Abstract
Background
The so-called Philadelphia (Ph) chromosome is present in almost all cases with chronic myeloid leukemia (CML). Around 5-10% of these patients show complex translocations involving other chromosomes in addition to and/or besides chromosomes 9 and 22. As nowadays most CML cases are treated with Imatinib, variant rearrangements have in general no specific prognostic significance, though events of therapy resistance remain to be studied.
Results
Here we report a Ph chromosome positive patient with hematological typical chronic phase CML. Untypically, an unbalanced complex rearrangement involving chromosomes 16 and 17 leading to a deletion of 16pter and partial trisomy of 17q21 to 17qter, was identified besides a trisomy 8 and an additional Ph chromosome in a part of malignant cells.
Conclusion
Here a novel and cytogenetically unique case of a Ph chromosome positive CML clinically in chronic phase is reported, having complex secondary chromosomal aberrations. Thus, CML patients with complex chromosomal changes are nonetheless treatable by Imatinib.
Similar content being viewed by others
Background
Chronic myeloid leukemia (CML) is a clonal malignant disorder of a pluripotent hematopoetic stem cell characterized by the presence of the Philadelphia (Ph) chromosome in more than 90% of patients. The Ph chromosome is a product of the reciprocal translocation t(9;22)(q34;q11), which transposes the 3' portion of the ABL oncogene from 9q34 to the 5' portion of the BCR gene on 22q11.2. The crucial pathogenetic consequence of this translocation is the creation of a chimeric BCR/ABL gene on the derivative chromosome 22 [1]. The expression of the BCR/ABL chimeric protein with an increased tyrosine kinase activity plays an essential role in the pathogenesis of CML [2].
The progression of CML from chronic phase (CP) to blast crisis (BC) is frequently associated with nonrandom secondary chromosomal aberrations such as +8, i(17q), +19 and an extra Ph chromosome [3]. Imatinib mesylate (Glivec, formerly STI571) was designed specifically to inhibit the tyrosine kinase activity of the BCR/ABL protein and other tyrosine kinases such as cABL, c-KIT and PDGF (platelet-derived growth factor receptor). By binding to an active site of the tyrosine kinase, Glivec switches off downstream signaling, cells stop proliferating and apoptosis ensues [4]. Many studies have shown a high efficiency of Imatinib therapy to achieve a complete or major cytogenetic response, i.e. a reduction to 0-34% Ph-positive cells. This positive effect may be attained in cases with a simple t(9;22), in such with complex translocations resulting in a BCR/ABL fusion gene, as well as in cases with cytogenetic clonal evolution [5, 6].
Herein we report a rare case of a Ph chromosome positive CML with a derivative chromosome 16 leading to a partial trisomy 17q21 to 17qter and partial deletion of 16pter, which was nonetheless successfully treatable by Imatinib.
Case report
A 30-year old male was diagnosed to suffer from CML in CP after a blood cell count was initiated in June 2009 due to splenomegaly and severe loss of weight. The patient was treated with Imatinib mesylate at a 400 mg/day for overall 12 months and previous relevant symptoms disappeared. Hematologic parameters were as follows: hemoglobin 129 g/l; platelets count was 103 × 109/l; and white blood cells (WBC), 5 × 109/l with 57.2% neutrophils, 36.3% lymphocytes, 4.8% monocytes, 1.3% eosinophiles and 0.4% basophiles.
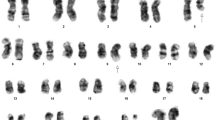
Karyotyping was done after initiation of chemotherapy treatment, showing the following karyotypic changes. A complex karyotype 48,XY,+8,t(9;22)(q34;q11),der(16),t(16;17),+der(22)/47,XY,+8,t(9;22)(q34;q11),der(16),t(16;17)/46,XY,t(9;22)(q34;q11) was determined in GTG-banding (Fig. 1) and further specified by molecular cytogenetic studies (Fig. 2, 3). Dual-color-FISH using a probe specific for BCR and ABL revealed a typical Philadelphia-chromosome with BCR/ABL fusion gene was present and the second derivative chromosome 22 was a second Ph chromosome (Fig. 2). M-FISH, was applied to exclude further cryptic rearrangements (Fig. 3A). As no other additional changes were found by that approach, array-proven high-resolution multicolor banding (aMCB) (10) probe sets for chromosomes 16 and 17 were applied. Thus, an intrachromosomal rearrangement on the derivative chromosome 16 was detected additionally to the translocation of chromosome 17 material to the derivative chromosome 16 (Figs. 3B and 3C). Finally, a subtelomeric probe for 16pter revealed a partial deletion on the derivative chromosome 16 (Fig. 3D). Thus, the following final karyotype was obtained: 48,XY,+8,t(9;22)(q34;q11),der(16)t(16;17)(16qter→16q22::16p13.3→16q22::17q21→qter),+der(22)t(9;22)(q34;q11)[8]/47,XY,+8,t(9;22)(q34;q11),der(16)t(16;17)(16qter→16q22::16p13.3→16q22::17q21→qter)[8]/46,XY,t(9;22)(q34;q11)[4].
Karyotype and chromosomal aberrations were confirmed using molecular cytogenetic approaches. (A) M-FISH confirmed the complexity of the karyotype: 47,XY,t(9;22),der(16)t(16;17),+8. (B) and (C) The application of MCB 16 and 17 revealed the chromosomal breakpoints on the corresponding derivative chromosomes as 16p13.3 and 16 q22 and 17q21. (D) The deletion of the subtelomeric region on the der(16) and the translocation of chromosome 17 material was also demonstrated by the application of a subtelomeric probe for 16p (Abbott Molecular/Vysis, USA) and a whole chromosome painting (wcp) probe for chromosome 17.
Discussion
In a case of a CP Ph chromosome positive CML additional chromosomal changes were detected. Apart from a trisomy 8 and an additional derivative chromosome 22 two additional chromosomal alterations were present: an intrachromosomal rearrangement involving 16q22, 16p11.3 and an interchromosomal one with breakpoints in 16q22 and 17q21. To our knowledge, this translocation has been never observed in CML before [7].
In 5-10% Ph chromosome CML cases have complex translocations in addition to those and/or besides chromosomes 9 and 22 [1]. At present it appears that in such rearrangements any other chromosome may be involved. However, it has been suggested that distribution of chromosomes and breakpoints is non-random with the chromosomal bands most susceptible to breakage being 1p36, 3p21, 5q31, 6p21, 9q22, 10q22, 11q13, 12p13, 17p13, 17q21, 17q25, 19q13, 21q22, 22q12 and 22q13 [8], showing one match with the present case, i.e. 17q21.
Additionally, the two breakpoints in chromosome 16 have previously been reported to be associated with acute nonlymphocytic leukemia (ANLL) type M4 [9]. 16q22 is frequently found in the ANLL typical inversion (16)(p13q22) and the related interchromosomal translocation (16;16)(p13;q22). These chromosome 16 rearrangements resulted in the disruption of the myosin heavy chain (MYH11) gene mapping to chromosome 16p13.13 to 16p13.12 [10]. MYH11 encodes the smooth-muscle myosin heavy chain and belongs to the family of conventional myosins. The well-characterized biological function of myosins is their ability to use the energy of ATP hydrolysis to move actin filaments and produce muscle force. Recently, myosins have been implicated in a variety of other intra-cellular functions, including cell migration, adhesion, control of cell shape, and membrane traffic but are implicated also in a variety of other cellular functions, many of which are relevant for cancer formation [11]. Unfortunately, in the present case no material was available to test if MYH11 was involved in the rearrangement.
During CML progression trisomy of chromosomes 8 and a second Ph chromosome are frequent anomalies in CML [3]. Also, another leukemogenic effect of the der(16) may be due to increase copy number of the 17q21 to 17qter region. Morerio et al. [12] have reported that trisomies for this region represent a negative prognostic indicator in myeloid neoplasia. Also an isochromosome 17q is a well-known progression step in CML.
In conclusion, here we reported a novel and cytogenetically unique case of a Ph chromosome positive CML in CP. Especially the latter fact being still a CML in CP and not in BC when having such complex secondary chromosomal aberrations is intriguing. We suggest that the reported patient had nonetheless such a good response to Imatinib as irrespective of complex chromosomal changes he was treated 'just in time' before further mutations appeared leading to BC.
Materials and methods
Chromosome analysis
Chromosome analysis using GTG-banding was done according to standard procedures [13]. 20 metaphases analyzed from unstimulated bone marrow culture were analyzed. Karyotypes were described according to the International System for Human Cytogenetic Nomenclature [14].
Molecular cytogenetics
Fluorescence in situ hybridization (FISH) using LSI BCR/ABL dual color dual fusion translocation probe (Abbott molecular/Vysis, USA) and subtelomeric probe for 16pter (Abbott molecular/Vysis, USA) were applied according to manufacturer's instructions together with a whole chromosome painting (WCP) probe for chromosome 17 [15]. Array-proven multicolor banding probe (aMCB) sets based on microdissection derived region-specific libraries for chromosome 16 and 17 were applied as described [16] 20 metaphase spreads were analyzed, each using a fluorescence microscope (AxioImager.Z1 mot, Zeiss) equipped with appropriate filter sets to discriminate between a maximum of five fluorochromes and the counterstain DAPI (Diaminophenylindol). Image capturing and processing were carried out using an ISIS imaging system (MetaSystems, Altlussheim, Germany) for the evaluation aMCB.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
La Starza R, Testoni N, Lafage-Pochitaloff M, Ruggeri D, Ottaviani E, Perla G, Martelli MF, Marynen P, Mecucci C: Complex variant Philadelphia translocations involving the short arm of chromosome 6 in chronic myeloid leukemia. Haematologica 2002, 87: 143–147.
Lugo T, Pendergast A, Müller A, Witte O: Tyrosine kinase activity and transformation potency of bcr-abl oncogene products. Science 1990, 247: 1079–1082. 10.1126/science.2408149
Sandberg AA: The chromosomes in Human Cancer and leukemia. 2nd edition. Elsevier Science, New York; 1990:151–172.
Griffen J: The biology of signal transduction The biology of signal transduction inhibition: basic science to novel therapies. Semin Oncol 2001, 28: 3–8.
Kantarjian H, Sawyers C, Hochhaus A, Guilhot F, Schiffer C, Gambacorti-Passerini C, Niederwieser D, Resta D, Capdeville R, Zoellner U, Talpaz M, Druker B, Goldman J, O'Brien SG, Russell N, Fischer T, Ottmann O, Cony-Makhoul P, Facon T, Stone R, Miller C, Tallman M, Brown R, Schuster M, Loughran T, Gratwohl A, Mandelli F, Saglio G, Lazzarino M, Russo D, Baccarani M, Morra E, International STI571 CML Study Group: Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N Engl J Med 2002, 346: 645–652. 10.1056/NEJMoa011573
Cortes JE, Talpaz M, Giles F, O'Brien S, Rios MB, Shan J, Garcia-Manero G, Faderl S, Thomas DA, Wierda W, Ferrajoli A, Jeha S, Kantarjian HM: Prognostic significance of cytogenetic clonal evolution in patients with chronic myelogenous leukemia on imatinib mesylate therapy. Blood 2003, 101: 3794–3800. 10.1182/blood-2002-09-2790
Mitelman F, Johansson B, Mertens F (Eds): Mitelman Database of Chromosome Aberrations in Cancer (2009) In [http://cgap.nci.nih.gov/Chromosomes/Mitelman]
Johansson B, Fioretos T, Mitelman F: Cytogenetic and molecular genetic evolution of chronic myeloid leukemia. Acta Haematol 2002, 107: 76–94. 10.1159/000046636
Liu P, Tarle SA, Hajra A, Claxton DF, Marlton P, Freedman M, Siciliano MJ, Collins FS: Fusion between transcription factor CBF beta/PEBP2 beta and a myosin heavy chain in acute myeloid leukemia. Science 1993, 261: 1041–1044. 10.1126/science.8351518
Deng Z, Liu P, Marlton P, Claxton DF, Lane S, Callen DF, Collins FS, Siciliano MJ: Smooth muscle myosin heavy chain locus (MYH11) maps to 16p13.13-p13.12 and establishes a new region of conserved synteny between human 16p and mouse 16. Genomics 1993, 18: 156–159. 10.1006/geno.1993.1443
Krendel M, Mooseker MS: Myosins: tails (and heads) of functional diversity. Physiology (Bethesda) 2005, 20: 239–251.
Morerio C, Russo I, Rosanda C, Rapella A, Leszl A, Basso G, Maserati E, Pasquali F, Panarello C: 17q21-qter trisomy is an indicator of poor prognosis in acute myelogenous leukaemia. Cancer Genet Cytogenet 2001, 124: 12–15. 10.1016/S0165-4608(99)00229-0
Claussen U, Michel S, Mühlig P, Westermann M, Grummt UW, Kromeyer-Hauschild K, Liehr T: Demystifying chromosome preparation and the implications for the concept of chromosome condensation during mitosis. Cytogenet Genome Res 2002, 98: 136–146. 10.1159/000069817
Shaffer L, Slovak M, Cambell L, (eds): ISCN (2009): An International System for Human Cytogenetic Nomenclature. S. Karger, Basel 2009.
Liehr T, Starke H, Weise A, Lehrer H, Claussen U: Multicolor FISH probe sets and their applications. Histol Histopathol 2004, 19: 229–237.
Liehr T, Heller A, Starke H, Rubtsov N, Trifonov V, Mrasek K, Weise A, Kuechler A, Claussen U: Microdissection based high resolution multicolor banding for all 24 human chromosomes. Int J Mol Med 2002, 9: 335–339.
Acknowledgements
We thank Dr. I. Othman, the Director General of Atomic Energy Commission of SYRIA (AECS) and Dr. N. Mirali, Head of Molecular Biology and Biotechnology Department for their support. This work was supported by the AECS, in parts by the Stefan-Morsch-Stiftung, Monika-Kutzner-Stiftung and the DAAD (D/07/09624).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
AW and FM performed the cytogenetic studies in the present case and collected the data relative to this case report. WA supervised the cytogenetic analysis as Director of the HGD. HM, AW, FM TL did the molecular cytogenetic analysis and interpretation. TL drafted the paper and all authors contributed to the finalizing of the manuscript and read and approved it also in its final version.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Al Achkar, W., Wafa, A., Mkrtchyan, H. et al. A rare case of chronic myeloid leukemia with secondary chromosomal changes including partial trisomy 17q21 to 17qter and partial monosomy of 16p13.3. Mol Cytogenet 3, 6 (2010). https://doi.org/10.1186/1755-8166-3-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1755-8166-3-6