Abstract
An experiment was performed during the grazing seasons of 1998, 1999 and 2000 to study the influence of the antiparasitic drug ivermectin and the nematophagous fungus Duddingtonia flagrans on cattle dung disintegration. The faeces originated from groups of animals that were part of a separate grazing experiment where different control strategies for nematode parasite infections were investigated. Each group consisted of 10 first-season grazing cattle that were either untreated, treated with the ivermectin sustained-release bolus, or fed chlamydospores of D. flagrans. Faeces were collected monthly on 4 occasions and out of pooled faeces from each group, 4 artificial 1 kg dung pats were prepared and deposited on nylon mesh on an enclosed pasture and protected from birds. The position of the new set of pats was repeated throughout the 3 years of the study. Each year, the dung pats were weighed 4, 6, 8 and 10 weeks after deposition and immediately afterwards replaced to their initial positions.
Results showed that there was no difference in faecal pat disintegration between groups. However, the time-lag between deposition and complete disintegration of the faeces varied significantly between deposition occasions. Dung pats disappeared within 2 weeks (visual observation) when subjected to heavy rainfall early after deposition, whereas an extended dry period coincided with faeces still remaining 12 months after deposition.
Svensk sammanfattning
Nedbrytning av träck från kalvar behandlade med avmaskningsmedlet ivermektin eller rovsvampen Duddingtonia flagrans.
I föreliggande studie undersöktes nedbrytningshastigheten av komockor experimentellt. Under betessäsongerna 1998 till 2000 preparerades träck årligen vid 4 tillfällen från 3 grupper om vardera 10 förstagångsbetande kalvar. Kalvarna var antingen obehandlade, behandlade med en vomkapsel innehållande avmaskningsmedlet ivermektin eller utfodrades med sporer av rovsvampen Duddingtonia flagrans. Fyra konstgjorda komockor tillverkades av en blandning träck från respektive djurgrupp och placerades på nylonnät på en betesyta som hölls fri från betesdjur och skyddade från fågelangrepp. Vägningar utfördes 4, 6, 8 och 10 veckor efter utplacering. Placeringen av komockorna för de olika behandlingarna och de olika deponeringstillfällena upprepades under de 3 åren.
Resultaten visade att ihållande regn i samband med, eller kort efter utplacering medförde att synlig träck försvann helt inom 2 veckor (visuell observation). I samband med långvarig torka fanns däremot rester av komockorna kvar upp till 12 månader efter deponering. Nedbrytningshastigheten påverkades dock inte av om träcken kom från kalvar behandlade med ivermektin eller rovsvamp.
Studien visar att träcknedbrytningen framförallt påverkades av nederbördsmängd och nederbördsintensitet medan komockornas innehåll av ivermektin eller rovsvamp inte påverkade nedbrytningshastigheten jämfört med mockorna preparerade från obehandlade kalvar. Detta utesluter emellertid inte att ivermektin eller D. flagrans kan inverka negativ på nedbrytningshastigheten av träck, men denna möjliga effekt överskuggades i föreliggande studie av meteorologiska faktorer.
Similar content being viewed by others
Introduction
There is probably no other habitat where so many organisms in such large numbers act simultaneously in the processes of biological decomposition, than in the dung of grazing livestock [30]. However, there is also a vast number of abiotic factors, including pat size and shape, composition, moisture, pH and location as well as prevailing weather and mechanical disturbance, which influence the process of dung breakdown [5]. Obviously, the numerous variables involved in the process of dung degradation and the differences in methodology and study designs require attention to detail in the interpretation and comparison of results between such studies [5].
Avermectins are pesticides effective against a wide range of internal and external parasites of livestock [7] of which ivermectin was the first drug in this class to be marketed in 1981. Later in the mid 1990's, when ivermectin administered through a sustained-release device (bolus) was introduced, veterinarians and farmers were provided with a highly effective tool for nematode parasite control of cattle. The ivermectin bolus is claimed by the manufacturer to release 12 mg ivermectin daily for 135 d and thus maintains the treated cattle virtually worm-free for most of the grazing season. However, concern has been raised about possible environmental and economic consequences when using avermectins [11], particularly if administered through the sustained-release system [20, 38]. Clearly, certain developmental stages of some coprophilic invertebrates (dung beetles and flies) are particularly sensitive to ivermectin residues in ruminant dung [35, 36]. A reasonable interpretation is that an absence of dung degrading organisms may account for a delay, or failure, in the disintegration of dung derived from bolus treated cattle [37]. Ivermectin could also have adverse effects on the soil dwelling nematode populations [26, 34]. However, [6] reported no significant difference in total numbers of soil nematodes in dung pats from cattle treated with the ivermectin bolus when compared with non-treated animals.
Recent research has shown that biological control of nematode parasites in livestock with the nematophagous fungus Duddingtonia flagrans may become part of an organically acceptable alternative (for review, see [29]). The resting spores (chlamydospores) of the fungus are capable of surviving the gut passage in ruminants and with warm and moist conditions they germinate and spread in the faecal deposits. Parasite control is obtained by the trapping hyphal structures of this fungus, which have the ability to capture and destroy larval stages of the nematodes before they migrate to herbage and become available to grazing animals (for review, see [28]).
Saprophytic nematodes is an important and abundant taxonomic component of the grassland fauna (for review, see [3]) that rapidly colonise the fresh dung pats and play key roles in nutrient recycling [13, 25, 40]. Understandably, soil nematodes may also fall prey to D. flagrans.
Saprophytic soil nematode investigations have been conducted on pasturelands in Australia grazed by sheep fed D. flagrans [27, 43] and in Denmark [12]. In Sweden, two soil nematode studies have been performed on pasturelands grazed by cattle subjected to parasite control by the ivermectin sustained-release bolus and D. flagrans [41, 42]. Both these studies were complementary to the cattle grazing experiment [9] that served as source of faeces in the present trial. However, no investigation has yet been conducted on deployment of D. flagrans with respect to dung degradation.
The aim of the present 3-year plot trial was to compare the decomposition rate of uniform, artificial dung pats derived from 3 groups of cattle that were either treated with the ivermectin bolus, fed chlamydospores of D. flagrans, or were maintained untreated.
Materials and methods
Experimental pasture plots
This study was conducted at the Kungsängen Research Centre, Swedish University of Agricultural Sciences (SLU), Uppsala, Sweden, between 1998 and 2000. The experimental site was a uniformly flat improved pasture, which consisted predominantly of smooth meadow grass (Poa pratensis) with smaller proportions of meadow fescue (Festuca pratensis), white clover (Trifolium repens), tussock grass (Deschampsia caespitosa) and couch grass (Agropyron repens). An enclosed area of 20 × 8 m was designated for deposition of the faecal pats. Prior to deposition of the pats, the pasture was mowed to approximately 5 cm. The plot area was divided into 48 separate 80 × 80 cm sub-plots with 20 cm buffer zones between the 4 replicates. An extra 2 m buffer zone was created between treatments and deposition occasions. These buffer zones were mowed regularly to a sward height of approximately 2 cm.
Experimental design and source of faeces
Each year, faeces were obtained from 10 first-season grazing cattle per treatment group that were maintained on improved pastures at the Research Centre. These cattle were primarily part of a grazing experiment where alternative strategies for gastrointestinal nematode parasite control were investigated. The strategies studied were biological control using D. flagrans, grazing management with turnout on cow pasture in combination with mid-summer move to aftermath and treatment with a copper oxide wire particle bolus. These alternatives were compared with an untreated control group and cattle treated with the ivermectin sustained-release bolus (for details, see [9]). For the purpose of this study, the following treatments were included:
-
Control: No anthelmintic or other medical treatment
-
IVM: Ivermectin (1.72 g, 12 mg/d or 40–65 μg/kg bw/d for 135 d) intraruminal bolus (Ivomec SR vet., Merial, Paris, France) administered to each animal at turnout
-
Fungus: Duddingtonia flagrans chlamydospores (Christian Hansen Biosystems A/S, Copenhagen, Denmark) administered daily for 90 d (d 21–111) mixed in concentrate (1 × 106 spores/kg body weight/d 1998; 0.5 × 106 spores/kg body weight/d 1999 and 2000)
During days 21 to 111 after turnout, all cattle were daily fed a 1 kg grain supplement from troughs with 0.5 m space per animal.
Faeces were collected per rectum from all animals in each treatment 4, 8, 12 and 16 weeks after turnout in mid May each year, representing June, July, August and September depositions, respectively. From each of the groups, pooled faeces were thoroughly mixed and its dry matter (DM) determined based on 3 sub-samples of 10 g. From the mixed faeces for each treatment, 4 artificial 1.0 kg dung pats were prepared in aluminium pie dishes (21 cm ∅). The blocks of 4 pats per treatment and deposition occasion were assigned to sub-plots by random allocation the first year of the trial (1998). Each pat was deposited on stretchable, quadratic (approximately 25 cm) 8 mm nylon mesh in the middle of the sub-plots, marked and protected from disturbance by birds by covering with individual wide-meshed wire cages. Weighing of the faecal pats was performed 4, 6, 8 and 10 weeks after deposition by gently lifting the nylon mesh supporting the dung pat from the ground on to a field scale and then replacing as close as possible to the same alignment on the sub-plot. Visual examination of the plot area was performed daily for two weeks following deposition of each new set of faecal pats. The year-to-year pat location for faecal pats from the different treatments and deposition occasions was maintained during the following 2 years of the trial (1999 and 2000).
Meteorology
Precipitation and temperature data were recorded continuously at a meteorological station, located 2.5 km from the experimental site. Monthly precipitation and 10 d average temperature values were expressed in relation to the long-term (1961–1990) average (LTA) as shown in Figure 1.
Meteorological data of the experimental site between May 1998 and December 2001. Comparisons between (A) the long-term (30-year) average (LTA) precipitation (dotted line) and the precipitation during the trial period (bars). (B) LTA monthly mean temperature (dotted line) compared with 10-day mean temperature during the trial period (solid line).
In the summer of 1998, the weather conditions were wetter and cooler than the LTA. In 1999, the precipitation during the summer was exceptionally low while the temperature was above the LTA. In 2000, precipitation was above the LTA, whilst the temperature was normal. Daily mean temperature and precipitation during the sampling periods are shown in Figure 2.
The change of mass of original artificial 1 kg dung pats derived from cattle that were either untreated, (Control; triangle), treated with the ivermectin bolus, (IVM Bolus; square), or fed the fungus Duddingtonia flagrans (Fungus; circle). The dung pats were protected from birds and deposited on a nylon mesh on 4 occasions during the grazing seasons 1998–2000, respectively. The year-to-year pat location for the different treatments and deposition occasions were repeated during the 3 years of the trial. Weighing of the pats was performed 4, 6, 8, and 10 weeks after deposition where the nylon mesh supporting the dung pat was lifted on to a scale and immediately afterwards replaced to its initial position. The corresponding daily mean temperature (solid line) and precipitation (bars) for the study periods are displayed below.
Statistical analysis
Data were summarised using Microsoft Excel® 2000 and the statistical analysis was performed with Intercooled Stata 7.0 for Windows NT (Stata Corporation, College Station, Texas, USA). To obtain normal distribution and equal variances, weight of the dung pat was transformed to the 1.5 root prior to analysis according to the formula (Weight-1.5). Dung degradation was subsequently analysed in a repeated measurement ANOVA-model with treatment, year and month of deposition as independent variables, and weighing occasion as the repeated variable. The June 1998 and July 2000 depositions were excluded from the analysis as all dung pats for all treatments had disappeared at the time of the first weighing occasion. Dry matter content of the faeces was analysed using one-way ANOVA.
Results
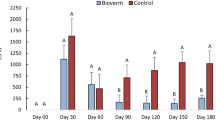
The complete degradation time of 1 kg artificial dung pats varied between 2 weeks and 12 months in this experiment. No significant difference in dung degradation was detected between treatments during the 3-year study (p = 0.47). However, differences were found between deposition months (p < 0.0001) and years (p < 0.0001) (Fig. 2). Faeces DM (Table 1) did not differ significantly between treatments (p = 0.10).
In 1998, all pats deposited in June had vanished in less than 4 weeks. The pats deposited in July, August and September showed a similar pattern where approximately 400 g remained after 4 weeks and all visible faecal material had disappeared within 10 weeks after deposition.
In 1999, 14 mm of rainfall in 2 days after the June deposition prevented formation of a crust on the surface of the dung pats and extensive pat erosion was observed within 3 days. Rehydration due to 66.8 mm rainfall in 10 d in mid September caused an increase in weight of the remaining parts of the 1999 faecal pats deposited in June and July (week 10 sampling) and August (week 6 sampling). Remnants of all deposited faecal pats were still present 10 weeks after deposition and even in May 2000, small fragments of faeces (<20 g) remained on all sub-plots.
In 2000, the dung pats deposited in July had completely disappeared in less than 2 weeks while approximately 300 g of the pats deposited in June, August and September remained 4 weeks after deposition. The dung pats deposited in June and September had all vanished 10 weeks after deposition whereas approximately 150 g of the pats deposited in August remained 10 weeks after deposition. However, no faeces remained in early May 2001.
Discussion
Results from this 3-year experiment failed to show any significant differences in the disintegration rate of dung pats derived from cattle that were either treated with the ivermectin sustained-release bolus, or fed the nematophagous fungus D. flagrans, compared with untreated animals. However, significant differences in the faecal pat disappearance were detected between years and months of pat deposition. For example, the dung pats deposited in June 1998 and July 2000 disappeared in less than 2 weeks, while complete disappearance was prolonged for 9 to 12 months for the pats deposited throughout 1999. It is evident that these differences were attributed to variation in rainfall.
The formation of a crust on the surface of the cattle dung pat is of particular importance in the process of disintegration, and if this is prevented by continuous rainfall shortly after deposition, disintegration occurs more rapidly [24] as do simulation of continuously wet weather by irrigation [8]. In contrast, a prolonged period of dry weather, which was a feature of summer and early autumn of 1999, extended the time-lag between dung pat deposition and complete disappearance for up to 12 months. Dry, sunny and hot weather conditions retard pat degradation [2, 8] due to the formation of an impervious hard outer crust that renders the pats relatively unattractive to coprophilic invertebrates and micro-organisms, as well as impeding their entry [17]. Further, microbial and earthworm activity is slower during extended dry conditions [17].
In Scandinavia, dung beetles seem to play a minor role in the dung degrading process [18], whereas earthworms are more important [21, 22]. The toxicity of ivermectin to the earthworm Eisenia foetida was investigated by [16] who found that the 28 d LC50 was 315 mg/kg soil. This is much higher than the 1.18 mg/kg faeces found by [1] in dung from cattle treated with the Ivomec SR bolus device (MSD AGVET, Paris, France), which is equivalent to the ivermectin bolus used in this trial. Indeed, no effect on lumbricoid earthworms of ivermectin in cattle dung was reported either by [37] or [31]. However, in contrast to these findings are the observations in the laboratory by [15], who tested soil contaminated with a drug formulation containing 0.08% w/v ivermectin. Based on results from different concentrations of ivermectin in soil, the 14 d EC50 for decreased growth rate of E. fetida was calculated to 4.7 mg/kg dry soil and the corresponding 14 d LC50 for mortality was estimated to 15.8 mg/kg dry soil. Although the concentration in dung from animals treated with injectable or topical formulations may reach 16.3 mg/kg organic matter of dung [33], this is still much higher than the 1.18 mg/kg faeces from ivermectin bolus treated animals [1]. Nevertheless, excreted ivermectin undergoes both oxidative degradation under aerobic conditions and rapid photodegradation if exposed to sunlight [17], which will diminish the environmental concentration of ivermectin over time. However, drug residues are excreted in faeces continuously for the period of 135 d when the bolus is active and are initially protected from sunlight within the pat [17].
For the first time, the rate of dung degradation of faeces produced by cattle fed D. flagrans was investigated. No statistical difference was found compared with faeces produced by untreated cattle. The trapping network is capable of capturing soil or dung inhabiting nematodes, which are in the order of 1–1.5 mm in length [4, 32]. In a companion study to this work where herbage was clipped around the same faecal pats that was used in this study, D. flagrans significantly reduced herbage larval availability of gastrointestinal nematodes of cattle [10]. However, the nematode trapping activity of D. flagrans is not specific to parasitic larvae and the possible perturbation of the soil nematode community needs to be carefully examined. Two investigations in Sweden recently focussed on this issue. These were trials conducted on pastureland grazed by the cattle that served as donors of faeces in this experiment [41], as well as in a plot study where soil samples were taken directly underneath the dung pats of this experiment [42]. The results of these investigations showed no effect on total numbers, or diversity and functional groups, of soil nematodes. Similarly, in the study by [12], no statistical difference was found in the soil nematode population or the nematode composition fauna in soil surrounding sheep faeces. Moreover, [12] could not detect any spread of D. flagrans to the soil underneath the dung pats in the trial described by [42], neither did [27] from soil profiles in Australia where sheep faeces containing D. flagrans had been deposited.
The short-term effect of D. flagrans on the lumbricoid earthworm Aporrectodea longa was examined by [14]. They found no indication of mycosis when exposed to cattle faeces during 20 d at a concentration of 800 chlamydospores/g faeces. Additionally, the larger size earthworms compared with soil nematodes would prevent them from falling prey to D. flagrans.
Results from studies on dung degradation from ivermectin treated cattle in temperate regions are inconclusive. [19] reviewed the literature and identified factors and discussed possible reasons for the diversity of results. For example, [37] and [36] reported that dung pats from cattle treated with the ivermectin sustained-release bolus failed to degrade normally and claimed that this was due to the toxic effects on some key dung-colonising insects. On the other hand, [39] and [6] were unable to detect differences in the decomposition rate or in the organic matter content between dung pats from cattle treated with the ivermectin bolus and those from untreated cattle. Indeed, the study by [39] was subject for critical analysis by [23] where they attributed major experimental deficiencies for the absence of an effect on dung decomposition. Sound methodology in studies of dung degradation has been emphasised by [5] who pointed out the importance to measure moisture content of the dung in relation to dung pat disappearance. Similarly, when no retardation of dung degradation from ivermectin treated cattle was observed, [35] considered that this was due to flaws in methodology, statistics, and/or extremes of climatic conditions. Therefore, it is surprising that no data on moisture of dung were presented in the paper by the same authors [37], which also makes interpretation of their results open to question.
In conclusion, excreted ivermectin, or D. flagrans, at concentrations associated with specific nematode parasite control methods in cattle, had no significant effect on the rate of dung degradation in this experiment. As the aim of this study was to examine the change of dung mass only, the actual level of ivermectin in dung was not analysed and no specific investigation of the dung insect fauna was included. Consequently, the absence of a treatment effect does not exclude the possibility of an underlying detrimental effect on specific components of the dung insect fauna assemblage. However, prevailing weather conditions accounted for significant difference in dung degradation between deposition months and years and overruled any possible negative effect on the dung degrading fauna. In addition, these observations were consistent over 3 consecutive years and under both dry and wet weather conditions.
References
Alvinerie M, Sutra JF, Galtier P, Lifschitz A, Virkel G, Sallovitz J, Lanusse C: Persistence of ivermectin in plasma and faeces following administration of a sustained-release bolus to cattle. Res Vet Sci. 1999, 66: 57-61. 10.1053/rvsc.1998.0240.
Anderson JR, Merritt RW, Loomis EC: The insect-free cattle dropping and its relationship to increased dung fouling of rangeland pastures. J Econ Entomol. 1984, 77: 133-141.
Bardgett RD, Cook R, Yeates GW, Denton CS: The influence of nematodes on below-ground processes in grassland ecosystems. Plant Soil. 1999, 212: 23-33. 10.1023/A:1004642218792.
Barron GL: Predators and parasites of microscopic animals. Biology of conidial fungi. Edited by: Cole GT, Kendrick B. 1981, Academic Press Inc., New York, 167-200.
Barth D: Importance of methodology in the interpretation of factors affecting degradation of dung. Vet Parasitol. 1993, 48: 99-108. 10.1016/0304-4017(93)90148-G.
Barth D, Heinze-Mutz EM, Roncalli RA, Schlüter D, Gross SJ: The degradation of dung produced by cattle treated with an ivermectin slow-release bolus. Vet Parasitol. 1993, 48: 215-227. 10.1016/0304-4017(93)90157-I.
Campbell WC: Ivermectin and abamectin. 1989, Springer, New York, ISBN 0-387-96944-6 (New York)
Dickinson CH, Underhay VSH, Ross V: Effect of season, soil fauna and water content on the decomposition of cattle dung pats. New Phytol. 1981, 88: 129-141.
Dimander S-O, Höglund J, Uggla A, Spörndly E, Waller PJ: Evaluation of gastrointestinal nematode parasite control strategies for first-season grazing cattle in Sweden. Vet Parasitol. 2003, 111: 193-209. 10.1016/S0304-4017(02)00380-1.
Dimander S-O, Höglund J, Waller PJ: Seasonal translation of infective larvae of gastrointestinal nematodes of cattle and the effect of Duddingtonia flagrans: a 3-year plot study. Vet Parasitol. 2003, 117: 99-116. 10.1016/j.vetpar.2003.07.016.
Edwards CA, Atiyeh RM, Römbke J: Environmental impact of avermectins. Rev Environ Contam Toxicol. 2001, 171: 111-137.
Faedo M, Larsen M, Dimander S-O, Yeates GW, Höglund J, Waller PJ: Growth of the fungus Duddingtonia flagrans in soil surrounding faeces deposited by cattle or sheep fed the fungus to control nematode parasites. Biol Control. 2002, 23: 64-70. 10.1006/bcon.2001.0987.
Griffiths BS, Young IM, Caul S: Nematode and protozoan population dynamics on decomposing barley leaves incubated at different soil matric potentials. Pedobiologia. 1995, 39: 454-461.
Grønvold J, Wolstrup J, Larsen M, Nansen P, Bjørn H: Absence of obvious short-term impact of the nematode-trapping fungus Duddingtonia flagrans on survival and growth of the earthworm Aporrectodea longa. Acta Vet Scand. 2000, 41: 147-151.
Gunn A, Sadd JW: The effect of ivermectin on the survival, behaviour and cocoon production of the earthworm Eisenia fetida. Pedobiologia. 1994, 38: 327-333.
Halley BA, Jacob TA, Lu AYH: The environmental impact of the use of ivermectin: environmental effects and fate. Chemosphere. 1989, 18: 1543-1563. 10.1016/0045-6535(89)90045-3.
Halley BA, VandenHeuvel WJ, Wislocki PG: Environmental effects of the usage of avermectins in livestock. Vet Parasitol. 1993, 48: 109-125. 10.1016/0304-4017(93)90149-H.
Hanski I, Cambefort Y: Dung beetle ecology. 1991, Princeton University Press, Princeton, ISBN 0-691-08739-3
Herd R: Endectocidal drugs: ecological risks and counter-measures. Int J Parasitol. 1995, 25: 875-885. 10.1016/0020-7519(95)00018-W.
Herd RP, Sams RA, Ashcraft SM: Persistence of ivermectin in plasma and faeces following treatment of cows with ivermectin sustained-release, pour-on or injectable formulations. Int J Parasitol. 1996, 26: 1087-1093. 10.1016/S0020-7519(96)00102-6.
Holter P: An experiment on dung removal by Aphodius larvae (Scarabaeidae) and earthworms. Oikos. 1977, 28: 130-136. 10.2307/3543332.
Holter P: Effect of dung-beetles (Aphodius spp.) and earthworms on the disappearance of cattle dung. Oikos. 1979, 32: 393-402. 10.2307/3544751.
Holter P, Strong L, Wall R, Wardhaugh K, Herd R: Effects of ivermectin on pastureland ecology. Vet Rec. 1994, 135: 211-212. discussion 212–213
Hypolite G, Morhain B, Vignon B: Influence des bousats et des pissats d'un troupeau de vaches laitières sur la production, la composition et la consommation de l'herbe (Influence of faecal pat and urine contamination from dairy cows on herbage production, composition and pasture consumption). Bull Tech CRZV Theix, INRA. 1984, 58: 11-17. (In French)
Ingham RE, Trofymow JA, Ingham ER, Coleman DC: Interactions of bacteria, fungi and their nematode grazers on nutrient cycling and plant growth. Ecol Monogr. 1985, 55: 119-140. 10.2307/1942528.
Jansson RK, Rabatin S: Curative and residual efficacy of injection applications of avermectins for control of plant-parasitic nematodes of banana. J Nematol. 1997, 29: 695-702.
Knox MR, Josh PF, Anderson LJ: Deployment of Duddingtonia flagrans in an improved pasture system: dispersal, persistence, and effects on free-living soil nematodes and microarthropods. Biol Control. 2002, 24: 176-182. 10.1016/S1049-9644(02)00012-9.
Larsen M: Biological control of helminths. Int J Parasitol. 1999, 29: 139-146. 10.1016/S0020-7519(98)00185-4. discussion 153–134
Larsen M: Prospects for controlling animal parasitic nematodes by predacious micro fungi. Parasitology. 2000, 120: S121-131. 10.1017/S0031182099005739.
Lodha BC: Decomposition of digested litter. Biology of plant litter decomposition. Edited by: Dickinson CH, Pugh GJF. 1974, Academic Press, London, 213-241.
Madsen M, Nielsen BO, Holter P, Pedersen OC, Brøchner Jespersen J, Vagn Jensen K-M, Nansen P, Grønvold J: Treating cattle with ivermectin: effects on the fauna and decomposition of dung pats. J Appl Ecol. 1990, 17: 1-15.
Mankau R: Soil fungistasis and nematophagous fungi. Phytopathology. 1962, 52: 611-615.
Sommer C, Steffansen B, Nielsen BO, Grønvold J, Vagn Jensen K-M, Jespersen JB, Springborg J, Nansen P: Ivermectin excreted in cattle dung after subcutaneous injection or pour-on treatment: concentration and impact on dung fauna. B Entomol Res. 1992, 82: 257-264.
Stretton AOW, Campbell WC, Babu JR: Biological activity and mode of action of avermectins. Vistas on Nematology. Edited by: Veech JA, Dickson DW. 1987, Society of Nematologists, Hyattsville, MD, 136-146.
Strong L: Overview: the impact of avermectins on pastureland ecology. Vet Parasitol. 1993, 48: 3-17. 10.1016/0304-4017(93)90140-I.
Strong L, Wall R, Woolford A, Djeddour D: The effect of faecally excreted ivermectin and fenbendazole on the insect colonisation of cattle dung following the oral administration of sustained-release boluses. Vet Parasitol. 1996, 62: 253-266. 10.1016/0304-4017(95)00890-X.
Wall R, Strong L: Environmental consequences of treating cattle with the antiparasitic drug ivermectin. Nature. 1987, 327: 418-421. 10.1038/327418a0.
Wiktelius S: Ivermectin bot eller hot? (Environmental effects of the use of ivermectin). Sv VetTidn. 1996, 48: 653-658. (In Swedish)
Wratten SD, Mead-Briggs M, Gettinby G, Ericsson G, Baggott DG: An evaluation of the potential effects of ivermectin on the decomposition of cattle dung pats. Vet Rec. 1993, 133: 365-371.
Yeates GW: Variation in soil nematode diversity under pasture with soil and year. Soil Biol Biochem. 1984, 16: 95-102. 10.1016/0038-0717(84)90098-1.
Yeates GW, Dimander S-O, Waller PJ, Höglund J: Environmental impacts on soil nematodes following the use of either the ivermectin sustained release bolus or the nematophagous fungus Duddingtonia flagrans to control nematode parasites of cattle in Sweden. Acta Agric Scand, Sect A, Anim Sci. 2002, 52: 233-242. 10.1080/090647002762381113.
Yeates GW, Dimander S-O, Waller PJ, Höglund J: Soil nematodes beneath faecal pats from cattle treated with either the ivermectin sustained-release bolus or the nematophagous fungus Duddingtonia flagrans to control nematode parasites. Acta Agric Scand, Sect A, Anim Sci. 2003, 54: 197-206. 10.1080/09064700310012962.
Yeates GW, Waller PJ, King KL: Soil nematodes as indicators of the effect of management of grasslands in the New England Tablelands (NSW): effect of measures for control of parasites of sheep. Pedobiologia. 1997, 41: 537-548.
Acknowledgements
We wish to acknowledge professor Arvid Uggla for valuable comments on the manuscript, and competent technical assistance of Mr. Abdul Wahab Mehdi and Mss. Elisabeth Wilhelmsson and Maria Moberg. Supply of Duddingtonia flagrans spores by Christian Hansen Biosystems A/S and the Ivomec SR vet. bolus by Veter AB is gratefully acknowledged. The project was financially supported by the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (Formas), contracts nos. 670.092/96 and 30.0214/99.
Author information
Authors and Affiliations
Additional information
Reprints may be obtained from: S.-O. Dimander, Department of Parasitology, National Veterinary Institute, SE-751 89 Uppsala, Sweden. E-mail: sten-olof.dimander@sva.se, tel: +46 18 67 41 53, fax: +46 18 30 91 62.
Rights and permissions
About this article
Cite this article
Dimander, SO., Höglund, J. & Waller, P. Disintegration of Dung Pats from Cattle Treated with the Ivermectin Anthelmintic Bolus, or the Biocontrol Agent Duddingtonia flagrans. Acta Vet Scand 44, 171 (2003). https://doi.org/10.1186/1751-0147-44-171
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1751-0147-44-171