Abstract
Background
The repeated freeze-thaw events during cold season, freezing of soils in autumn and thawing in spring are typical for the tundra, boreal, and temperate soils. The thawing of soils during winter-summer transitions induces the release of decomposable organic carbon and acceleration of soil respiration. The winter-spring fluxes of CO2 from permanently and seasonally frozen soils are essential part of annual carbon budget varying from 5 to 50%. The mechanisms of the freeze-thaw activation are not absolutely clear and need clarifying. We investigated the effect of repeated freezing-thawing events on CO2 emission from intact arable and forest soils (Luvisols, loamy silt; Central Germany) at different moisture (65% and 100% of WHC).
Results
Due to the measurement of the CO2 flux in two hours intervals, the dynamics of CO2 emission during freezing-thawing events was described in a detailed way. At +10°C (initial level) in soils investigated, carbon dioxide emission varied between 7.4 to 43.8 mg C m-2h-1 depending on land use and moisture. CO2 flux from the totally frozen soil never reached zero and amounted to 5 to 20% of the initial level, indicating that microbial community was still active at -5°C. Significant burst of CO2 emission (1.2–1.7-fold increase depending on moisture and land use) was observed during thawing. There was close linear correlation between CO2 emission and soil temperature (R2 = 0.86–0.97, P < 0.001).
Conclusion
Our investigations showed that soil moisture and land use governed the initial rate of soil respiration, duration of freezing and thawing of soil, pattern of CO2 dynamics and extra CO2 fluxes. As a rule, the emissions of CO2 induced by freezing-thawing were more significant in dry soils and during the first freezing-thawing cycle (FTC). The acceleration of CO2 emission was caused by different processes: the liberation of nutrients upon the soil freezing, biological activity occurring in unfrozen water films, and respiration of cold-adapted microflora.
Similar content being viewed by others
Background
The repeated freeze-thaw events during cold season, freezing of soils in autumn and thawing in spring are typical for the tundra, boreal, and temperate soils [1–5]. The winter-spring fluxes of CO2 from permanently and seasonally frozen soils are essential part of annual carbon budget varying from 5 to 50% and they should be not ignored [6–16].
Global warming scenarios predict a milder winter in high and middle latitude regions [17]. Changes in the over-winter temperature regime, including frequency of freezing-thawing and snowmelt processes will be more pronounced in open cropland in comparison with forest or grassland soils [18–20]. The freezing-thawing of soils induces the release of decomposable organic carbon, affects the composition and function of microbial communities, and thus has a profound influence on the overall functioning of ecosystems [21, 22]. It was found that the decomposition of organic matter increased 10 times upon thawing in the forest soils of North America, which remained frozen most of the year [23]. In arctic soils, the carbon losses caused by repeated FTC amounted to 20–30 g C m-2 during non-growing season [22].
The significant acceleration of soil respiration during thawing is well known [16, 23–30]. The most common explanation for the increasing of respiratory activity upon freeze-thaw events is that the soil microbes are killed, releasing nutrient into the soil [31–33]. It was shown that a single FTC may kill up to 50% of the microbial biomass [16]. Later Herrmann and Witter [33] reported that only 5% of microbial biomass is destroyed during freezing-thawing, but this contributes 65% to the total C-flush. Using the 14C labelling plant residues and glucose, Kuzyakov and Sapronov [34] showed that the significant part of CO2 flush after thawing originated from root respiration (80% and 46% of total CO2 efflux from arable and forest soil, respectively), and the contribution of C from microbial biomass killed by freezing was the second important source of C-flush. Substantial over-winter losses of aboveground materials from various catch and cover crops have also been found [30, 35, 36]. The freeze-thaw-released organic C from microbes and plants are readily available for living microorganisms and may play the significant role in freeze-thaw-induced N2O emission [30, 37–39]. Some mathematical models are developed to simulate the observed activation dynamics of gas emission, which account the burst of microbial growth on nutrients released into soil from frost destroyed cells [11, 40]. Moreover, in recent study of Koponen et al. [3] it was shown that freezing and thawing of boreal soils does not have a strong effect on microbial biomass and structure of microbial community. Thus, the mechanisms of freeze-thaw activation are not absolutely clear and need future clarification.
The soil moisture is a key factor regulating the respiratory activity of soil [41]. Land use of soil has a profound effect on CO2 emission rate as well, governing the main soil properties (C and N content, pH, microbial activity, structure etc). However, the influence of soil moisture and land use on the freezing-thawing-induced CO2 emission is still poorly studied. For instance, Koponen and Martikainen [2] did not find the significant difference in freeze-thaw-released CO2 from soils at moisture corresponding to 56% and 85% of water filled pore space (WFPS). The influence of site location and land use were investigated by Prieme and Christensen [27] and Dorsch et al. [20]. They showed that total surplus of CO2 emission from grassland and fallow sites following thawing was generally higher than from arable and forest sites probably due to decomposition of carbon sources liberated from stressed grass roots [27] and residual plant activity at low temperature [20].
In this article we try to display how the soil moisture and land use affect CO2 production during repeated FTC. The present study was aimed to investigate the dynamics of CO2 emission from intact arable and forest soils (Luvisols, loamy silt; Low Saxony, Germany) at different water content during two FTC. The "dry" soil corresponds to 65% of WHC, and "wet" soil corresponds to 100% of WHC. We have chosen these contrast levels of soil moisture to simulate the natural soil conditions during cold season after the dry and wet autumn.
Results
Duration of soil freezing and thawing
Our investigation showed that the pattern of soil freezing and thawing depended on soil moisture, land use, and temperature during the freezing period (deep of frost). Thus, the duration of soil freezing varied from 1.3 to 2.7 days and the order of precedence was: ADS < FDS < AWS < FWS. We also found that the time to thaw the soils was shorter than to freeze and changed between 0.9–1.6 days. During the second cycle, the duration of freezing and thawing was shorter due to more mild frost (-3°C) compared to the first one (-5°C).
CO2 dynamics during freezing-thawing cycles
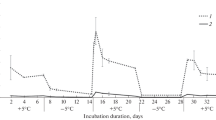
Due to the measurement of CO2 flux in two hours intervals, we described the dynamics of CO2 emissions during repeated FTC in a detailed way. Although the initial levels of CO2 production were different in the forest and arable soils, the patterns of CO2 emission were generally similar for soils studied during both FTCs (Fig. 1). When the soil temperature decreased from +10 to negative temperature during freezing, soil respiration rate reduced immediately. In arable soils (pHH2O = 7.9), the evident rise of CO2 emission (from 1.0–2.8 to 5.6–6.9 mg C m-2h-1) have been observed soon after starting of freezing. This effect was very weak in the forest dry soil (FDS) and was not observed in the forest wet soil (FWS, pHH2O = 4.3–5.6).
The CO2 flux from the totally frozen soils never reached zero. During each thawing period we registered a sharp and two-levelled increase of CO2 emission. It started soon after soil thawing and lasted 1–2 days (Fig. 1). The CO2 emission rate increased up to 75 mg C m-2h-1 in the forest soils and to 28 mg C m-2h-1 in the arable soils during the first thawing. The acceleration of CO2 emissions lasted about 1–2 days; the subsequent decrease of the soil respiration was very slow. Before the second FTC, the CO2 emission level was higher than the CO2 emission levels at the beginning of the experiment. There was close correlation between CO2 emission rate and soil temperature during the freezing and thawing. Linear and polynomial models described these relationships satisfactory. The determination coefficients (R2) were 0.86–0.97 for linear regressions (P < 0.001) and changed between 0.94 and 0.99 (P < 0.001) for polynomial models.
Carbon dioxide emission rates during different periods of FTC
We calculated mean values of CO2 fluxes and relative CO2 emission rates during different periods of freezing-thawing cycles (Table 1, 2). The emission rate at +10°C was taken as control level. Carbon dioxide emissions from soils at +10°C changed from 7.4 to 43.8 mg C m-2day-1 and depended on the soil moisture and land use. The CO2 emission rates of forest soils with the higher contents of microbial biomass, soluble and total soil organic matter (SOM), were 2–3 times higher than SOM-poor agricultural soils. We observed the depressive effect of high soil moisture on respiration of arable soils: CO2 flux from arable wet soil (AWS) was twice less than from arable dry soil (ADS). In the forest soils, the depressive effect of high moisture on CO2emission was not observed. There is a close positive linear correlation between the initial CO2 emission rate and total C content in the topsoil (R2 = 0.96; P < 0.01).
The respiration rate of completely frozen soil varied from 0.8 to 4.5 mg C m-2h-1 and constituted 5–20% of the CO2 flux at +10°C. It indicates that microbial community was still active at -5°C and hence, evidences the existence of winter CO2 fluxes from tundra and boreal soils. During freezing and thawing, when the mean soil temperature was about 0°C, the CO2 emission rate varied strongly and depended on soil moisture and land use. Thus, CO2 fluxes from FDS, FWS and ADS amounted to 18–36% from initial respiration rate during freezing and were higher (35–70%) during thawing (Table 2). This pattern was reverse in AWS: respiration rate reached 63–93% of the initial level during freezing and constituted 19–35% during thawing. This contradiction may be explained by discovered increase of CO2 emission rate in arable soils immediately after starting of freezing. The mean values of CO2 emission after the first thawing were 1.3–1.7 times higher than before starting of freezing (Table 2). This difference was smaller after the second thawing and did not exceed 30%.
Total and extra CO2 fluxes during FTCs
The total CO2 flux during two FTCs changed from 3.2 to 14.4 g C·m-2, the order of precedence was: AWS < ADS < FDS < FWS. These fluxes were approximately equal during the first and second FTCs (Table 3). There was a close positive correlation between the total CO2 flux values, total C content in the topsoil (0–4 cm) and WFPS of 0–4 cm and 4–8 cm layers (R2 = 0.77 to 0.90; P < 0.01).
The sum of extra CO2 fluxes induced freezing-thawing processes varied through 0.5–2.2 g C·m-2. The largest amount of extra CO2 emission was found in the FDS and the smallest one was observed in the AWS. The FWS and ADS provided the approximately equal extra CO2 fluxes: 1.0 and 1.3 g C·m-2. The freeze-thaw-induced CO2 emissions from forest soils were 2–3 times higher during the first FTC than for the second one. The extra CO2 fluxes from arable soils did not differ significantly during 1-st and 2-d FTC (Table 3). We found the significant negative correlation between the total extra CO2 fluxes and WFPS in 0–4 and 4–8 cm layers (R2 = -0.81; P < 0.01). The share of extra CO2 fluxes in the total CO2 fluxes changed from 7.2 to 22.5% (Table 4). It was most significant in ADS (lowest level of initial CO2 emission), and was smallest in FDS (highest initial carbon dioxide emission).
Discussion
The results of our experiment well agree with the observations of Willis et al. [42]. They also found that the dry soils freeze faster and deeper than moist ones. The wet soils with the higher specific heat capacity required more energy consumption for cooling and subsequent freezing. The heat conductivity of mineral soils has been also found to be approximately an order higher than the heat conductivity of dry peat [43]. In our experiment, the freezing rate of arable soils with a low C content was also higher than in forest soil due to insulating function of the humus layer.
We observed two uncommon phenomena during our laboratory study: (1) the significant increase of CO2 emission from AWS soon after starting of freezing and (2) two-levelled pulse of CO2 emission during the thawing. We suppose that the first phenomenon may be caused by the higher dissolution of CO2 in the water phase of arable weak-alkaline soils during cooling and its subsequence release during freezing [44]. This physicochemical process takes place only in neutral and alkaline soils: HCO3 - + OH- ⇔ CO2 ↑+ H2O. The evident acceleration of CO2 emission was also observed immediately after starting of freezing in the laboratory experiment with sterilized cultivated soils (pH = 6.8–7.2) [45]. It was shown that the sterilized weak-alkaline cultivated soil emitted 0.22–0.35 mg C-CO2 per 1 kg of soil during the freezing process. At the same time in sterilized forest soil with acidic reaction, the freezing-thawing events did not influence the dynamics of CO2 dissolution and emission. In field conditions, Zimov et al. [7, 8] observed similar increase of CO2 emission rate during freezing of moist tundra soils due to physical release of trapped CO2 from soil pores and frozen water.
The two-levelled CO2 emission peak observed during soil thawing may be explained by two different processes. The first CO2-pulse lasted a short time and was governed by physical release of trapped CO2. The second one was higher and longer and was caused by an acceleration of microbial activity due to the temperature increase. This CO2-pulse possibly ensured from the organic substrates releases caused by microbial death during freezing [21, 27, 30, 32, 46]. Thus, it was found that thawing of soils produced an initial pulse (< 24 hours) in microbial respiration and that the total amount of carbon respired in each thaw period was largest during the first cycle and decreased in successive cycles [21, 27]. We also observed similar decrease in surplus of CO2 emission and extra CO2 fluxes during the second FTC in comparison with the first one (Table 1 and 3). Schimel and Clien [21] believe that total respiration over the first cycle appears to dominate by the flush from microbial biomass, while the respiration over the second and next FTC is driven by the reduction in attack on the soil organic matter resulting from a reduced microbial population. It was found, that freezing-thawing induces stronger destabilization of microbial communities and it takes more time to restore in comparison with the drying of soils [46]. The restoration of frost-damaged microbial biomass takes about two weeks.
Our investigation showed that the respiration rate of completely frozen soils never reached zero indicating that the microbial community was still active at -5°C. These results well agree with other laboratory studies. The CO2 production of permanently frozen soils from northern regions has been found to remain positive and measurable at -16°C and even though -39°C [4, 11]. The winter CO2 emission to atmosphere were also observed in a number of tundra and forest ecosystems under the field conditions [6–16, 47]. The cold-season emission of CO2 from northern soils was a significant source of atmospheric CO2 that can account for up to half of the annual CO2 flux from Arctic and boreal forest ecosystems. The mechanism for the observed cold-season CO2 emission is not absolutely clear and could presumably result from variety of processes [1, 7–9, 19, 25, 48]: (1) the physical release of summer accumulated gases, (2) the biological activity occurring in a warm unthawed soil layer; (3) the microbial metabolism in unfrozen water films on the surface of soil particles; (4) the respiration of cold-adapted microbes and plant roots within the bulk of frozen soil.
We also showed that respiratory activation of forest soils induced by freezing-thawing was higher compared to arable soils. Such a different response of arable and forest soils to the repeated freezing-thawing events is governed by the differences in substrate availability, the size of microbial pool and root mass in soils. Thus, soil fungi dominating in forest soils have been considered the main source of CO2 release at low temperatures [1, 4]. They are the cold-tolerant organisms and display a wider temperature range of metabolic activity than bacteria [49]. Besides, forest soils contained some amount of fresh leaves and roots (living and dead), while these materials were absent in arable soil. Therefore, the more significant acceleration of CO2 emission in forest soil during thawing could be caused by decomposition of carbon sources liberated from frost-damaged fresh organic materials [20, 27].
Conclusion
Our results showed the importance of soil moisture and land use on freeze-thaw-induced CO2 emission. These factors governed the initial rate of soil respiration, duration of freezing and thawing of soil, pattern of CO2 dynamics, and extra CO2 flux. As a rule, the freezing-thawing-induced emissions of CO2 were more significant in dry soils and during the first FTC. The forest soils demonstrated high respiratory activity at low temperatures and the more significant acceleration of CO2 emission during the thawing due to the predominance of fungi in microbial biomass and C-release from frost-damaged fresh organic matter. The cold soil respiration and acceleration of CO2 emission during the freezing-thawing was caused by different processes: the liberation of nutrients upon the soil freezing, biological activity occurring in unfrozen water films, and respiration of cold-adapted microflora. Since the cold season CO2 emission is mainly caused by the microbial activity (with some possible contribution of roots), our results can be used to develop the mathematical model of future winter CO2 emission under a warmer climate. The mechanisms of freeze-thaw activation also need future clarification.
Methods
Soils
The soil monoliths (diameter -15 cm, height 10–12 cm, weight 2.5–3.1 kg) were taken in October, 1999 from a beech forest and arable site (winter barley) both located in the central part of Germany (52° 30'N, 9°55'E). The field moisture of soils corresponds to 44% and 61% of WHC for forest and arable soils, respectively. The intact soil cores were adjusted to two contrast levels of water content: 65% of their water holding capacity (WHC) – dry soils and 100% of WHC – wet soils. To reach 100% of WHC moisture, the soil monoliths were placed in the tank with water. The water level in tank was equal to the height of monoliths. After 1–2 days, when soil cores were completely saturated with water, they were replaced in another tank for trickling the gravitational (free) water. To reach the soil moisture corresponding the 65% of WHC, we drained off the odd water from soil monoliths at 100% of WHC by means of a special vacuum-pumping devise. It was connected to the bottom of soil cores and kept the constant negative pressure corresponding to soil moisture 65% of WHC. This procedure allowed us to wet monoliths uniformly. The number of replications was five for each of four variants: (1) – Forest Dry Soil (FDS); (2) – Forest Wet Soil (FWS); (3) – Arable Dry Soil (ADS); (4) – Arable Wet Soil (AWS),
The forest and arable soils (Luvisols) were characterised by similar texture (loamy silt). The chemical properties of soils depended on the land use (Table 4). The pH of the forest and agricultural soil was 4.3 and 7.9, respectively. The humus layer of forest soils was characterised by higher content of total and dissolved carbon and nitrogen compared to arable topsoil. The C and N contents in microbial biomass were also higher in the forest soils.
CO2 emission measurements
The automated gas chromatographic systems equipped with a 63Ni electron-capture detector [50] was used for CO2 emission measurements. The laboratory experiment was carried out in the microcosm systems [51] with intact soil monoliths, which allowed us to measure the CO2 fluxes from soils every two hours.
Freezing-thawing cycles
Twenty soil columns were placed in the freezer and incubated at about +10°C during 4–5 days until the CO2 emission reached the constant level and then subjected to two FTC. To control the temperature in the freezer, the temperature sensors with automatic data logging were installed into the soil monoliths. There were 5 temperature sensors in each column. They were inserted on 1, 5, and 10 cm depths at the centre of monoliths, and 1, 5 cm depths near the side of monoliths. The temperature of soils was measured in one hour intervals with the data loggers. For our calculations, we used the mean values of soil temperature, since 5 sensors in each column demonstrated the similar temperature during experiment. To simulate freezing and thawing events in the soils the temperature in the freezer was changed from +10°C to -5°C during the first FTC and to -3°C during the second FTC. The initial level of CO2 fluxes at +10°C was a control to check the effect of FTC on CO2 emission. The duration of each freezing-thawing cycle was about 14 days. For future calculations we divided each FTC into five different periods depending on soil temperature (ST): (1) – before freezing, ST = +10°C; (2) – soil freezing, ST changed from +10 to -5°C; (3) – constant freezing temperature, ST = -5°C (for 1-st FTC) and ST = -3°C (for 2-d FTC); (4) – soil thawing, ST changed from -5 to +10°C; (5) – after thawing, ST = +10°C.
Chemical and microbiological analysis of the soils
In the end of experiment each monolith of forest soil was divided into two layers: 0–4 cm (humus) and 4–8 cm (mineral). The forest litter was analysed separately. Arable cores were divided identically. The soil moisture, WHC and WFPS were determined by conventional methods of soil physics. The content of total C and N were determined using CN-analyser. Water soluble organic carbon, NO3 - and NH4 + concentrations were measured colorimetrically in 0.5 M K2SO4 extracts (TRAACS 800 auto-analyser). Microbial C and N were determined by fumigation-extraction method and calculated as a difference between C and N contents in 0.5 M K2SO4 extracts before and after fumigation procedure. To estimate the amount of C immobilized in soil microbial biomass, we used kEC = 0.51 and kEC = 0.42 for forest and arable soils, respectively [52]. To calculate the amount of N immobilized in soil microbial biomass, we used kEN = 0.54 both for forest and arable soils [53]. We did not determine microbial C and N in forest litter since fumigation-extraction method was not applied for determination of microbial biomass in fresh organic materials [54].
Data analyses and statistics
The mean CO2 emission rates (per hour) and fluxes (per period) were calculated for 5 different periods of each FTC. The extra CO2 fluxes (CO2 fluxes induced by freezing-thawing) were estimated for each period of FTC according to the following equations:
EFi = (Fi - Fo)* Di (1)
where EFi is extra CO2 flux for i-period of FTC (mg C m-2period-1); Fi is mean CO2 flux during the i-period of FTC (mg C m-2day-1); Fo is mean CO2 flux at +10°C (before freezing, mg C m-2day-1); Di is duration of the i-period, days.
The results presented are arithmetic mean and standard deviation (STD). The correlation analysis was carried out using linear regression function. Statistical differences between treatments were tested by Student's T-test.
Abbreviations
- FTC:
-
freezing-thawing cycle
- WHC:
-
water holding capacity
- WFPS:
-
water filled pore space
- ST:
-
soil temperature
- SOM:
-
soil organic matter
- EF:
-
extra CO2 flux
- TEF:
-
total extra CO2 flux
- FWS:
-
forest wet soil
- FDS:
-
forest dry soil
- AWS:
-
agricultural wet soil
- ADS:
-
agricultural dry soil
References
Panikov NS: Fluxes of CO 2 and CH 4 in high latitude wetlands: measuring, modelling and predicting response to climate change. Polar Research 1999, 18: 237–244.
Koponen HT, Martikainen PJ: Soil water content and freezing temperature affect freeze-thaw related N 2 O production in organic soil. Nutrient Cycling in Agroecosystems 2004, 69: 213–219. 10.1023/B:FRES.0000035172.37839.24
Koponen HT, Jaakkola T, Keinanen-Toivola MM, Kaipainen S, Tuomainen J, Servomaa K, et al.: Microbial communities, biomass, and activities in soils as affected by freeze thaw cycles. Soil Biology and Biochemistry 2006, 38: 1861–1871. 10.1016/j.soilbio.2005.12.010
Panikov NS, Flanagan PW, Oechel WC, Mastepanov MA, Christensen TR: Microbial activity in soils frozen to below -39°C. Soil Biology and Biochemistry 2006, 38: 785–794. 10.1016/j.soilbio.2005.07.004
Song CC, Wang YS, Wang YY, Zhao ZC: Emission of CO 2 , CH 4 and N 2 O from freshwater marsh during freeze-thaw period in Northeast of China. Atmospheric Environment 2006, 40: 6879–6885. 10.1016/j.atmosenv.2005.08.028
Sommerfeld RA, Mosier AR, Musselman RS: CO 2 , CH 4 and N 2 O flux through a Wyoming snowpark and implications for global budgets. Nature 1993, 361: 140–142. 10.1038/361140a0
Zimov SA, Zimova GM, Daviodov SP, Daviodova AI, Voropaev YV, Voropaeva ZV, Prosiannikov SF, Prosiannikova OV, Semiletova IV, Semiletov IP: Winter biotic activity and production of CO 2 in Siberian soils: a factor in the greenhouse effect. Journal of Geophysical Research 1993, 98: 5017–5023.
Zimov SA, Daviodov SP, Voropaev YV, Prosiannikov SF, Semiletov IP, Chapin MC, Chapin FS: Siberian CO 2 efflux in winter as a CO 2 source and cause of seasonality in atmospheric CO 2 . Climatic Change 1996, 33: 111–120. 10.1007/BF00140516
Oechel W, Vourlitis G, Hastings S: Cold season CO 2 emissions from arctic soils. Global Biogeochemical Cycles 1997, 11: 163–172. 10.1029/96GB03035
Winston GC, Sundquist ET, Stephens BB, Trumbore SE: Winter CO 2 fluxes in a boreal forest. Journal of Geophysical Research 1997,102(24):28795–28804. 10.1029/97JD01115
Panikov NS, Dedysh SN: Cold season CH 4 and CO 2 emission from boreal peat bogs (West Siberia): Winter fluxes and thaw activation dynamics. Global Biogeochemical Cycles 2000, 14: 1071–1080. 10.1029/1999GB900097
Lopes de Gerenyu VO, Kurganova IN, Rozanova LN, Kudeyarov VN: Annual emission of carbon dioxide from soils of the Southern Taiga soils of Russia. Eurasian Soil Science 2001, 34: 931–944.
Zamolodchikov DG, Karelin DV: An empirical model of carbon fluxes in Russian tundra. Global Change Biology 2001, 7: 147–161. 10.1046/j.1365-2486.2001.00380.x
Kurganova I, Lopes de Gerenyu V, Rozanova L, Sapronov D, Myakshina T, Kudeyarov V: Annual and seasonal CO 2 fluxes from Russian southern taiga soils. Tellus 2003, 55B: 338–344.
Kurganova IN, Rozanova LN, Myakshina TN, Kudeyarov VN: Monitoring of CO 2 emission from soils of different ecosystems in Southern part of Moscow region: data base analyses of long-term field observations. Eurasian Soil Science 2004, 37: 74–78.
Schimel JS, Bilbrough CB, Welker JM: The effect of changing snow cover on year-round soil nitrogen dynamics in arctic tundra ecosystems. Soil Biology and Biochemistry 2004, 36: 217–227. 10.1016/j.soilbio.2003.09.008
IPCC In Climate change 2001: The scientific basis. Cambridge University Press, Cambridge; 2001.
Edwards AC, Ceasey J, Cresser MS: Soil freezing effects on upland stream solute chemistry. Water Research 1986, 20: 831–834. 10.1016/0043-1354(86)90169-7
Teepe R, Brumme R, Beese F: Nitrous oxide emission from soil during freezing and thawing periods. Soil Biology and Biochemistry 2001, 33: 1269–1275. 10.1016/S0038-0717(01)00084-0
Dörsch P, Palojarvi A, Mommertz S: Overwinter greenhouse gas fluxes in two contrasting agricultural habitats. Nutrient Cycling in Agroecosystems 2004, 70: 117–133. 10.1023/B:FRES.0000048473.11362.63
Schimel JP, Clein JS: Microbial response to freeze-thaw cycles in tundra and taiga soils. Soil Biology and Biochemistry 1996, 28: 1061–1066. 10.1016/0038-0717(96)00083-1
Larsen KS, Jonasson S, Michelsen A: Repeated freeze-thaw cycles and their effect on biological processes in two arctic ecosystem types. Applied Soil Ecology 2002, 21: 187–195. 10.1016/S0929-1393(02)00093-8
Goulden ML, Wofsy SC, Harden JW, Trumbore SE, Crill PM, Gower ST, Fries T, Daube BC, Fan SM, Sutton DJ, Bazzaz A, Munger JW: Sensitivity of boreal forest carbon balance to soil thaw. Science 1998, 279: 214–217. 10.1126/science.279.5348.214
Soulides DA, Allison FE: Effects of drying and freezing soils on carbon dioxide production, available mineral nutrients, aggregation, and bacterial population. Soil Science 1961, 91: 291–298. 10.1097/00010694-196105000-00001
Burton DL, Beauchamp EG: Profile nitrous oxide and carbon dioxide concentrations in a soil subject to freezing. Soil Science Society of American Journal 1994, 58: 115–122.
Ross DJ: Effects of freezing and thawing on some grassland topsoils on oxygen uptakes and dehydrogenase activities. Soil Biology and Biochemistry 1972, 4: 115–117. 10.1016/0038-0717(72)90051-X
Prieme A, Christensen S: Natural perturbations, drying-wetting and freezing-thawing cycles, and the emission of nitrous oxide, carbon dioxide and methane from farmed organic soils. Soil Biology and Biochemistry 2001, 33: 2083–2091. 10.1016/S0038-0717(01)00140-7
Teepe R, Brumme R, Beese F: Nitrous oxide emission from frozen soils under agricultural, fallow and forest land. Soil Biology and Biochemistry 2000, 32: 1807–1810. 10.1016/S0038-0717(00)00078-X
Teepe R, Ludwig B: Variability of CO 2 and N 2 O emissions during freeze-thaw cycles: results of model experiments on undisturbed forest-soil cores. Journal of Plant Nutrition and Soil Science-Zeitschrift fur Pflanzenernahrung und Bodenkunde 2004, 167: 153–159. 10.1002/jpln.200321313
Morkved PT, Dörsch P, Henriksen TM, Bakken LR: N 2 O emissions and product ratios of nitrification and denitrification as affected by freezing and thawing. Soil Biology and Biochemistry 2006, 38: 3411–3420. 10.1016/j.soilbio.2006.05.015
Morley CR, Trofimov JA, Coleman DC, Cambardella C: Effects of freeze-thaw stress on bacterial population on soil microcosms. Microbial Ecology 1983, 9: 329–340. 10.1007/BF02019022
Skogland T, Lomeland S, Goksoyr J: Respiratory burst after freezing and thawing of soil: Experiments with soil bacteria. Soil Biology and Biochemistry 1988, 20: 851–856. 10.1016/0038-0717(88)90092-2
Herrmann A, Witter E: Sources of C and N contributing to the flush in mineralization upon freeze thaw cycles in soils. Soil Biology and Biochemistry 2002, 34: 1495–1505. 10.1016/S0038-0717(02)00121-9
Kuzyakov Ya, Sapronov DV: Partitioning of sourses of CO 2 flush induced by freezing-thawing of arable and forest soils. Abstracts of Open Science Conference on "The GHG Cycle in the Northern Hemisphere", Sissi-Lassithi, Crete, November, 14–18 77.
Dejoux JF, Recous S, Meynard JM, Trinsoutrot I, Leterme P: The fate of nitrogen from winter-frozen rapeseed leaves: mineralization, fluxes to the environmental and uptake by rapeseed crop in spring. Plant and Soil 2000, 218: 257–272. 10.1023/A:1014934924819
Koursæth A, Henriksen TM, Bakken LR: Temporal changes in mineralization and immobilization of N during degradation of plant materials: implications for the plant N-supply and nitrogen losses. Soil Biology and Biochemistry 2002, 34: 789–799. 10.1016/S0038-0717(02)00008-1
Flessa H, Dörsch P, Beese F: Seasonal variation of N 2 O and CH 4 fluxes in differently managed arable soils in southern Germany. Journal of Geophysical Research 1995,100(32):115–124.
Ludwig B, Wolf I, Teepe R: Contribution of nitrification and denitrification to the emission of N 2 O in a freeze-thaw event in an agricultural soil. Journal of Plant Nutrition and Soil Science-Zeitschrift fur Pflanzenernahrung und Bodenkunde 2004, 167: 678–684. 10.1002/jpln.200421462
Ludwig B, Teepe R, Lopes de Gerenyu V, Flessa H: N 2 O and CO 2 emissions from gleyic soils in the Russian tundra and a German forest during freeze-thaw periods – a microcosm study. Soil Biology and Biochemistry 2006, 38: 3815–3819. 10.1016/j.soilbio.2006.06.006
Grant RF, Pattey E: Mathematical modeling of nitrous oxide emission from an agricultural field during spring thaw. Global Biogeochemical Cycles 1999,13(2):679–694. 10.1029/1998GB900018
Rustad LE, Huntington TG, Boone RD: Controls on soil respiration: Implication for climate change. Biogeochemistry 2000, 48: 1–6. 10.1023/A:1006255431298
Willis WO, Carlson CW, Allessi J, Haas HJ: Depth of freezing and spring run off as reelated to full soil moisture levels. Canadian Journal of Soil Science 1961, 41: 115–123.
Brown RJE, Williams JP: The freezing of peatland. Technical paper 1972, 381: 128–138.
Smagin AV: Gas phase of soil. Moscow: Moscow State University Press; 1999. (in Russian).
Lopes de Gerenyu V, Kurganova I, Loftfield N, Teepe R: Contribution of biological and physicochemical processes to CO 2 efflux from soils during the freezing-thawing events. Abstracts of Symposium on Soil Processes under Extreme Meteorological Conditions, Bayreuth, Germany, 25th to 28th February 2007, in press.
Anan'eva ND, Blagodatskaya EV, Demkina TS: The effect of drying-moistering and freezing-thawing on soil microbial communities' resilience. Eurasian Soil Science 1997, 9: 1010–1014.
Pajary B: Soil respiration in a poor upland site of Scots pine stand subject to elevated temperatures and atmospheric carbon concentration. Plant and Soil 1995, 168–169: 563–570. 10.1007/BF00029369
Rivkina EM, Friedman EI, McKay CP, Gilichinsky DA: Metabolic activity of permafrost Bacteria below the freezing point. Applied and Environmental Microbiology 2000, Aug: 3230–3233. 10.1128/AEM.66.8.3230-3233.2000
Flanagan PW, Bunnel FL: Microfloral activities and decomposition. In An Arctic Ecosystem: the Coastal tundra of Northern Alaska. Edited by: Brown J, et al. New York: Van Nostrand Reinhold; 1980:291–335.
Loftfield N, Flessa H, Augustin J, Beese F: Automated gas chromatographic system for rapid analysis of atmospheric trace gases: methane, carbon dioxide and nitrous oxide. Journal of Environmental Quality 1997, 26: 560–564.
Hantschel R, Flessa H, Beese F: An automated microcosm system for studying soil ecological processes. Soil Science Society of American Journal 1994, 58: 401–404.
Joergensen RG: The fumigation-extraction method to estimate soil microbial biomass: calibration of the k EC value. Soil Biology and Biochemistry 1996,28(1):25–31. 10.1016/0038-0717(95)00102-6
Joergensen RG, Mueller T: The fumigation-extraction method to estimate soil microbial biomass: calibration of the k EN value. Soil Biology and Biochemistry 1996,28(1):33–37. 10.1016/0038-0717(95)00101-8
Anderson J, Domsch KH: A physiological method for the quantitative measurement of microbial biomass in soils. Soil Biology and Biochemistry 1978, 10: 215–221. 10.1016/0038-0717(78)90099-8
Acknowledgements
This study was supported by the Germany Foundation of Academy Exchange (DAAD) and Russian Foundation for Basic Researches. We would like to thank Dr. Heiner Flessa (Institute of Soil Science and Forest Nutrition, Goettingen University, Germany) for his participation in the design of this study and results discussion. We are grateful to Dr. T. Khomutova (Institute of Physicochemical and Biological Problems in Soil Science, Russian Academy of Science) for editing of manuscript. We graceful thahk the reviewers for their comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors' contributions
IK conceived of the study, carried out the freezing-thawing experiments, made the chemical and microbiological analyses of soils, and wrote the manuscript. RT conceived of the study, and participated in its design, coordination and interpretation of results. LN realised the technical support of equipment, carried out the freezing-thawing experiments, and participated in results interpretation. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Kurganova, I., Teepe, R. & Loftfield, N. Influence of freeze-thaw events on carbon dioxide emission from soils at different moisture and land use. Carbon Balance Manage 2, 2 (2007). https://doi.org/10.1186/1750-0680-2-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1750-0680-2-2