Abstract
Background
The most substantial and best preserved area of Atlantic Forest is within the biogeographical sub-region of Serra do Mar. The topographic complexity of the region creates a diverse array of microclimates, which can affect species distribution and diversity inside the forest. Given that Atlantic Forest includes highly heterogeneous environments, a diverse and medically important Culicidae assemblage, and possible species co-occurrence, we evaluated mosquito assemblages from bromeliad phytotelmata in Serra do Mar (southeastern Brazil).
Methods
Larvae and pupae were collected monthly from Nidularium and Vriesea bromeliads between July 2008 and June 2009. Collection sites were divided into landscape categories (lowland, hillslope and hilltop) based on elevation and slope. Correlations between bromeliad mosquito assemblage and environmental variables were assessed using multivariate redundancy analysis. Differences in species diversity between bromeliads within each category of elevation were explored using the Renyi diversity index. Univariate binary logistic regression analyses were used to assess species co-occurrence.
Results
A total of 2,024 mosquitoes belonging to 22 species were collected. Landscape categories (pseudo-F value = 1.89, p = 0.04), bromeliad water volume (pseudo-F = 2.99, p = 0.03) and bromeliad fullness (Pseudo-F = 4.47, p < 0.01) influenced mosquito assemblage structure. Renyi diversity index show that lowland possesses the highest diversity indices. The presence of An. homunculus was associated with Cx. ocellatus and the presence of An. cruzii was associated with Cx. neglectus, Cx. inimitabilis fuscatus and Cx. worontzowi. Anopheles cruzii and An. homunculus were taken from the same bromeliad, however, the co-occurrence between those two species was not statistically significant.
Conclusions
One of the main findings of our study was that differences in species among mosquito assemblages were influenced by landscape characteristics. The bromeliad factor that influenced mosquito abundance and assemblage structure was fullness. The findings of the current study raise important questions about the role of An. homunculus in the transmission of Plasmodium in Serra do Mar, southeastern Atlantic Forest.
Similar content being viewed by others
Background
The tropical forest of eastern South America, known as Atlantic Forest, is one of the world's most important biodiversity hotspots [1]. The most substantial and best preserved area of Atlantic Forest is found in the biogeographical sub-region of Serra do Mar [2], which contains approximately 30% of the remaining forest [3]. Although climatic conditions are relatively uniform across Serra do Mar, the topographic complexity of the region [4] creates a diverse array of microclimates. These topographical differences provide gradients of oxygen, humidity and temperature that can affect species distribution and diversity inside the forest [5, 6]. As such, topography is likely to be an important factor in shaping floral [4] and faunal [6] diversity in Atlantic Forest.
A rich diversity of species from the Culicidae family is found in Serra do Mar [7, 8]. Many of these mosquito species appear to have evolved in close association with bromeliads [9]. For example, Culex ocellatus Theobald and many species of Culex (Microculex) Theobald [10, 11] and the subgenera Hystatomyia Dyar and Phoniomyia Theobald of the genus Wyeomyia are highly dependent upon bromeliads for larval habitat [9]. With the exception of Anopheles (Kerteszia) bambusicolus Komp, which is associated with bamboo internodes, the larva and the pupa of Kerteszia Theobald species depend on bromeliad phytotelmata from preserved environments as their primary larval habitat [12]. The larvae of Anopheles (Kerteszia) cruzii Dyar & Knab, Anopheles (Kerteszia) bellator Dyar & Knab, and Anopheles (Kerteszia) homunculus Komp are frequently found in Nidularium and Vriesea bromeliads in Atlantic Forest [13].
Human malaria is endemic in Serra do Mar [14] where the primary vectors are An. cruzii and An. bellator[15]. Anopheles homunculus has also been incriminated as a vector of human Plasmodium parasites in Paraná and Santa Catarina states [16]. Despite the medical importance of these three species, many aspects of their biology are poorly known [15]. Consequently, studying the way in which environmental variables influence the presence and distribution of these species may help to determine the role of vectors in the dynamics of human plasmodium transmission in Serra do Mar.
Considering the existence of environmental determinants for mosquito assemblages in larval habitats [17], and a highly heterogeneous environment [4] and diverse medically important Culicidae assemblage in Serra do Mar [7], the main objectives of the study are to: (1) characterize bromeliad mosquito assemblage structure; (2) assess correlations between bromeliad mosquito assemblage structure and various environmental factors; (3) determine correlations between the most abundant species and various bromeliad characteristics; and (4) assess co-occurrence among species.
Methods
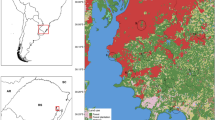
The study area is located in the Aroeira District (25° 0' 54"S and 47° 55' 37"W, SAD 69) of Cananéia, Serra do Mar, São Paulo state (Figure 1). Collection sites were divided into three distinct landscape categories based on elevation, slope and site accessibility. They were: 1) lowland (5-20 m altitude), 2) hillslope (33 to 54 m altitude), and 3) hilltop (81 to 263 m altitude). All three sites were of primary and considerably preserved forest. The lowland area was found adjacent to an estuarine channel and mangrove. The area was very humid, with a high tree density and little light penetration at ground level. Rocky outcrops were scattered in this area, but abundant at higher elevations (hillslope and hilltop), which may have lowered humidity and increased light penetration at the hilltop.
Study area and collection sites. A: Location of study area in South America. B: The remnants of Atlantic Forest in Serra do Mar, São Paulo and Rio de Janeiro states. C: The study area showing the collection sites according to landscape categories: lowland, hillslope and hilltop. The range of elevation (0 to 1,514 m) is relative to Serra do Mar (B).
Larvae and pupae were collected from terrestrial, epiphytic and saxicolous Nidularium (Subfamily: Bromelioideae) and Vriesea (Subfamily: Tillandsioideae) bromeliads in each of the three elevation categories at monthly intervals from July 2008 until June 2009. This yielded samples from 36 plants in each category, totaling 108 bromeliads. Monthly sampling sites varied spatially (by approximately 500 m) in order to increase the coverage area. Of the 108 bromeliads sampled, 94 were Nidularium and 14 Vriesea. Plants of both genera were equally sampled among elevation categories. Thirty Nidularium bromeliads were sampled on the lowland, 33 on the hillslope and 31 on the hilltop whilst 6 Vriesea were sampled on the lowland, 3 on the hillslope and 5 on the hilltop.
Data taken for each sampled plant were: type of bromeliad (terrestrial, epiphytic and saxicolous), bromeliad height, water pH, tank diameter, tank depth, and water volume. Water from each bromeliad was removed with a manual suction pump and measured for the water volume. Additional fresh water was poured into each plant and the removal was again repeated. Samples from each bromeliad were kept in separate plastic containers and the suction pump was washed with both 70% ethanol and fresh water to avoid cross contamination between plants. Climatic data from the two weeks preceding the collection date were obtained from the Centro Integrado de Informações Agrometeorológicas - CIIAGRO (http://www.ciiagro.sp.gov.br/) station in Cananéia.
Larvae and pupae were taken to the laboratory and raised to adulthood for species identification. Larval and pupal exuviae were mounted on microscopy slides with Canada balsam. Whenever possible, male genitalia were used for species identification. Morphological identification was based on Lane and Whitman [18], Lane [19], Correa and Ramalho [20], Cotrim and Galati [21] and Forattini [22]. Nine specimens that could not be identified using Lane's [19] keys were registered as Cx. (Mcx.) sp1 according to Marques' et al. [11] informal nomenclature. For the purpose of the present study, we identified larvae with evident subapical swelling as Cx. daumasturus[23], a species that was formerly synonymized with Cx. imitator imitator[19]. However, the validation of the species needs further investigation. Identification of An. cruzii and An. homunculus was based on characters of the fourth-instar larva, pupa and adults following the characteristics proposed by Forattini [22] and Sallum et al. [24]. Adults associated with either larval or pupal exuviae were deposited in Coleção Entomológica de Referência da Faculdade de Saúde Pública da Universidade de São Paulo (FSP-USP).
Statistical analyses were performed in the program R 2.12 (http://www.r-project.org/), using the packages BiodiversityR [25], MASS [26], and epicalc [27]. Species richness was extrapolated using the second order Jackknife [28] in order to assess the sampling effort. This estimator performs best with respect to accuracy in both even and uneven communities [29]. The multivariate redundancy analysis (RDA) [30] was used to assess correlations between bromeliad mosquito assemblage structure and various environmental variables. These variables included collection site landscape category (m), mean temperature (°C), total precipitation of rain two weeks prior to collection (mm), type of bromeliad (terrestrial, epiphytic and saxicolous), bromeliad height from the ground (m), tank diameter (cm), tank depth (cm), water pH, bromeliad water volume (ml), and an estimate of bromeliad tank fullness (ml/cm; water volume divided by tank height).
The Renyi diversity index [31] was used to explore differences in species diversity among bromeliads within each category of elevation (lowland, hillslope, and hilltop). This index provided four further diversity indices: Total richness, Shannon-Weiner index, Simpson-Yule index, and the Berger-Parker index. The differences between landscape categories for each of these indices were then tested for statistical significance using Kruskal-Wallis test (p < 0.05).
Correlations between species abundance and both bromeliad fullness and landscape category were assessed based on the results from the RDA analysis. Univariate Gaussian regression analyses were performed to determine whether the bromeliad fullness had either a positive or negative contribution to species abundance (n = 108, p < 0.05). Univariate binomial negative regression analyses were then carried out for landscape categories (n = 108). Relative species abundance (prevalence ratio) values for hillslope and hilltop were estimated using the lowland category as the baseline. A prevalence ratio of one indicates the given species is not associated with any landscape category, whereas values of greater than one and less than one indicate a positive and negative association, respectively (p < 0.05, CI 95%).
Univariate binary logistic regression analyses (n = 108) were used to assess species co-occurrence in bromeliads. Species abundance was transformed into dummy variables (absence = 0, presence = 1), and the analyses of these variables provided one of three possible results; an odds ratio value of one indicates species are associated randomly, whereas odds ratio values of greater than one and less than one indicate a positive and negative association, respectively (p < 0.05, CI 95%).
Results
Two thousand and twenty four mosquitoes belonging to 22 species were collected (Table 1). Culex ocellatus (429; 21.20%), Cx. (Mcx.) imitator retrosus (388; 19.17%), Cx. (Mcx.) neglectus (350; 17.29%), Cx. (Mcx.) imitator imitator (213; 10.52%), An. homunculus (201; 9.93%), Cx. (Mcx.) inimitabilis fuscatus (126; 6.23%), An. cruzii (106; 5.24%), Cx. (Mcx.) worontozowi (80; 3.95%) and Cx. (Mcx.) aphylactus (55; 2.52%) were the most abundant species.
The extrapolated species richness using the second-order jackknife estimator was 35 species. Of the 22 species collected, two were found to be singletons and four doubletons. Results of the RDA showed that landscape categories, bromeliad water volume and bromeliad fullness had a significant influence on the bromeliad mosquito assemblage structure (pseudo-F value = 1.89, p = 0.04; pseudo-F value = 2.99, p = 0.03; and pseudo-F value = 4.47, p < 0.01, respectively; Table 2).
The Renyi diversity curves showed that the lowland possesses the highest diversity indices, and that the values for the hillslope are more similar to those obtained for the hilltop (Figure 2; Additional file 1). The Kruskal-Wallis tests indicated significant difference in the Total richness (= 0) and the Shannon-Weiner diversity index (= 1; Figure 2) between lowland and hilltop (KWχ2 = 4.90, p = 0.03 and KWχ2 = 5.75, p = 0.02, respectively). Further results are included in Additional file 1.
Renyi index curves showing differences in species diversity between each bromeliad within each landscape category (lowland, hillslope, and hilltop). The Renyi index estimates total richness for α = 0, Shannon-Weiner index for α = 1, the inverse Simpson-Yule index for ?α= 2 and 1/Berger-Parker index for α = Inf.
Univariate Gaussian linear regression analyses found that bromeliad fullness was positively associated with Cx. imitator imitator (β1= 0.90; p < 0.001), An. cruzii (β1= 0.19; p < 0.01), Cx. neglectus (β1= 0.68; p < 0.01) and Cx. aphylactus (β1= 0.10; p = 0.03). Cx. inimitabilis fuscatus, however, showed a negative association (β1= - 0.32; p = 0.04) indicating this species was more associated with shallow water inside bromeliad tanks (Additional file 2).
Univariate negative binomial regression analyses found that An. homunculus (prevalence ratio = 0.42; CI 95% = 0.18 - 0.95) and Cx. neglectus (prevalence ratio = 0.19; CI 95% = 0.07 - 0.5) were more closely associated with lowland than hilltop, and that Cx. imitator imitator was more closely associated with lowland than either hilltop (prevalence ratio = 0.08; CI 95% = 0.03 - 0.22) or hillslope (prevalence ratio = 0.21; CI 95% = 0.08 - 0.53) (Additional file 3).
Univariate binary logistic regression analyses for co-occurrence showed that An. homunculus was associated with Cx. ocellatus and An. cruzii was associated with Cx. neglectus, Cx. inimitabilis fuscatus and Cx. worontzowi (Table 3). Although An. cruzii and An. homunculus were occasionally found in the same bromeliad, this association was not statistically significant (1.82, p = 0.14). Considering mosquito frequency distribution among bromeliad genera (Additional file 4), results of co-occurrence analyses were mainly because of Nidularium. The number of plants sampled of the Nidularium genus was approximately seven times higher than those of the Vriesea (94 Nidularium versus 14 Vriesea).
Discussion and conclusions
The difference between the observed (n = 22) and extrapolated species richness (n = 35) in our study can be explained by the high number of rare species present in the tropical forest of Serra do Mar [4]. According to the second order jackknife estimator, we detected six rare species in our study, which constitutes approximately one quarter of the observed species. It therefore appears that rarer species were under-represented in our study, which may have affected our ability to detect the significance of some determinants (Table 2). However, those determinants that were significant for the assemblage structure were also significant for the most abundant species.
Alves et al. [4] found significant differences in forest structure and biomass variation along a 0-1100 m altitude gradient of coastal Atlantic Forest in Serra do Mar. The authors also showed how small changes in elevation in tropical regions can significantly affect various environmental factors such as air temperature, solar radiation, light availability, edaphic discontinuities, both soil moisture and temperature, nutrients availability, ground evaporation and microbial decomposition. Considering that environmental variables may have an impact on the availability and suitability of mosquito habitats, including larval habitats (e.g. bromeliads), one should expect to find differences in Culicidae assemblages in different locations within Atlantic Forest. In the present study, differences in mosquito community structure in a small gradient of altitude (0-263 m) were found, showing that the complexity of Serra do Mar and elevational gradient may be considered in ecological studies of Culicidae. This finding is consistent with that of Navarro et al. [32] who found that elevation is an important landscape determinant for Culicidae fauna distribution in Venezuela.
One of the main findings in our study was that differences in species among mosquito assemblages were influenced by elevation categories. The higher total species richness found in the lowland (Table 1) is consistent with greater niche availability, and may be related to higher species abundance because of the decreased probability of local population extinction [33]. Micro-climatic variation could not be evaluated because such data was not available but is likely to be important in influencing mosquito fauna and their distribution. The macro-climatic data that was used in our study could not explain differences in assemblage structure (Table 2).
Previous studies showed that differences in species among mosquito assemblages can be explained by bromeliad characteristics [34, 35]. The quantity and quality of food resources, and physico-chemical properties of the water in bromeliads tanks may determine the species found in them [34]. It is noteworthy that pH, conductivity, temperature, and O2 concentration were found unrelated to both richness and species diversity of macro-invertebrate fauna inhabiting Tillandsia turneri Baker (Bromeliaces) in high altitude forest in Colombia [36]. However, bromeliad water volume and plant area were associated with abundance. Machado-Allison et al. [37] found a positive correlation between bromeliad structural complexity, habitat persistence, presence of predators and mosquito species richness. In another study, Araújo et al. [38] found that species abundance was positively associated with increased water volume, whereas richness was correlated with plant diameter. In considering that larval mosquito community structure may be influenced by both the volume of water inside a bromeliad tank and depth of the water, another variable named bromeliad fullness was assessed for Vriesea and Nidularium plants from Atlantic Forest. This variable was found to have a statistically significant influence on the mosquito community structure. Water fullness may create an array of micro-variation in the physico-chemical characteristics of water content, and thus in food resources and presence of predators that can affect mosquito larval community structure.
Mosquito species abundance may also be influenced by bromeliad taxa [9, 39–42]. Navarro et al. [43] found mosquito taxa association with bromeliad family in Venezuela. Similarly, in Panaquire, Venezuela, Machado-Allison et al. [44] found that some species of mosquitoes were strongly associated with species of bromeliads. In the present study, among the six rarest mosquito species found in Nidularium and Vriesea bromeliads, four belonged to the Wyeomyia (Phoniomyia). Similarly, Müller and Marcondes [45] collected only a single individual of Wyeomyia (Phoniomyia) in plants of Nidularium innocentii. However, Mocellin et al. [46] found Wyeomyia (Phoniomyia) to be the most abundant taxa in Neoregelia compacta (Mez) and Billbergia nana E. Pereira in the Botanical Garden of Rio de Janeiro. It therefore appears that plants of the genus Nidularium do not represent important larval habitat for Wyeomyia (Phoniomyia). Given the large numbers of adult Wyeomyia (Phoniomyia) previously found in the study area [47], it is likely that this subgenus favors alternative bromeliad genera, for example, Neoregelia and Billbergia. Moreover, it is noteworthy that at least 21 additional genera of Bromeliaceae can be found in Serra do Mar [48], some of which may provide important larval habitat for Wyeomyia (Phoniomyia).
The subgenus Kerteszia is comprised of 12 species, of which four have been implicated as malaria vectors [12]. Three of these, An. bellator, An. cruzii, and An. homunculus, are important vectors in the Atlantic Forest. While An. bellator and An. cruzii are widely distributed, the geographical distribution of An. homunculus is poorly known [15]. Sallum et al. [24] stated that the lack of An. homunculus records in Brazil may be a consequence of an inability to effectively identify the species based on female morphological characters. Larval and pupal characteristics, on the other hand, are highly effective at resolving this species. Despite the abundance of An. cruzii and An. bellator found in the study area by Forattini et al. [49], we only identified An. cruzii and An. homunculus. The absence of An. bellator may be because of a preference for larval habitat either in the canopy [13] or restinga [50], but plants from both environments were not sampled. Anopheles homunculus, on the other hand, was frequently encountered in our study and made up approximately 65% of all Kerteszia specimens collected. Anopheles homunculus was similarly found to be common in enclosed, humid forests in Serra do Mar in Santa Catarina state [50]. It is noteworthy that Harbach and Navarro [51] reported An. homunculus in forests at altitudes of up to 1700 m in Auyantepui, Venezuela.
Great importance lies in the ability to effectively identify the presence of malaria vectors, and understand mechanisms involved in multiple species coexistence. The identification of a surrogate species may be used as a tool to assess the presence of vector species. In our study area, three species from the genus Culex were found to be indicative of the presence of An. cruzii and one was found to be indicative of An. homunculus (Table 3), and mostly within Nidularium plants (Additional file 4). However, the field observations conducted for the present study do not allow addressing ecological and evolutionary mechanisms of mosquito species coexistence. Consequently, hypotheses of species co-occurrence need further investigations employing an experimental design that combines field investigation and also mathematical modeling.
There are few published accounts of An. homunculus in the Atlantic Forest, and this study is the first to include this species in an assessment of diversity among mosquito assemblages. Anopheles homunculus seems to be abundant in Nidularium and Vriesea bromeliads, sharing the same plants with An. cruzii, and presence of both species were found to be associated with distinct categories of elevation and slope. The findings of the current study open questions about the importance of An. homunculus in the transmission of Plasmodium sp. in Atlantic Forest. The species was incriminated as a vector on coastal areas of Santa Catarina state [16], but its current role in human malaria transmission is largely unknown. It is noteworthy that Alouatta monkeys were found infected with Plasmodium vivax, P. malariae and P. falciparum in Serra do Mar, and thus they may act as reservoirs for human Plasmodium[52, 53]. In considering that An. homunculus is found in areas with dense forest coverage [50] where Alouatta monkeys are present, it is plausible to propose that either the species, or other Kerteszia species, may be involved in the transmission of malaria parasites from monkeys to humans. The involvement of An. homunculus in the dynamics of malaria transmission in Atlantic Forest requires further investigation, with a particular emphasis on greater sampling and testing for infectivity across the region in different levels within the forest.
References
Myers N, Mittermeier RA, Mittermeier CG, Fonseca GAB, Kent J: Biodiversity hotspots for conservation priorities. Nature. 2000, 403: 853-858. 10.1038/35002501.
Silva JMC, Casteleti CHM: Status of the biodiversity of the Atlantic Forest of Brazil. The Atlantic Forest of South America: biodiversity status, threats, and outlook. Edited by: Galindo-Leal C, Câmara IG. 2003, Washington: Island Press, 43-59.
Galindo-Leal C, Câmara IG: Atlantic Forest hotspot status: an overview. The Atlantic Forest of South America: biodiversity status, threats, and outlook. Edited by: Galindo-Leal C, Câmara IG. 2003, Washington: Island Press, 3-11.
Alves LF, Vieira SA, Scaranello MA, Camargo PB, Santos FAM, Joly CA, Martinelli LA: Forest structure and live aboveground biomass variation along an elevational gradient of tropical Atlantic moist forest (Brazil). For Ecol Manage. 2010, 260: 679-691. 10.1016/j.foreco.2010.05.023.
Carnaval AC, Moritz C: Historical climate modelling predicts patterns of current biodiversity in the Brazilian Atlantic forest. J Biogeogr. 2008, 35: 1187-1201. 10.1111/j.1365-2699.2007.01870.x.
Fitzpatrick SW, Brasileiro CA, Haddad DFB, Zamudio KR: Geographical variation in genetic structure of an Atlantic Coastal Forest frog reveals regional differences in habitat stability. Mol Ecol. 2009, 18: 2877-2896. 10.1111/j.1365-294X.2009.04245.x.
Forattini OP, Lopes OS, Rabello EX: Investigações sobre o comportamento de formas adultas de mosquitos silvestres no estado de São Paulo, Brasil. Rev Saude Publica. 1968, 2: 111-173.
Marques GRAM, Santos RLC, Forattini OP: Aedes albopictus em bromélias de ambiente antrópico no Estado de Säo Paulo, Brasil. Rev Saude Publica. 2001, 35: 243-248.
Frank JH: Bromeliad phytotelmata and their biota, especially mosquitoes. Phytotelmata: Terrestrial Plants as Host for Aquatic Insect Communities. Edited by: Frank JH, Lounibos LP. 1983, New Jersey: Plexus, Meford, 101-128.
Müller GA, Marcondes CB: Bromeliad-associated mosquitoes from Atlantic forest in Santa Catarina Island, southern Brazil (Diptera, Culicidae), with new records for the State of Santa Catarina. Iheringia Ser Zool. 2006, 96: 315-319. 10.1590/S0073-47212006000300007.
Marques GRAM, Forattini OP: Culicídeos em bromélias: diversidade de fauna segundo influência antrópica, litoral de São Paulo. Rev Saude Publica. 2008, 42: 979-985. 10.1590/S0034-89102008000600001.
Zavortink TJ: Mosquito studies XXIX. A review of the subgenus Kerteszia of Anopheles. Contrib Amer Entomol Inst. 1973, 9: 1-54.
Frank JH, Lounibos LP: Insects and allies associated with bromeliads: a review. Terr Arthropod Rev. 2009, 1: 125-153. 10.1163/187498308X414742.
Oliveira-Ferreira J, Lacerda MVG, Brasil P, Ladislau JLB, Tauil PL, Daniel-Ribeiro CT: Malaria in Brazil: an overview. Malar J. 2010, 9: 115. 10.1186/1475-2875-9-115.
Marrelli MT, Malafronte RS, Sallum MAM, Natal D: Kerteszia subgenus associated with the Brazilian Atlantic forest: current knowledge and future challenges. Malar J. 2007, 6: 127. 10.1186/1475-2875-6-127.
Smith LB: Bromeliad malaria. Ann Rep Smithsonian Inst. 1952, 385-398.
Beketov MA, Yurchenko YA, Belevich OE, Liess M: What environmental factors are important determinants of structure, species richness, and abundance of mosquito assemblages?. J Med Entomol. 2010, 47: 129-139. 10.1603/ME09150.
Lane J, Whitman L: The Subgenus Microculex in Brazil (Diptera, Culicidae). Rev Bras Biol. 1951, 11: 341-366.
Lane J: Neotropical Culicidae. São Paulo: USP. 1953
Correa RR, Ramalho GR: Revisão de Phoniomyia (Theobald, 1903). Folia Clin Biol. 1956, 25: 5-176.
Cotrim MD, Galati EAB: Revisão da série Pleuristriatus do subgênero Microculex Theobald, 1907 (Diptera, Culicidae). Rev Bras Entomol. 1977, 20: 169-205.
Forattini OP: Culicidologia Médica. São Paulo: EDUSP. 2002
Dyar HG, Knab F: The larvae of Culicidae classified as independent organisms. J New Jersey Entomol Soc. 1906, 14: 169-230.
Sallum MAM, Santos CLS, Wilkerson RC: Studies on Anopheles (Kertes-zia) homunculus Komp (Diptera: Culicidae). Zootaxa. 2009, 2299: 1-18.
Kindt R, Coe R: Tree diversity analysis. A manual and software for common statistical methods for ecological and biodiversity studies. Nairobi: World Agroforestry Centre (ICRAF). 2005
Venables WN, Ripley BD: Modern applied statistics with S. 2002, New York: Springer Science Business Media
Chongsuvivatwong V: Analysis of epidemiological data using R and Epicalc. 2008, Songkhla, Thailand: Chanmuang Press
Magurran AE: Measuring biological diversity. 2004, Oxford: Blackwell Publishing company
Brose U, Martinez ND, Williams RJ: Estimating species richness: sensitivity to sample coverage and insensitivity to spatial patterns. Ecology. 2003, 84: 2364-2377. 10.1890/02-0558.
Gotelli NJ, Ellison AM: A primer of ecological statistics. 2004, Sunderland: Sinauer Associate Publishers
Henderson PA: Practical methods in ecology. 2006, Oxford: Blackwell Publishing Company
Navarro JC, Del Ventura F, Zorrilla A, Liria J: Registros de mayor altitud para mosquitos (Diptera: Culicidae) en Venezuela. Rev Biol Trop. 2010, 58: 245-254.
Yee DA, Juliano SA: Abundance matters: a field experiment testing the more individuals hypothesis for richness-productivity relationships. Oecologia. 2007, 153: 153-162. 10.1007/s00442-007-0707-1.
Ambruster P, Hutchinson RA, Cotgreave P: Factors influencing community structure in a South American tank bromeliad fauna. Oikos. 2002, 96: 225-234. 10.1034/j.1600-0706.2002.960204.x.
O'Meara GF, Cutwa MM, Evans LF: Bromeliad-inhabiting mosquitoes in south Florida: native and exotic plants differ in species composition. J Vector Ecol. 2003, 28: 37-46.
Ospina-Bautista F, Estévez-Varón JV, Betancur J, Realpe-Rebolledo E: Estructura y Composición de la comunidad de macro invertebrados acuáticos asociados a Tillandsia turneri Baker (Bromeliaceae) en un bosque Alto Andino Colombiano. Acta Zool Mex. 2004, 20: 153-166.
Machado-Allison CE, Barrera R, Frank JH, Delgado L, Gomez-Cova C: Mosquito communities in Venezuela phytotelmata. Ecology of mosquitoes: proceedings of a workshop. Edited by: Lounibos LP, Rey JR, Frank JH. 1985, Vero Beach: Florida Medical Entomology Laboratory, 79-93.
Araújo VA, Melo SK, Araújo APA, Gomes MLM, Carneiro MAA: Relationship between invertebrate fauna and bromeliad size. Braz J Biol. 2007, 67: 611-617. 10.1590/S1519-69842007000400004.
Fish D: Phytotelmata: Flora and Fauna. Phytotelmata: terrestrial plants as hosts for aquatic insect communities. Edited by: Frank JH, Lounibos LP. 1983, New Jersey: Plexus Publishing, 1-25.
Frank JH, Curtis GA: On the bionomics of bromeliad-inhabiting mosquitoes. VI. A review of the bromeliad inhabiting species. J Florida Anti-Mosquito Assoc. 1981, 52: 4-23.
Maguire B: Phytotelmata biota and community structure determination in plant-held waters. Annu Rev Ecol Syst. 1971, 2: 439-464. 10.1146/annurev.es.02.110171.002255.
Navarro JC, Ingunza J, Fernández Z, Barrera R: Mosquitoes and bromeliads: species-specific selectivity patterns on the northern coast and southern Guiana Shields in Venezuela. J Am Mosq Control Assoc. 1995, 11: 345-346.
Navarro JC, Liria J, Piñango H, Barrera R: Biogeographic area relationships in Venezuela: A Parsimony analysis of Culicidae - Phytotelmata distribution in National Parks. Zootaxa. 2007, 1547: 1-19.
Machado-Allison CE, Barrera R, Delgado L, Gomez-Cova C, Navarro JC: Mosquitos (Diptera: Culicidae) de los Fitotelmata de Panaquire, Venezuela. Acta Biol Ven. 1986, 12: 1-12.
Müller GA, Marcondes CB: Immature mosquitoes (Diptera: Culicidae) on the bromeliad Nidularium innocentii in ombrophilous dense forest of Santa Catarina Island, Florianópolis, Santa Catarina State, southern Brazil. Biotemas. 2007, 20: 27-31.
Mocellin MG, Simões TC, Nascimento TFS do, Teixeira MLF, Lounibos LP, Lourenço-de-Oliveira R: Bromeliad-inhabiting mosquitoes in an urban botanical garden of dengue endemic Rio de Janeiro. Are bromeliads productive habitats for the invasive vectors Aedes aegypti and Aedes albopictus?. Mem Inst Oswaldo Cruz. 2009, 104: 1171-1176. 10.1590/S0074-02762009000800015.
Forattini OP, Gomes AC, Natal D, Santos JLF: Rev Saude Publica. 1986, 20: 1-20. 10.1590/S0034-89101986000100001.
Martinelli G, Vieira CM, Gonzalez M, Leitman P, Piratininga A, Costa AF da, Forzza RC: Bromeliaceae da Mata Atlântica brasileira: lista de espécies, distribuição e conservação. Rodriguesia. 2008, 59: 209-258.
Forattini OP, Kakitani I, Massad E, Marucci D: Studies on mosquitoes (Diptera: Culicidae) and anthropic environment. 11 - Biting activity and blood- seeking parity of Anopheles (Kerteszia) in South-Eastern Brazil. Rev Saude Publica. 1996, 30: 107-114. 10.1590/S0034-89101996000200001.
Veloso HP, Moura JV de, Klein RM: Delimitação ecológica dos anofelíneos do subgênero Kerteszia na região costeira do sul do Brasil. Mem Inst Oswaldo Cruz. 1956, 54: 517-541.
Harbach RE, Navarro JC: A new species of Anopheles subgenus Kerteszia (Diptera: Culicidae) from Venezuela. Entomologica Scandinavica. 1996, 27: 207-216. 10.1163/187631296X00052.
Curado I, Malafronte RS, Duarte AMRC, Kirchgatter K, Branquinho MS, Galati EAB: Malaria epidemiology in low-endemicity areas of the Atlantic forest in the Vale do Ribeira, Sao Paulo, Brazil. Acta Trop. 2006, 100: 54-62. 10.1016/j.actatropica.2006.09.010.
Duarte AMRC, Malafronte RS, Cerutti C, Curado I, Paiva BR, Maeda AY, Yamasaki T, Summaf MEL, Neves DVDA, Oliveira SG, Gomes AC: Natural Plasmodium infections in Brazilian wild monkeys: Reservoirs for human infections?. Acta Trop. 2008, 107: 179-185. 10.1016/j.actatropica.2008.05.020.
Acknowledgements and funding
We are in debt to the reviewers for suggestions and comments that greatly improved the first draft of the manuscript; to A. Fernandes for assistance in the specimens identification; to field team from Departamento de Epidemiologia for helping with field collections. To Fundação de Amparo à Pesquisa do Estado de São Paulo, FAPESP (Processo n° 05/53973-0, and 2011/22088-1), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq BPP 300351/2008-9) for financial support. TCM was a recepient of CNPq fellowship no. 136557/2008-2e.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
TCM and MAMS conceived and designed the experiments. TCM did the field collections and identified the specimens. GZL performed the statistical analysis in the R package with contributions by BPB, MAMS and TCM. MAMS, TCM, BPB, GZL wrote the paper. All authors read and approved the final manuscript.
Electronic supplementary material
13071_2011_559_MOESM1_ESM.DOC
Additional file 1: Differences between landscape categories for each of α value of the Renyi index tested for statistical significance using Kruskal-Wallis test. Results of Kruskal-Wallis test to assess statistical significance of Renyi index values. (DOC 36 KB)
13071_2011_559_MOESM2_ESM.DOC
Additional file 2: Results of univariate Gaussian regression analyses performed with species abundance against the bromeliad fullness (volume of water divided by depth of bromeliad tank). Results of univariate regression analysis to determine correlation between species abundance and bromeliad fulness defined as volume of water divided by depth of the bromeliad tank. (DOC 33 KB)
13071_2011_559_MOESM3_ESM.DOC
Additional file 3: Univariate negative binomial models regression analyses of species abundance as a function of elevation categories with lowland as the baseline. Results of negative binomial regression analysis showing correlations between species abundance and elevation.(DOC 34 KB)
13071_2011_559_MOESM4_ESM.DOC
Additional file 4: Species of Culicidae taken from the genera Nidularium and Vriesea. *Number of plants in which a species was found/number of plants sampled in a specific landscape category. List of species of Culicidae collected from Nidularium and Vriesea showing the number of plants in which a species was found and the number of plants sampled in a specific landscape category. (DOC 60 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Marques, T.C., Bourke, B.P., Laporta, G.Z. et al. Mosquito (Diptera: Culicidae) assemblages associated with Nidularium and Vriesea bromeliads in Serra do Mar, Atlantic Forest, Brazil. Parasites Vectors 5, 41 (2012). https://doi.org/10.1186/1756-3305-5-41
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1756-3305-5-41