should be recalled that GHD leads to the decrease of peripheral T4 to T3 deiodination [20–23]. Thus, the observed lower FT3 concentrations in both groups with decreased IGF-I, despite even higher FT4 levels in them seem to correspond with GHD in these patients. Moreover, the very similar FT4, FT3 and TSH concentrations in both groups with normal IGF-I levels (i.e., in ISS Group and low GH Group), independently from either normal or subnormal results of GHST, should be stressed. We are convinced that these observations speak against the disorders of thyroid function related to GHD in both groups with normal IGF-I secretion, especially since we certainly do not expect such disorders in patients with ISS.
According to previous suggestions that IGF-I may be a mediator of GH action on stimulating peripheral T4 deiodination [24], the results of current study seem quite reliable. The quoted observation allows to explain the results obtained in GHD Group, ISS Group, and - to some extent - in low IGF Group. In fact, in the latter group, either decreased GH sensitivity or other completely overlooked diseases, present in most of children, should be assumed as the only possible cause of decreased IGF-I secretion. Taking into account both the relatively low incidence of GH insensitivity and the exclusion criteria, such possibility seems, however, poorly justified. Next, the results, obtained in low GH Group do not seem to be explained by anything - except for the falsely positive (i.e., falsely decreased) GH peaks in both performed GHST. In these patients, not only IGF-I concentrations are normal but also they correspond to the similar thyroid status as we observed in ISS Group.
As it was mentioned before, the discrepancies between the results of GHST and IGF-I secretion were reported in numerous studies [9–12], being explained either by the individual differences in GH sensitivity [32] or by the lack of concordance between GH secretion under physiological conditions and the results of GHST [33–35]. The conclusions, derived from these observations were also non-consistent. For instance, Rasat et al. [36] proposed IGF-I assessment as a screening procedure in diagnosing GHD. Similar was the statement of Rosenfeld [37–39]. However, there is also a strong evidence that it may be impossible to predict the results of GHST on the ground of IGF-I concentration [9, 12–14, 16, 40–42]. It seems very important to adequately answer the question, which (if any) of these procedures is the most reliable one in GHD diagnosing. According to current recommendations [1, 2], GHST are the main tools for the assessment of GH secretion. However, some other options were also proposed. Thus, Badaru and Wilson [43] stated that IGF-I assessment had of no less importance than the results of GHST. Similarly, Loche at al. [7] suggested that the diagnosis of GHD should not exclusively be based on the results of GHST. In 2009, Lemaire et al. [44] proposed the assessment of growth rate and IGF-I concentration as the screening procedures in children suspected for GHD, in order to reduce the necessity of subjecting the patients to GHST. The results of our previous studies [19, 45] also speak for the significance of IGF-I assessment in GHD diagnosing, thus bringing into question the credibility of GHST.
Another issue, however not analyzed in the current study, is an assessment of spontaneous GH secretion. Although - up to now - this procedure has not been recommended for clinical practice according to international guidelines [2], the data exist that GH administration to short children with decreased stimulated but normal spontaneous GH secretion is not associated with an increase of final height [46]. It seems that not only decreased spontaneous GH secretion should be taken into account in short children with low IGF-I concentrations (leading to the diagnosis of neurosecretory dysfunction) but also normal spontaneous GH secretion must be considered in the patients with normal IGF-I levels, despite decreased GH peak in GHST. It should also be taken into account that the relatively high incidence of falsely positive results of the two (2) GHST, performed in an individual patient was revealed in one of our previous studies [47]. As in Poland the assessment of spontaneous GH secretion after falling asleep was introduced as an obligatory procedure (screening) in diagnosing GHD in children, our research team intends to assess prospectively both IGF-I secretion and thyroid function with respect to nocturnal GH secretion.
Conclusion
It seems that the assessment of thyroid function, while diagnosing GHD in children with short stature, provides the evidence that IGF-I concentration may be no less reliable indicator of GH action than the results of GHST, at least in children suspected for idiopathic, isolated GHD and after exclusion of other causes of impaired IGF-I secretion.
Abbreviations
- CV:
-
coefficient of variation
- FT4 :
-
free thyroxine
- FT3 :
-
free triiodothyronine
- GH:
-
growth hormone
- GHD:
-
growth hormone deficiency
- GHST:
-
growth hormone stimulating tests
- hSDS:
-
height standard deviation score
- Hypo-T:
-
hypothyroidism
- IGF-I:
-
insulin-like growth factor-I
- IGFBP-3:
-
insulin-like growth factors binding protein-3
- ISS:
-
idiopathic short stature
- SD:
-
standard deviation
- SDS:
-
standard deviation score
- T4 :
-
thyroxine
- T3 :
-
triiodotyronine
- TSH:
-
thyroid stimulating hormone (thyrotropin)
References
Saggese G, Ranke MB, Saenger P, Rosenfeld RG, Tanaka T, Chaussain JL, Savage MO: Diagnosis and treatment of growth hormone deficiency in children and adolescents: towards a consensus. Horm Res 1998, 50: 320–340. 10.1159/000023298
GH Research Society: Consensus guidelines for the diagnosis and treatment of growth hormone deficiency in childhood and adolescence: summary statement of the GH Research Society. J Clin Endocrinol Metab 2000, 85: 3990–3993. 10.1210/jc.85.11.3990
Cianfarani S, Liguori A, Germani D: IGF-I and IGFBP-3 assessment in the management of childhood onset growth hormone deficiency. Endocr Dev 2005, 9: 66–75. full_text
Rosenfeld RG, Albertsson-Wikland K, Cassorla F, Frasier SD, Hasegawa Y, Hintz RL, Lafranch S, Lippe B, Loriaux L, Melmed S, Preece MA, Ranke MB, Reiter EO, Rogol AD, Underwood LE, Werther GA: Diagnostic controversy: the diagnosis of childhood growth hormone deficiency revisited. J Clin Endocrinol Metab 1995, 80: 1532–1540. 10.1210/jc.80.5.1532
Price DA: GH testing in KIGS: the clinical reality. In Growth Hormone Therapy in KIGS - 10 Years' Experience. Edited by: Ranke MB, Wilton P. Johann Ambrosius Barth Verlag, Leipzig, Hiedelberg; 1999:73–80.
Zadik Z, Chalew SA, Gilula Z, Kowarski AA: Reproducibility of growth hormone testing procedures: a comparison between 24-hour integrated concentration and pharmacological stimulation. J Clin Endocrinol Metab 1990, 71: 1127–1130. 10.1210/jcem-71-5-1127
Loche S, Bizzarri C, Maghine M, Faedda A, Tziala C, Autelli M, Casini MR, Cappa M: Results of early reevaluation of growth hormone secretion in children with apparent growth hormone deficiency. J Pediatr 2002, 140: 445–449. 10.1067/mpd.2002.122729
Thomas M, Massa G, Maes M, Beckers D, Craen M, Francois I, Heinrichs C, Bourguignon JP, Belgian Study Group for Paediatric Endocrinology (BSGPE): Growth hormone (GH) secretion in patients with childhood-onset GH deficiency: retesting after one year of therapy and at final height. Horm Res 2003, 59: 7–15. 10.1159/000067936
Rosenfeld RG, Wilson DM, Lee PD, Hintz RL: Insulin-like growth factors I and II in evaluation of growth retardation. J Pediatr 1986, 109: 428–433. 10.1016/S0022-3476(86)80112-3
Nunez SB, Municchi G, Barnes KM, Rose SR: Insulin-like growth factor I (IGF-I) and IGF-binding protein-3 concentrations compared to stimulated and night growth hormone in the evaluation of short children - a clinical research center study. J Clin Endocrinol Metab 1996, 81: 1927–1932. 10.1210/jc.81.5.1927
Koch A, Dorr HG: Insulin-like growth factor-I and its binding protein-3 in serum: are they good screening properties for the diagnosis of growth hormone deficiency? Eur J Clin Chem Clin Biochem 1997, 35: 379–385.
Tillmann V, Buckler JM, Kibirige MS, Price DA, Shalet SM, Wales JK, Addison MG, Gill MS, Whatmore AJ, Clayton PE: Biochemical tests in the diagnosis of childhood growth hormone deficiency. J Clin Endocrinol Metab 1997, 82: 531–535. 10.1210/jc.82.2.531
Hindmarsh PC, Swift PGF: An assessment of growth hormone provocation tests. Arch Dis Child 1995, 72: 362–368. 10.1136/adc.72.4.362
Mitchell H, Dattani V, Nanduri P, Hindmarsh PC, Preece MA, Brook CDG: Failure of IGF-I and IGFBP-3 to diagnose growth hormone insufficiency. Arch Dis Child 1999, 80: 443–447. 10.1136/adc.80.5.443
Bouquete HR, Sobrado PG, Fideleff HL, Sequera AM, Giaccio AV, Suarez MG, Rubial GF, Miras M: Evaluation of diagnostic accuracy of insulin-like growth factor (IGF)-I and IGF-binding protein-3 in growth hormone-deficient children and adults using ROC plot analysis. J Clin Endocrinol Metab 2003, 88: 4702–4708. 10.1210/jc.2003-030412
Haghshenas Z, Sotoudeh K, Karamifar H, Karamizadeh Z, Amirhakimi G: The role of insulin like growth factor (IGF)-1 and IGF-binding protein-3 in diagnosis of Growth Hormone Deficiency in short stature children. Indian J Pediatr 2009, 76: 699–703. 10.1007/s12098-009-0115-0
Rosenfeld RG, Lamson G, Pham H, Oh Y, Conover C, De-Leon DD, Donovan SM, Ocrant I, Guidice L: Insulin-like growth factor binding proteins. Recent Prog Horm Res 1990, 46: 99–164.
Shalet SM, Toogood A, Rahim A, Brennan BMD: The diagnosis of growth hormone deficiency in children and adults. Endocrine Rev 1998, 19: 203–223. 10.1210/er.19.2.203
Hilczer M, Smyczynska J, Stawerska R, Lewinski A: Stability of IGF-I concentration despite divergent results of repeated GH stimulating tests indicates poor reproducibility of test results. Endocr Regul 2006, 40: 37–45.
Gács G, Bános C: The effect of growth hormone on the plasma levels of T4, free-T4, T3, reverse T3 an TBG in hypopituitary patients. Acta Endocrinol (Copenh) 1981, 96: 475–479.
Rezvani I, DiGeorge AM, Dowshen SA, Bourdony CJ: Action of human growth hormone (hGH) on extrathyroidal conversion of thyroxine (T4) to triiodothyronine (T3) in children with hypopituitarism. Pediatr Res 1981, 15: 6–9.
Jørgensen JO, Pedersen SA, Laurberg P, Weeke J, Skakkebaek NE, Christiansen JS: Effects of growth hormone therapy on thyroid function of growth hormone-deficient adults with and without concomitant thyroxine-substituted central hypothyroidism. J Clin Endocrinol Metab 1989, 69: 1127–1132.
Jørgensen JO, Møller J, Laursen T, Orskov H, Christiansen JS, Weeke J: Growth hormone administration stimulates energy expenditure and extrathyroidal conversion of thyroxine to triiodothyronine in a dose-dependent manner and suppresses circadian thyrotrophin levels: studies in GH-deficient adults. Clin Endocrinol (Oxf) 1994, 41: 609–614.
Jørgensen JO, Møller J, Skakkebaek NE, Weeke J, Christiansen JS: Thyroid function during growth hormone therapy. Horm Res 1992,38(Suppl 1):63–67.
Akin F, Yaylali GF, Turgut S, Kaptanoglu B: Growth hormone/insulin-like growth factor axis in patients with subclinical thyroid dysfunction. Growth Horm IGF Res 2009, 19: 252–255. 10.1016/j.ghir.2008.11.003
Näntö-Salonen K, Muller HL, Hoffman AR, Vu TH, Rosenfeld RG: Mechanisms of thyroid hormone action on the insulin-like growth factor system: all thyroid hormone effects are not growth hormone mediated. Endocrinology 1993, 132: 781–788.
Inukai T, Takanashi K, Takebayashi K, Fujiwara Y, Tayama K, Takemura Y: Thyroid hormone modulates insulin-like growth factor-I(IGF-I) and IGF-binding protein-3, without mediation by growth hormone, in patients with autoimmune thyroid diseases. Horm Metab Res 1999, 31: 576–579. 10.1055/s-2007-978798
Iglesias P, Bayón C, Méndez J, Gancedo PG, Grande C, Diez JJ: Serum insulin-like growth factor type 1, insulin-like growth factor-binding protein-1, and insulin-like growth factor-binding protein-3 concentrations in patients with thyroid dysfunction. Thyroid 2001, 11: 1043–1048. 10.1089/105072501753271734
Purandare A, Co Ng L, Godil M, Ahnn SH, Wilson TA: Effect of hypothyroidism and its treatment on the IGF system in infants and children. J Pediatr Endocrinol Metab 2003, 16: 35–42.
Soliman AT, Omar M, El Awwa A, Rizk MM, El Alaily RK, Bedair EM: Linear growth, growth-hormone secretion and IGF-I generation in children with neglected hypothyroidism before and after thyroxine replacement. J Trop Pediatr 2008, 54: 347–349. 10.1093/tropej/fmn030
Palczewska I, Niedźwiecka Z: Indices of somatic development of Warsaw children and adolescents. Medycyna Wieku Rozwojowego 2001,5(suppl. I/2):17–118. (in Polish)
Clayton PE: The Role of Insulin-Like Growth Factors in the Diagnosis of Growth Hormone Deficiency. In Growth Hormone Therapy in KIGS - 10 Years' Experience. Edited by: Ranke MB, Wilton P. Johann Ambrosius Barth Verlag, Leipzig, Hiedelberg; 1999:53–64.
Bercu BB, Schulman D, Root AW, Spiliotis BE: Growth hormone provocative testing frequently does not reflect endogenous GH secretion. J Clin Endocrinol Metab 1986, 63: 709–716. 10.1210/jcem-63-3-709
Donaldson DL, Pan F, Hollowell JG, Stevenson JL, Gifford RA, Moore WV: Reliability of stimulated and spontaneous growth hormone levels in identifying the child with low GH secretion. J Clin Endocrinol Metab 1991, 72: 647–652. 10.1210/jcem-72-3-647
Ropelato MG, Martinez A, Heinrich JJ, Bergada C: Reproducibility of growth hormone secretion tests. J Pediatr Endocrinol Metab 1996, 9: 41–50.
Rasat R, Livesey JL, Espiner EA, Abbott D, Donald RA: IGF-1 and IGFBP-3 screening for disorders of growth hormone secretion. N Z Med J 1996, 109: 156–159.
Rosenfeld RG: Biochemical diagnostic strategies in the evaluation of short stature: the diagnosis of insulin-like growth factor deficiency. Horm Res 1996, 46: 170–173. 10.1159/000185018
Rosenfeld RG: An endocrinologist's approach to the growth hormone - insulin-like growth factor axis. Acta Paediatr Suppl 1997, 423: 17–19.
Rosenfeld RG: Editorial: Is growth hormone deficiency a viable diagnosis? J Clin Endocrinol Metab 1997, 82: 349–351. 10.1210/jc.82.2.349
Juul A, Dalgaard P, Blum WF, Bang P, Hall K, Michaelsen KF, Müller J, Skakkebaek N: Serum levels of insulin-like growth factor (IGF) binding protein 3 (IGFBP-3) in healthy infants, children and adolescents: the relation to IGF-I, IGF-II, IGFBP-1, IGFBP-2, age, sex, body mass index, and pubertal maturation. J Clin Endocrinol Metab 1995, 80: 2534–2542. 10.1210/jc.80.8.2534
Ranke MB, Schweitzer R, Elmlinger MW, Weber K, Binder G, Schwarze CP, Wollmann HA: Significance of basal IGF-I, IGFBP-3 and IGFBP-2 measurements in the diagnostics of short stature in children. Horm Res 2000, 54: 60–68. 10.1159/000053233
Bouquete HR, Sobrado PG, Fideleff HL, Sequera AM, Giaccio AV, Suarez MG, Rubial GF, Miras M: Evaluation of diagnostic accuracy of insulin-like growth factor (IGF)-I and IGF-binding protein-3 in growth hormone-deficient children and adults using ROC plot analysis. J Clin Endocrinol Metab 2003, 88: 4702–4708. 10.1210/jc.2003-030412
Badaru A, Wilson DM: Alternatives to growth hormone stimulation testing in children. Trends Endocrinol Metab 2004, 15: 252–258. 10.1016/j.tem.2004.06.004
Lemaire P, Brauner N, Hammer P, Trivin C, Souberbielle JC, Brauner R: Improved screening for growth hormone deficiency using logical analysis data. Med Sci Monit 2009, 15: 5–10.
Smyczyńska J, Lewiński A, Hilczer M, Stawerska R, Karasek M: Partial growth hormone deficiency (GHD) in children has more similarities to idiopathic short stature than to severe GHD. Endokrynol Pol - Pol J Endocr 2007, 58: 182–187.
Radetti G, Buzi F, Cassar W, Paganini C, Stacul E, Maghnie M: Growth hormone secretory pattern and response to treatment in children with short stature followed to adult height. Clin Endocrinol (Oxf) 2003, 59: 27–33. 10.1046/j.1365-2265.2003.01773.x
Hilczer M, Smyczyńska J, Lewinski A: Limitations of clinical utility of growth hormone stimulating tests in diagnosing children with short stature. Endocr Reg 2006, 40: 69–75.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
JS designed the study, qualified the patients and performed statistical analysis. RS participated in performing hormonal studies and in statistical analysis. AL participated in drawing up the study protocol and coordinated the study. MH, the senior author, wrote the manuscript. All authors read and approved the final manuscript
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Smyczyńska, J., Stawerska, R., Lewiński, A. et al. Do IGF-I concentrations better reflect growth hormone (GH) action in children with short stature than the results of GH stimulating tests? Evidence from the simultaneous assessment of thyroid function. Thyroid Res 4, 6 (2011). https://doi.org/10.1186/1756-6614-4-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1756-6614-4-6