Abstract
Background
Normal thyroid hormone secretion or appropriate L-thyroxine (L-T4) substitution is necessary for the optimal effect of the growth hormone (GH) administration on growth rate. The decrease of free thyroxine (FT4) levels at recombinant human GH (rhGH) therapy onset has been reported in several studies. The aim of the present study was to evaluate the effect of rhGH administration on thyrotropin (TSH) and FT4 serum concentrations in children with GH deficiency (GHD) during the 1st year of therapy, as well as to assess potential indications to thyroid hormone supplementation in them.
Patients and methods
The analysis involved data of 75 children (59 boys, 16 girls) with disorders of GH secretion (GHD, neurosecretory dysfunction - NSD) and partial GH inactivity (inactGH), who were treated with rhGH for - at least - one year. In all the children, body height and height velocity (HV) were assessed before and after 1 year of therapy, while TSH, FT4, IGF-I and IGFBP-3 before treatment and after 3-6 months and 1 year of treatment. In the patients, who revealed hypothyroidism (HypoT), an appropriate L-T4 substitution was introduced immediately. The incidence of HypoT, occurring during the initial phase of rhGH therapy, was assessed, as well as its influence on the therapy effectiveness.
Results
Before rhGH substitution, there were no significant differences in either auxological indices or TSH and FT4 secretion, or IGF-I concentration and its bioavailability among the groups of patients. During the initial 3-6 months of rhGH administration, a significant decrease of FT4 serum concentration, together with a significant increase of IGF-I SDS and IGF-I/IGFBP-3 molar ratio was observed in all the studied groups. In 17 children, HypoT was diagnosed and L-T4 substitution was administered. Despite similar IGF-I secretion increase, the improvement of HV presented significantly lower in children with HypoT than in those who remained euthyroid all the time.
Conclusions
The incidence of HypoT during the initial phase of GH treatment in children with GHD and the negative effect of even transient thyroid hormone deficiency on the growth rate should be taken into account.
Similar content being viewed by others
Background
Growth hormone (GH) deficiency (GHD) in children with short stature is an unchallenged indication to the therapy with recombinant human GH (rhGH). The main goal of the treatment is to increase patients' height velocity (HV) and to improve the attained final height (FH). The most important peripheral mediator of GH activity is the insulin-like growth factor-I (IGF-I). The insulin-like growth factor binding protein-3 (IGFBP-3) is the main carrier protein binding to IGF-I in plasma, thus determining its bioavailability. Besides, either normal thyroid hormone secretion or appropriate substitution of L-thyroxine (L-T4) is necessary for the optimal effect of both endogenous GH and rhGH substitution on the growth rate.
The relationships between GH secretion and thyroid function, as well as the effects of rhGH administration on thyroid hormone levels have been the subject of numerous studies. The data of Cacciari et al. [1], presented 30 years ago, indicated that the risk of inducing an alteration in thyroid function in hypopituitary patients during rhGH therapy was only slight and that the abnormal values of thyroxine (T4) and triiodothyronine (T3) returned to normal limits during follow-up. Next, Gács and Bános [2] reported that rhGH therapy in children with idiopathic GHD reduced T4 secretion and affected the peripheral metabolism of thyroid hormones, resulting in an increase of T3. In 1994, Jørgensen et al. [3] reported that, in GH-deficient adults, rhGH administration stimulated peripheral T4 to T3 conversion in a dose-dependent manner and influenced circadian rhythm of thyrotropin (TSH) secretion. Moreover, in some of those patients before rhGH administration, serum T3 levels were subnormal despite T4 substitution and normalised during the therapy. As it was shown that rhGH administration might induce a fall in serum T4, it seemed probable that GHD could mask secondary hypothyroidism in some patients with hypopituitarism. Recently, Agha et al. [4] proved that rhGH administration really led to „unmasking” hypotyroidism in hypopituitary adults. Similar were the observations of Losa et al. [5], who reported that, in adults with GHD, administration of rhGH therapy was associated with a significant decrease of free T4 (FT4) in first 6 months of treatment.
First reports, concerning the effects of rhGH therapy on thyroid hormone levels in children also confirmed an increase of extrathyroidal conversion of T4 to T3 during the therapy [6]. Conversely than described for adults [5], as early as in 1994, Laurberg et al. [7] stated that children with GHD, evaluated thoroughly to exclude secondary thyroid failure before rhGH administration, did not develop hypothyroidism during rhGH substitution.
Up to now, several interesting studies have been published on long-term effects of rhGH replacement therapy on thyroid function, both in adults [8–10] and in children [11–18].
The aim of current study was to evaluate the effect of rhGH substitution on TSH and FT4 serum concentrations in children with GHD during the 1st year of therapy, as well as to assess potential indications to thyroid hormone supplementation in them.
Patients and methods
The retrospective analysis involved the data of 75 children (59 boys, 16 girls) with GHD, who were qualified to rhGH therapy. At therapy onset, the patients' height was below the 3rd centile, according to Polish reference charts [19], HV was slow (below -1.0 SD/year), bone age was delayed, according to Greulich-Pyle's standards [20]. Thyroid function was normal in most of children (67 cases). In the remaining 8 patients, L-T4 supplementation had been administered, due to either hyperthyrotropinemia or relatively low (normal but close to the lower limit of reference range) FT4 concentration and pharmacological euthyrodism was then confirmed. In all the children IGF-I and IGFBP-3 secretion was measured in a single blood sample during in morning hours. In most of the children, IGF-I concentration was either decreased or close to lower limit of normal range. It should be mentioned that - though IGF-I is the main peripheral mediator of GH action - children with normal IGF-I secretion may be diagnosed as GH-deficient and qualified to rhGH therapy in the light of current national recommendations, enclosed in the programme of therapy of GHD in children with rhGH [21]. In all the patients, nocturnal GH secretion was assessed during 3 hours after falling asleep (5 samples every 30 minutes from the 60th to the 180th minute) and 2 stimulating tests were performed (with clonidine 0.15 mg/m2 orally and with glucagon 30 μg/kg, not exceeding 1 mg, i.m.). The diagnosis of GHD was established when GH peak during nocturnal assessment and in both stimulating tests was below 10 ng/ml. Neurosecretory dysfunction (NSD) was diagnosed in children with normal results of stimulating tests but decreased nocturnal GH secretion (that observation had to be confirmed by documenting decreased GH secretion in prolonged, 6-hour nocturnal profile). In children with decreased IGF-I secretion and normal GH peak (both in nocturnal profile and after pharmacological stimulation), IGF-I generation test was performed after exclusion of other causes of IGF-I deficiency, not related to GH secretion disorders and GH action (like malabsorption syndromes, liver diseases, malnutrition, other severe chronic diseases). Interestingly enough, a good response to rhGH administration - at least, twofold increase of IGF-I secretion, leading to normalisation of its level in plasma - was observed in each case, thus allowing exclusion of GH insensitivity and pointing at decreased bioactivity of endogenous GH in these children. The obtained results of that test supported the indications to rhGH administration in them. Children with either other hormonal deficiencies or severe and/or chronic, growth influencing diseases, as well as those with acquired GHD, were not included to the studied group. All the girls had normal female karyotype.
At rhGH therapy onset, patients' age was 12.2 ± 2.4 years (mean ± SD). The therapy with rhGH in a dose of 0.21 ± 0.02 mg (0.63 ± 0.05 IU)/kg/week was administered for, at least, 1 year. In every patient, TSH and FT4 concentration was assessed three times: before the first rhGH injection, after 3-6 months and at after 1 year of treatment; all the blood samples were taken in morning hours. Additionally, IGF-I and IGFP-3 concentrations were measured at the same time points and IGF-I standard deviation score (SDS) for age and sex, as well as IGF-I/IGFBP-3 molar ratio was calculated.
Plasma TSH and FT4 concentrations were measured by the electroimmunochemiluminescent method (ECLIA), Roche, Elecsys®Systems 1010/2010/modular analytics E170. For TSH, analytical sensitivity was 0.005 μIU/ml, range - up to 100 μIU/ml, intra-assay coefficient of variance (CV) - 1.5-8.6%, accuracy - 1.1-3.0%. Analytical range for FT4 was 0.023-7.77 ng/dl, intra-assay CV - 1.4-2.9%, accuracy - 2.7-6.6%.
Growth hormone concentrations were measured by hGH Immulite, DPC assay, calibrated to WHO IRP 80/505 standard, with analytical sensitivity up to 0.01 ng/ml, calibration range up to 40 ng/ml, sensitivity of 0.01 ng/ml, intra-assay CV - 5.3-6.5% and inter-assay CV - 5.5-6.2%.
Both IGF-I and IGFBP-3 concentration was assessed by Immulite, DPC assays. For IGF-I, WHO NIBSC 1st IRR 87/518 standard was applied, with analytical sensitivity 20 ng/ml, calibration range up to 1600 ng/ml, intra-assay CV - 3.1-4.3% and inter-assay CV - 5.8-8.4%. For comparison among children of different age and sex, IGF-I concentrations were expressed as IGF-I SDS, according to DPC reference data. The assay for IGFBP-3 assessment was calibrated to WHO NIBSC Reagent 93/560 standard, with analytical sensitivity 0.02 μg/ml, the calibration range up to 426 μg/ml, the intra-assay CV - 3.5-5.6% and the total CV - 7.5-9.9%. For calculation of IGF-I/IGFBP-3 molar ratio, the following molecular masses were used: 7.5 kDa for IGF-I and 42.0 kDa for IGFBP-3 [22]. For comparison among children with different age and sex, IGF-I concentrations were expressed as SD score (IGF-I SDS), according to DPC reference data.
Statistical analysis included comparison of thyroid function (FT4 and TSH concentrations), IGF-I secretion (as IGF-I SDS) and its bioavailability (expressed as IGF-I/IGFBP-3 molar ratio) in particular time points, before and during rhGH therapy. Non-parametric Wilcoxon's test for dependent samples was applied, as the distribution of the analysed parameters (assessed with Kolmogorov-Smirnov's test) presented not consistent with normal distribution. The differences among particular subgroups of patients in the same time point were assessed with non-parametric Kruskall-Wallis' test for independent samples. The level of statistical significance was at p < 0.05.
Results
Before rhGH substitution, there were no significant differences in either auxological indices or TSH and FT4 secretion, or IGF-I concentration and its bioavailability among the groups of patients with GHD, NSD and inactGH. Moreover, all the differences among the groups at particular time points (i.e. both after 3-6 months and after 1 year of rhGH therapy) still remained insignificant, except for significantly lower TSH in inactGH than in both GHD and NSD after 1 year of rhGH treatment (see Table 1). Interestingly enough, the changes in FT4 and TSH concentration were similar in children with previously normal thyroid function (67 cases) and in those on L-T4 substitution at rhGH therapy onset (8 cases). Detailed comparisons of the above-mentioned groups of children are presented in Table 2. Among the patients who had L-T4 substitution, administered before rhGH therapy onset, the decrease of FT4 below the lower limit of normal range was observed in 2 out of 8 cases and in those children, an increased dose of L-T4 was necessary to restore euthyroidism. A significant decrease of FT4 serum concentration was observed during the initial 3-6 months of rhGH administration, together with insignificant increase of TSH in all the studied groups, as well as in particular subgroups of patients. For more detailed data see Table 1 and Figure 1 and Figure 2.
Simultaneously, a significant increase of IGF-I SDS was observed not only on the 3-6th month of rhGH therapy, with respect to pre-treatment values, but also after 1 year of therapy vs. the values obtained on the 3-6th month of treatment. Significant differences in IGF-I SDS in particular time points were observed for all the subgroups, expect for that between the values on the 3-6th month and after 1 year in the subgroup with inactGH. Similarly, a significant increase of IGF-I/IGFBP-3 molar ratio was observed on the 3-6th month of rhGH therapy, with respect to pre-treatment values, however, with only a very slight further increase (insignificant) after 1 year of therapy, both in all the studied groups and in the subgroups of patients. The more detailed data are presented in Figure 3 and Figure 4. Interestingly enough, there were no significant differences in either IGF-I SDS or IGF-I/IGFBP-3 molar ratio, observed among the particular subgroups of children at any time point (see Table 1).
According to our observations, the therapy with rhGH led to a similar increase of IGF-I secretion and of its bioavailability in the patients with different forms of disorders of GH secretion and activity. During the initial phase of rhGH replacement therapy, a transient decrease of FT4 concentration was observed in all the subgroups of patients, in most subjects being connected with an increase of TSH secretion. Spontaneous normalisation of FT4 concentration on the level close to the values obtained before rhGH administration, observed in the majority of patients, presented parallel to further increase of IGF-I secretion.
In 17 children, out of 67 previously untreated with L-T4 (25.4%), either FT4 concentration decreased below the lower limit of normal range (8 cases) or TSH increased above the upper limit of the normal range (8 cases), or both (1 case). In those patients, L-T4 therapy was administered just after the diagnosis of hypothyroidism was established, in the initial daily dose of 25 μg, individually adjusted under control of TSH and FT4 levels. In the remaining 50 patients, TSH and FT4 concentrations returned to pretreatment values at the end of the 1st year of rhGH administration. Thus, in all the children both TSH and FT4 concentrations after 1 year of rhGH therapy were normal. It should be reminded that all the children were euthyroid at rhGH therapy onset.
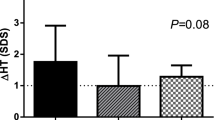
In the patients, in whom hormonal evaluation after 3-6 months of rhGH administration led to the diagnosis of HypoT (at least in subclinical form), TSH levels were significantly higher, while FT4 concentrations - significantly lower than in those children who remained euthyroid during rhGH administration. There were no significant differences between them in either IGF-I SDS or IGF-I/IGFBP-3 molar ratio at that time point. Moreover, after 1 year of rhGH administration there were no significant differences either in IGF-I secretion or in IGF-I/IGFBP-3 molar ratio between the groups of children who were euthyroid all the time and the other one, grouping children who required L-T4 substitution. However, it should be emphasized that TSH and FT4 levels in all of them were normal at the latter time point. Nevertheless, some differences between the groups were observed and should be mentioned. Surprisingly, IGF-I SDS presented lower (however only insignificantly) in those children who remained euthyroid during all the study period, than in those, who presented with hypothyroidism after 3-6 months of rhGH replacement. Another important observation was a further increase of IGF-I SDS in both groups after 1 year of therapy with respect to the values obtained after 3-6 months. Nevertheless, after 1 year of rhGH therapy, HV improvement (expressed both as the difference and as the ratio between HV during and before the therapy) was significantly lower in those children who were hypothyroid even for a relatively short period of time during the initial phase of rhGH therapy. It should be stressed that L-T4 substitution was administered as soon as possible when hypothyroidism was diagnosed. For detailed data see Table 3 and Figure 5, Figure 6 and Figure 7.
Discussion
The phenomenon of T4 and FT4 concentration decrease after rhGH administration in GH-deficient subjects has been reported in several studies [1, 2, 5, 9, 12, 14]. Also, the return of thyroid function to baseline during follow-up has been quite well documented [13, 15, 17, 18]. Our results confirm previous observations, concerning these problems.
Moreover, it has been suggested that rhGH therapy might disclose previously unrecognised thyroid insufficiency rather than induce hypothyroidism [4, 7, 14]. The data, concerning the development of central hypothyroidism in terms of rhGH substitution, seem to be rather scarce and non-consistent. In 2007, Agha et al. [7] stated that GHD masked central hypothyroidism in a significant proportion of hypopituitary adults. Conversely, in 2008, Lose et al. [5] reported only a low incidence of hypothyroidism in GH-deficient adults on rhGH substitution. Similar were the observations of Laurberg et al. [7] and Giavoli et al. [16] in GH-deficient children, beginning rhGH therapy. In our study, in 15% of previously euthyroid children, a decrease of FT4 level with no adequate increase of TSH was observed, (it should be stressed that children with evident central hypothyroidism before rhGH administration, being a component of multiple pituitary hormone deficiency, were not included in current study). Thus, our observations confirm the phenomenon of „unmasking” central hypothyroidism after rhGH therapy administration in some of children with previous diagnosis of isolated GHD. Changes in TSH secretion during initial period of rhGH substitution are less evident than fluctuations of FT4 concentration. Most researchers reported either a lack of significant changes in TSH secretion [1, 4, 6, 9, 14, 16, 18] or a decrease of TSH level in terms of rhGH administration [8, 15, 17]. The last phenomenon has been explained by an increase of somatostatin (being a natural TSH inhibitor) in the patients on rhGH therapy [15]. In our study, the mean TSH serum concentration presented a slight increase, followed by a recovery to pre-treatment level. It seems that the differences among the results obtained in different studies might be related to different study protocols and analysed time points.
The most frequently quoted mechanism of changes in thyroid hormone levels is GH-mediated increase of peripheral T4 to T3 deiodination [7–9, 12, 14]. Moreover, a potential role of IGF-I in stimulating that process has been suggested by Jørgensen et al. [9]. The relationships between GH, IGF-I and thyroid hormone secretion have been subject of numerous studies. More than 25 years ago, in 1983, Chernausek et al. [23] documented that plasma somatomedin C (i.e. IGF-I) concentrations were diminished in hypothyroid patients, however, the pathogenesis of this phenomenon remained unclear. Either diminished GH secretion or direct effects of hypothyroidism upon somatomedin production were considered. In early 1990s, Näntö-Salonen et al. [24] stated that the mechanisms of thyroid hormone action on the insulin-like growth factor system were not GH-mediated. Similar were the observations of Inukai et al. [25], concerning the patients with autoimmune thyroid diseases. In 2003, Iglesias et al. [26] stated that hypothyroidism is associated with significant reductions of IGF-1 and IGFBP-3. Next, Purandare et al. [27] documented that in infants with hypothyroidism both total and free IGF-I levels were lower than those in healthy ones and increased significantly after L-T4 therapy, while in older children with acquired hypothyroidism they were not significantly lower than in age- and sex-matched controls. However, during L-T4 treatment an increase of total IGF-I but not of free IGF-I was observed. Similarly, Bona et al. [28] documented that in the patients with hypothyroidism - both congenital and caused by thyroiditis - L-T4 replacement led to physiological increase of IGF-I and IGFBP-3 secretion. Moreover, Schmid et al. [29] showed that, during L-T4 replacement, IGF-I and acid-labile subunit secretion increased in the patients with both primary and central hypothyroidism, while IGFBP-3 - only in those with primary hypothyroidism. In 2008, Akin et al. [30] reported that GH-IGF axis was affected in the patients with subclinical hypothyroidism and that L-T4 replacement therapy could prevent abnormalities related to GH-IGF axis in them. Moreover, at the same time, Soliman et al. [31] proved that, in children with neglected congenital hypothyroidism, even after long period of hypothyroidism, L-T4 replacement improved the growth rate, leading to a partial recovery of GH-IGF-I axis.
In our study, differences were found between the improvement of the growth rate in the patients with normal thyroid function and in those with even transient hypothyroidism. As a matter of fact, a direct effect of thyroid function on IGF-I secretion was not fully confirmed in our study, as there were no significant differences in an increase of IGF-I secretion between the euthyroid children and those, who presented with hypothyroidism during rhGH administration. However, further increase of IGF-I secretion on the same rhGH dose was observed after 1 year of rhGH therapy, with respect to the values obtained after 3-6 months of treatment. This phenomenon might be explained either by the improvement of thyroid function (i.e. recovery to pre-rhGH-treatment values of FT4 and TSH) or by the appropriate L-T4 substitution.
Obligatory L-T4 supplementation from the beginning of rhGH therapy in euthyroid patients has not been recommended [17] due to a little evidence for the development of clinically significant hypothyroidism in most of previously euthyroid patients [13] and spontaneous recovery to pre-treatment thyroid function in most cases [13, 15, 17, 18].
Our findings speak for the important role of maintaining euthyroid status of the patients for the best effectiveness of rhGH therapy, as even short-term, transient hypothyroidism presented to be a cause of lower increase of HV in 1st year of rhGH administration. Thus, the incidence of revealing (or "unmasking") hypothyroidism should be taken into account, while starting rhGH administration, as hypothyroidism may worsen the response to the therapy. It seems that either earlier assessment of TSH and FT4 concentration after rhGH therapy onset or L-T4 administration from the beginning of rhGH therapy in children with normal but relatively low FT4 secretion and/or normal but relatively high TSH levels should be taken into account. Further studies seem necessary to fully assess the influence of thyroid function (and thyroid hormone substitution) on the effectiveness of rhGH therapy in children with disorders of GH secretion. It seems also important to establish - if possible - the threshold values of pre-rhGH-treatment TSH and/or FT4 levels predictive for revealing hypothyroidism during rhGH administration.
Conclusions
The incidence of HypoT during the initial phase of rhGH treatment in children with GHD and the negative effect of even transient thyroid hormone deficiency on growth rate should be taken into account while beginning rhGH administration in them.
Authors' information
AL - Professor; Head of Chair of Endocrinology and Metabolic Diseases, Medical University of Lodz, Poland, Head of Department of Endocrinology and Metabolic Diseases, Polish Mother's Memorial Hospital - Research Institute. MH - Ass. Professor; Head of Department of Pediatric Endocrinology, University of Lodz, Poland. JS - PhD, MD, endocrinologist. RS - PhD, MD, endocrinologist.
Abbreviations
- CV:
-
coefficient of variance
- FH:
-
final height
- FT4 :
-
free thyroxine
- GH:
-
growth hormone
- GHD:
-
growth hormone deficiency
- HypoT:
-
hypothyroidism
- HV:
-
height velocity
- IGF-I:
-
insulin-like growth factor-I
- IGFBP-3:
-
insulin-like growth factors binding protein-3
- L-T4 :
-
L-thyroxine (levothyroxine)
- NSD:
-
neurosecretory dysfunction
- inactGH:
-
growth hormone inactivity
- RhGH:
-
recombinant human growth hormone
- SDS:
-
standard deviation score
- T3 :
-
triiodotyronine
- T4 :
-
thyroxine
- TSH:
-
thyrotropin.
References
Cacciari E, Cicognani A, Pirazzoli P, Bernardi F, Zappulla F, Salardi S, Mazzanti L, Biasini A, Valenti E: Effect of long-term GH administration on pituitary-thyroid function in idiopathic hypopituitarism. Acta Paediatr Scand 1979, 68: 405–409. 10.1111/j.1651-2227.1979.tb05028.x
Gács G, Bános C: The effect of growth hormone on the plasma levels of T4, free-T4, T3, reverse T3 an TBG in hypopituitary patients. Acta Endocrinol (Copenh) 1981, 96: 475–479.
Jørgensen JO, Møller J, Laursen T, Orskov H, Christiansen JS, Weeke J: Growth hormone administration stimulates energy expenditure and extrathyroidal conversion of thyroxine to triiodothyronine in a dose-dependent manner and suppresses circadian thyrotrophin levels: studies in GH-deficient adults. Clin Endocrinol (Oxf) 1994, 41: 609–614. 10.1111/j.1365-2265.1994.tb01826.x
Agha A, Walker D, Perry L, Drake WM, Chew SL, Jenkins PJ, Grossman AB, Monson JP: Unmasking of central hypothyroidism following growth hormone replacement in adult hypopituitary patients. Clin Endocrinol (Oxf) 2007, 66: 72–77.
Losa M, Scavini M, Gatti E, Rossini A, Madaschi S, Formenti I, Caumo A, Stidley CA, Lanzi R: Long-term effects of growth hormone replacement therapy on thyroid function in adults with growth hormone deficiency. Thyroid 2008, 18: 1249–1254. 10.1089/thy.2008.0266
Rezvani I, DiGeorge AM, Dowshen SA, Bourdony CJ: Action of human growth hormone (hGH) on extrathyroidal conversion of thyroxine (T4) to triiodothyronine (T3) in children with hypopituitarism. Pediatr Res 1981, 15: 6–9.
Laurberg P, Jakobsen PE, Hoeck HC, Vestergaard P: Growth hormone and thyroid function: is secondary thyroid failure underdiagnosed in growth hormone deficient patients? Thyroidology 1994, 6: 73–79.
Jørgensen JO, Pedersen SA, Laurberg P, Weeke J, Skakkebaek NE, Christiansen JS: Effects of growth hormone therapy on thyroid function of growth hormone-deficient adults with and without concomitant thyroxine-substituted central hypothyroidism. J Clin Endocrinol Metab 1989, 69: 1127–1132. 10.1210/jcem-69-6-1127
Jørgensen JO, Møller J, Skakkebaek NE, Weeke J, Christiansen JS: Thyroid function during growth hormone therapy. Horm Res 1992,38(Suppl 1):63–67.
Martins MR, Doin FC, Komatsu WR, Barros-Neto TL, Moises VA, Abucham J: Growth hormone replacement improves thyroxine biological effects: implications for management of central hypothyroidism. J Clin Endocrinol Metab 2007, 92: 4144–4153. 10.1210/jc.2007-0941
Saggese G, Cesaretti G, Di Spigno G, Cinquanta L, Giannessi N, Cioni C, Bracaloni C: Thyroid and thyrotropin functions in subjects with pituitary nanism treated with growth hormone. Pediatr Med Chir 1990, 12: 483–488.
Pirazzoli P, Cacciari E, Mandini M, Sganga T, Capelli M, Cicognani A, Gualandi S: Growth and thyroid function in children treated with growth hormone. J Pediatr 1992, 121: 210–213. 10.1016/S0022-3476(05)81190-4
Wyatt DT, Gesundheit N, Sherman B: Changes in thyroid hormone levels during growth hormone therapy in initially euthyroid patients: lack of need for thyroxine supplementation. J Clin Endocrinol Metab 1998, 83: 3493–3497. 10.1210/jc.83.10.3493
Portes ES, Oliveira JH, MacCagnan P, Abucham J: Changes in serum thyroid hormones levels and their mechanisms during long-term growth hormone (GH) replacement therapy in GH deficient children. Clin Endocrinol (Oxf) 2000, 53: 183–189. 10.1046/j.1365-2265.2000.01071.x
Kalina-Faska B, Kalina M, Koehler B: Assessment of thyrotropin concentrations in children with somatotropin deficiency treated with growth hormone. Endokrynol Diabetol Chor Przemiany Materii Wieku Rozw 2002, 8: 17–21. (article in Polish)
Giavoli C, Porretti S, Ferrante E, Cappiello V, Ronchi CL, Travaglini P, Epaminonda P, Arosio M, Beck-Peccoz P: Recombinant hGH replacement therapy and the hypothalamus-pituitary-thyroid axis in children with GH deficiency: when should we be concerned about the occurrence of central hypothyroidism? Clin Endocrinol (Oxf) 2003, 59: 806–810. 10.1046/j.1365-2265.2003.01892.x
Kalina-Faska B, Kalina M, Koehler B: Effects of recombinant growth hormone therapy on thyroid hormone concentrations. Int J Clin Pharmacol Ther 2004, 42: 30–34.
Seminara S, Stagi S, Candura L, Scrivano M, Lenzi L, Nanni L, Pagliai F, Chiarelli F: Changes of thyroid function during long-term hGH therapy in GHD children. A possible relationship with catch-up growth? Horm Metab Res 2005, 37: 751–756. 10.1055/s-2005-921104
Palczewska I, Niedźwiecka Z: Indices of somatic development of Warsaw children and adolescents. Medycyna Wieku Rozwojowego 2001.,5(suppl I/2): (in Polish)
Greulich WW, Pyle SI: Radiographic Atlas of Skeletal Development of the Hand and Wrist. Stanford University Press, Stanford, California; 1993.
Therapy of short children with growth hormone deficiency. Therapeutic Programme of National Health Fund in Poland Regulation of the President of the National Health Fund 98/2008/DGL, Appendix 20 (in Polish)
Tillmann V, Patel L, Gill MS, Whatmore AJ, Price DA, Kibirige MS, Wales JK, Clayton PE: Monitoring serum insulin-like growth factor-I (IGF-I), IGF binding protein-3 (IGFBP-3), IGF-I/IGFBP-3 molar ratio and leptin during growth hormone treatment for disordered growth. Clin Endocrinol (Oxf) 2000, 53: 329–336. 10.1046/j.1365-2265.2000.01105.x
Chernausek SD, Underwood LE, Utiger RD, Van Wyk JJ: Growth hormone secretion and plasma somatomedin-C in primary hypothyroidism. Clin Endocrinol (Oxf) 1983, 9: 337–344. 10.1111/j.1365-2265.1983.tb00007.x
Näntö-Salonen K, Muller HL, Hoffman AR, Vu TH, Rosenfeld RG: Mechanisms of thyroid hormone action on the insulin-like growth factor system: all thyroid hormone effects are not growth hormone mediated. Endocrinology 1993, 132: 781–788. 10.1210/en.132.2.781
Inukai T, Takanashi K, Takebayashi K, Fujiwara Y, Tayama K, Takemura Y: Thyroid hormone modulates insulin-like growth factor-I(IGF-I) and IGF-binding protein-3, without mediation by growth hormone, in patients with autoimmune thyroid diseases. Horm Metab Res 1999, 31: 576–579. 10.1055/s-2007-978798
Iglesias P, Bayón C, Méndez J, Gancedo PG, Grande C, Diez JJ: Serum insulin-like growth factor type 1, insulin-like growth factor-binding protein-1, and insulin-like growth factor-binding protein-3 concentrations in patients with thyroid dysfunction. Thyroid 2001, 11: 1043–1048. 10.1089/105072501753271734
Purandare A, Co Ng L, Godil M, Ahnn SH, Wilson TA: Effect of hypothyroidism and its treatment on the IGF system in infants and children. J Pediatr Endocrinol Metab 2003, 16: 35–42.
Bona G, Rapa A, Boccardo G, Silvestro L, Chiorboli E: IGF-1 and IGFBP in congenital and acquired hypothyroidism after long-term replacement treatment. Minerva Endocrinol 1999, 24: 51–55.
Schmid C, Zwimpfer C, Brändle M, Krayenbühl PA, Zapf J, Wiesli P: Effect of thyroxine replacement on serum IGF-I, IGFBP-3 and the acid-labile subunit in patients with hypothyroidism and hypopituitarism. Clin Endocrinol (Oxf) 2006, 65: 706–711. 10.1111/j.1365-2265.2006.02652.x
Akin F, Yaylali GF, Turgut S, Kaptanoglu B: Growth hormone/insulin-like growth factor axis in patients with subclinical thyroid dysfunction. Growth Horm IGF Res 2009, 19: 252–255. 10.1016/j.ghir.2008.11.003
Soliman AT, Omar M, El Awwa A, Rizk MM, El Alaily RK, Bedair EM: Linear growth, growth-hormone secretion and IGF-I generation in children with neglected hypothyroidism before and after thyroxine replacement. J Trop Pediatr 2008, 54: 347–349. 10.1093/tropej/fmn030
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
JS participated in acquisition of data, performed the statistical evaluation and drafted the manuscript, MH participated in acquisition of data and in design of the study, RS participated in acquisition of data, AL conceived of the study, participated in its design and revised the text of manuscript. All authors read and approved the manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Smyczynska, J., Hilczer, M., Stawerska, R. et al. Thyroid function in children with growth hormone (GH) deficiency during the initial phase of GH replacement therapy - clinical implications. Thyroid Res 3, 2 (2010). https://doi.org/10.1186/1756-6614-3-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1756-6614-3-2