Abstract
Background
In recent years, there has been a considerable research effort concerning the integration of magnetic resonance imaging (MRI) into the external radiotherapy workflow motivated by the superior soft tissue contrast as compared to computed tomography. Image registration is a necessary step in many applications, e.g. in patient positioning and therapy response assessment with repeated imaging. In this study, we investigate the dependence between the registration accuracy and the size of the registration volume for a subvolume based rigid registration protocol for MR images of the prostate.
Methods
Ten patients were imaged four times each over the course of radiotherapy treatment using a T2 weighted sequence. The images were registered to each other using a mean square distance metric and a step gradient optimizer for registration volumes of different sizes. The precision of the registrations was evaluated using the center of mass distance between the manually defined prostates in the registered images. The optimal size of the registration volume was determined by minimizing the standard deviation of these distances.
Results
We found that prostate position was most uncertain in the anterior-posterior (AP) direction using traditional full volume registration. The improvement in standard deviation of the mean center of mass distance between the prostate volumes using a registration volume optimized to the prostate was 3.9 mm (p < 0.001) in the AP direction. The optimum registration volume size was 0 mm margin added to the prostate gland as outlined in the first image series.
Conclusions
Repeated MR imaging of the prostate for therapy set-up or therapy assessment will both require high precision tissue registration. With a subvolume based registration the prostate registration uncertainty can be reduced down to the order of 1 mm (1 SD) compared to several millimeters for registration based on the whole pelvis.
Similar content being viewed by others
Introduction
The role of magnetic resonance imaging (MRI) in modern prostate external radiotherapy treatments has in recent years attracted a lot of scientific attention. The applications span from MRI based treatment planning [1–4] to assessment of treatment response using different MRI techniques such as dynamic contrast enhanced MRI (DCE-MRI) [5, 6], diffusion weighted imaging (DWI) [7, 8] and magnetic resonance spectroscopy (MRS) [9]. It is widely accepted in the radiotherapy community that MRI is the preferred choice for target delineation of e.g. prostate, due to its superior soft tissue contrast [10]. It has also been shown that multi-modal registration between MRI and computed tomography (CT) increases the systematic uncertainty of the treatment [11]. It is therefore desirable to develop an MR only workflow where the treatment planning, patient positioning and treatment response evaluation is based on MR imaging. The soft tissue contrast and non-ionizing properties of the MRI scanner make it ideal for daily patient positioning. Several solutions on integration of MRI into the external radiotherapy procedure for this purpose have been suggested in literature, e.g. integrated MR scanner-accelerator solutions [12, 13] or using a patient transport solution from a nearby MR scanner [14].
Image registration is an essential part of medical image analysis. It can be used to combine multi-modal images via image fusion [15, 16], align four dimensional images [17], correct for patient setup errors [18], respiratory tracking [19], automatic image segmentation [20], contour propagation [21] and many other purposes. All of these applications are present in a modern radiotherapy department during treatment planning, the treatment delivery as well as during patient follow-up and tumor response evaluation.
In patients with clinically localized prostate cancer, traditional rigid registration between image volumes acquired at different times may not perform adequately with respect to the tumor shape and position, since the prostate can move with respect to the bony anatomy and external patient contour [22]. This makes ordinary rigid registration, based on the entire patient anatomy, imprecise. In order to align the prostate volume with high precision, there is a need for a registration of the prostate only. One way of accomplishing this is the use of intra-prostatic fiducial markers. The radio opaque markers are implanted into the prostate gland, and can thereafter be visualized using most imaging modalities. By manually defining the markers in the two image sets, the images can be registered so that the markers are as close to each other as possible. This implicitly registers the images with focus on the prostate area, provided that the markers have not migrated within the prostate gland.
A non-invasive path to localized registration of mobile organs is subvolume based rigid registration taking only the volume of interest into account. For patient positioning, the subvolume based rigid registration approach has the advantage that the registration results can be readily interpreted as couch movements, making instant adjustment of patient position possible. The properties of subvolume based registration have been investigated for repeat CT [23] and cone beam CT (CBCT) [24], but to our knowledge not yet for MRI.
In the present study we investigate the precision of subvolume based rigid registration of the prostate for ten patients with four repeat MR scans each. The aim was to quantify the registration precision and its dependence of the registration volume for a mean square metric based algorithm, i.e. determine the optimal size of the registration volume to be used for alignment of MR images for treatment response evaluation and external radiotherapy purposes.
Methods
Patients
Ten patients with median age 58 years (range 52-69 years) scheduled for pre-treatment pelvic MRI scans were included in the study. All patients were treated with fractionated external radiotherapy using three different protocols. The choice of radiotherapy protocol did not influence the prostate delineation to be used in the study.
Imaging
Prior to treatment the patients were imaged with an Espree 1.5 T MR scanner (Siemens Medical, Erlangen, Germany) using a T2 weighted high resolution 3D sequence (SPACE) with axial slices (repetition time was 1500 ms, echo time was 209 ms, number of slice averages was 1, slice thickness 1.7 mm, 120 slices, pixel bandwidth 590 Hz/pixel, flip angle 150 degrees, matrix size 384 × 348, in-plane pixel size 1.17 × 1.17 mm). This MR sequence is part of the normal clinical protocol and is used for target definition. The same MR sequence was repeated three times during the treatment duration, yielding a total of four MR image sets for each patient. The patients were placed on a flat tabletop insert during the MR imaging, and the images were acquired with the body matrix and spine coil.
During the MR imaging, the patients were placed in the scanner in supine position with the standard treatment fixation devices, which consist of a knee cushion that prevents rotation of the pelvis.
Delineation
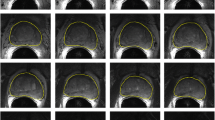
The prostate gland registration volume, defined as RV0, was delineated by a hospital physicist in collaboration with a radio oncologist on the pre-treatment image sets. RV0 included the entire prostate gland excluding the seminal vesicles. 3D margins of 1, 2 and 3 cm were added to RV0 to create different registration volumes denoted as RV1, R2V and RV3, see Figure 1. A volume corresponding to RV0 was delineated on the treatment image sets. This volume did not affect the registration in any way, but was used solely for analysis purposes.
Registration
In order to register the images with respect to the soft tissue in the target and not take the bony anatomy and external patient contour into account, the metric calculation needs to be constructed in such a way that only values within a specific region of interest, i.e. the registration volume, are taken into account. This was accomplished by use of binary volumes, i.e. masks, which define in what region the metric values should be calculated. These masks were created by converting the contours delineated by the authors to binary volumes.
We used MATLAB (MathWorks, Natick, MA) and the Insight Toolkit (ITK) to develop a method for MR-MR image registration. Since it was a single modality registration problem, we used a mean square metric. A step gradient descent approach, the VersorTransformOptimizer, was used for the optimization.
We registered the pre-treatment MRI to the other 3 image sets for each patient, using the complete volume, RV0 mask, RV1 mask, RV2 mask and RV3 mask. This yielded a total number of 150 MR-MR registrations.
Analysis
We quantified the registration uncertainty as the standard deviation of the center of mass distance between the prostate gland (RV0) binary masks for each pair of registered images. This measure has a clinical relevance as the center of mass distance vector corresponds to the couch shift vector when positioning the patient. The registration uncertainty was scored for each main direction x (right-left), y (anterior-posterior) and z (cranio-caudal) and for the norm of this vector. We used F-tests to test for significance in the difference of variance in registrations between different pairs of registration volumes.
Results
The registrations were performed for all patients and all registration volumes for the MR series, see Figure 2. The standard deviation of the center of mass distance post registration was reduced with a decrease in registration volume. The reduction was most pronounced in the anterior-posterior direction, from 5.2 mm with full volume registration to 1.3 mm (p < 0.001) using RV0. In the cranio-caudal direction the standard deviation was reduced from 3.2 mm to 1.7 mm (p < 0.001), and in the right-left direction the reduction of the standard deviation was modest, from 0.7 mm to 0.5 mm (p = 0.08), also using RV0. The standard deviation of the norm of the vector was reduced from 2.8 mm to 0.8 mm (p < 0.001). The mean, median and range of the norm improvement are presented in table 1, together with p-values for difference in variance between the specific registration volumes compared to the full volume registrations. Negative numbers indicate that the subvolume based registration failed to produce a better result than the full volume registration. The numbers indicated in the min row all occurred for the same patient image set where registration failed, see Figure 3. Exclusion of this atypical image set would have led to a minimum improvement around -1 mm.
Registration results. Center of mass standard deviations per coordinate, grouped by registration volume. The colored bar represents the mean center of mass distance and the error bars displays ± 1 standard deviation. The variance in center of mass distance is stable for the right-left direction, but increases with increasing registration volume size for the other directions.
Failed registration. The failed registration reflected in the min row in table 1. The fixed image is displayed in grayscale and the moving image is displayed using a green overlay. The full volume registration can be seen to the left and the subvolume based registration using RV2 to the right. The misregistration is obvious and is easily detected by visual inspection.
Figure 2 shows that the registration uncertainty in the anterior-posterior direction is more sensitive to the size of the registration volume, compared to the cranio-caudal and right-left directions. For the largest registration volumes RV2 and RV3, as well as full volume registration, the anterior-posterior direction contributes to the largest part of the total registration uncertainty. This is likely due to the increase in rectal volume included in the registration volume.
The registration volume that gave the most precise results was RV0 for 77% of the image pairs, RV1 and RV2 for 10% of the pairs each, and RV3 was most precise only for 3% of the cases. These results are not surprising, since the larger registration volumes include more of the rectum and bladder. Hence, the registration algorithm includes changes in these areas, leading to a degradation of the registration with respect to the prostate.
Discussion
The results in this study clearly demonstrate that subvolume based rigid registration improves the registration precision within the area of interest. However, as with all registration protocols, there is a need for quality control such as visual inspection to make sure that the registration has not failed. The subvolume based protocol has applications within patient positioning using image guided radiotherapy and when using multiple imaging for treatment response evaluation.
The MR-MR subvolume based registration protocol described in the present study performs optimally when applied to a registration subvolume with no margin added to the prostate gland. In a study by Mclaughlin et al [25] regarding subvolume based registration between MR and CT, the prostate volume with no margin did not result in a successful registration due to the lack of information in the prostate area of the CT. In this study, a 2 cm margin added to the prostate was required to ensure a successful registration.
An alternative approach is non-rigid image registration for treatment adaptation. Chao et al [26] used deformable registration to warp a narrow shell and map contours from a planning CT to CBCT images. Wang et al [27] used deformable registration over the entire volume to map contours from a planning CT to 25 repeat CTs for a prostate patient. A problem with deformable registration for image guided radiotherapy is that it requires online replanning or some other form of plan modification. There is no obvious way to interpret the deformation field into a table movement that can be applied immediately. Instead, the multi leaf collimator must be adapted to the new contour, and the dose distribution should be recalculated. This problem does not occur when using localized rigid registration since the registration transform can be readily interpreted as couch movement to reposition the patient. While online plan modification may increase the accuracy of the delivered dose, it is currently time consuming and not easily implemented in a clinical setting.
The implantation of fiducial gold markers into the prostate for localized rigid registration, while accurate if applied properly, has disadvantages compared to the proposed method of registration; it is invasive and the position of the gold markers in the MR images does not necessarily correspond to the markers actual position, depending on sequence parameters [28]. The proposed method is automatic with no need for user interaction and does not require any additional steps in the workflow. In an external radiotherapy workflow, the registration volume can simply be set to the prostate volume defined by the radio oncologist during target definition.
The resulting uncertainties from this study indicate that a standard deviation of approximately 1 mm can be achieved in an automatic procedure. Data from the CT-based study [23] indicate similar results, based on more registrations but with outlier removal, which was not performed in the current study.
Conclusions
The subvolume based rigid registration of MR scans of the prostate improves the precision significantly as compared to full volume registration. Our results indicate that the optimal registration volume is the prostate itself without any additional surrounding tissue. The subvolume based registration procedure can be applied in an image guided radiotherapy protocol and can be used for registration of repeated MR-imaging of the prostate.
References
Chen L, Price RA Jr, Nguyen TB, Wang L, Li JS, Qin L, Ding M, Palacio E, Ma CM, Pollack A: Dosimetric evaluation of MRI-based treatment planning for prostate cancer. Phys Med Biol 2004, 49: 5157-5170. 10.1088/0031-9155/49/22/010
Chen L, Price RA Jr, Wang L, Li J, Qin L, McNeeley S, Ma CM, Freedman GM, Pollack A: MRI-based treatment planning for radiotherapy: dosimetric verification for prostate IMRT. Int J Radiat Oncol Biol Phys 2004, 60: 636-647.
Jonsson JH, Karlsson MG, Karlsson M, Nyholm T: Treatment planning using MRI data: an analysis of the dose calculation accuracy for different treatment regions. Radiat Oncol 5: 62.
Lee YK, Bollet M, Charles-Edwards G, Flower MA, Leach MO, McNair H, Moore E, Rowbottom C, Webb S: Radiotherapy treatment planning of prostate cancer using magnetic resonance imaging alone. Radiother Oncol 2003, 66: 203-216. 10.1016/S0167-8140(02)00440-1
Franiel T, Ludemann L, Taupitz M, Bohmer D, Beyersdorff D: MRI before and after external beam intensity-modulated radiotherapy of patients with prostate cancer: the feasibility of monitoring of radiation-induced tissue changes using a dynamic contrast-enhanced inversion-prepared dual-contrast gradient echo sequence. Radiother Oncol 2009, 93: 241-245. 10.1016/j.radonc.2009.08.016
Lee KC, Sud S, Meyer CR, Moffat BA, Chenevert TL, Rehemtulla A, Pienta KJ, Ross BD: An imaging biomarker of early treatment response in prostate cancer that has metastasized to the bone. Cancer Res 2007, 67: 3524-3528. 10.1158/0008-5472.CAN-06-4236
Jennings D, Hatton BN, Guo J, Galons JP, Trouard TP, Raghunand N, Marshall J, Gillies RJ: Early response of prostate carcinoma xenografts to docetaxel chemotherapy monitored with diffusion MRI. Neoplasia 2002, 4: 255-262. 10.1038/sj.neo.7900225
Song I, Kim CK, Park BK, Park W: Assessment of response to radiotherapy for prostate cancer: value of diffusion-weighted MRI at 3 T. AJR Am J Roentgenol 194: W477-482.
Carroll PR, Coakley FV, Kurhanewicz J: Magnetic resonance imaging and spectroscopy of prostate cancer. Rev Urol 2006,8(Suppl 1):S4-S10.
Khoo VS, Padhani AR, Tanner SF, Finnigan DJ, Leach MO, Dearnaley DP: Comparison of MRI with CT for the radiotherapy planning of prostate cancer: a feasibility study. Br J Radiol 1999, 72: 590-597.
Nyholm T, Nyberg M, Karlsson MG, Karlsson M: Systematisation of spatial uncertainties for comparison between a MR and a CT-based radiotherapy workflow for prostate treatments. Radiat Oncol 2009, 4: 54. 10.1186/1748-717X-4-54
Kron T, Eyles D, John SL, Battista J: Magnetic resonance imaging for adaptive cobalt tomotherapy: A proposal. J Med Phys 2006, 31: 242-254. 10.4103/0971-6203.29194
Raaymakers BW, Lagendijk JJ, Overweg J, Kok JG, Raaijmakers AJ, Kerkhof EM, van der Put RW, Meijsing I, Crijns SP, Benedosso F, et al.: Integrating a 1.5 T MRI scanner with a 6 MV accelerator: proof of concept. Phys Med Biol 2009, 54: N229-237. 10.1088/0031-9155/54/12/N01
Karlsson M, Karlsson MG, Nyholm T, Amies C, Zackrisson B: Dedicated magnetic resonance imaging in the radiotherapy clinic. Int J Radiat Oncol Biol Phys 2009, 74: 644-651. 10.1016/j.ijrobp.2009.01.065
Maes F, Collignon A, Vandermeulen D, Marchal G, Suetens P: Multimodality image registration by maximization of mutual information. IEEE Trans Med Imaging 1997, 16: 187-198. 10.1109/42.563664
Pluim JP, Maintz JB, Viergever MA: Mutual-information-based registration of medical images: a survey. IEEE Trans Med Imaging 2003, 22: 986-1004. 10.1109/TMI.2003.815867
Makela T, Clarysse P, Sipila O, Pauna N, Pham QC, Katila T, Magnin IE: A review of cardiac image registration methods. IEEE Trans Med Imaging 2002, 21: 1011-1021. 10.1109/TMI.2002.804441
van Herk M: Different styles of image-guided radiotherapy. Semin Radiat Oncol 2007, 17: 258-267. 10.1016/j.semradonc.2007.07.003
Coselmon MM, Balter JM, McShan DL, Kessler ML: Mutual information based CT registration of the lung at exhale and inhale breathing states using thin-plate splines. Med Phys 2004, 31: 2942-2948. 10.1118/1.1803671
Ellingsen LM, Chintalapani G, Taylor RH, Prince JL: Robust deformable image registration using prior shape information for atlas to patient registration. Comput Med Imaging Graph 34: 79-90.
van der Put RW, Kerkhof EM, Raaymakers BW, Jurgenliemk-Schulz IM, Lagendijk JJ: Contour propagation in MRI-guided radiotherapy treatment of cervical cancer: the accuracy of rigid, non-rigid and semi-automatic registrations. Phys Med Biol 2009, 54: 7135-7150. 10.1088/0031-9155/54/23/007
Balter JM, Sandler HM, Lam K, Bree RL, Lichter AS, ten Haken RK: Measurement of prostate movement over the course of routine radiotherapy using implanted markers. Int J Radiat Oncol Biol Phys 1995, 31: 113-118. 10.1016/0360-3016(94)00382-U
Smitsmans MH, Wolthaus JW, Artignan X, de Bois J, Jaffray DA, Lebesque JV, van Herk M: Automatic localization of the prostate for on-line or off-line image-guided radiotherapy. Int J Radiat Oncol Biol Phys 2004, 60: 623-635.
Smitsmans MH, de Bois J, Sonke JJ, Betgen A, Zijp LJ, Jaffray DA, Lebesque JV, van Herk M: Automatic prostate localization on cone-beam CT scans for high precision image-guided radiotherapy. Int J Radiat Oncol Biol Phys 2005, 63: 975-984. 10.1016/j.ijrobp.2005.07.973
McLaughlin PW, Narayana V, Kessler M, McShan D, Troyer S, Marsh L, Hixson G, Roberson PL: The use of mutual information in registration of CT and MRI datasets post permanent implant. Brachytherapy 2004, 3: 61-70. 10.1016/j.brachy.2004.06.001
Chao M, Xie Y, Xing L: Auto-propagation of contours for adaptive prostate radiation therapy. Phys Med Biol 2008, 53: 4533-4542. 10.1088/0031-9155/53/17/005
Wang H, Garden AS, Zhang L, Wei X, Ahamad A, Kuban DA, Komaki R, O'Daniel J, Zhang Y, Mohan R, Dong L: Performance evaluation of automatic anatomy segmentation algorithm on repeat or four-dimensional computed tomography images using deformable image registration method. Int J Radiat Oncol Biol Phys 2008, 72: 210-219. 10.1016/j.ijrobp.2008.05.008
Jonsson JH, Garpebring A, Karlsson MG, Nyholm T: Internal Fiducial Markers and Susceptibility Effects in MRI-Simulation and Measurement of Spatial Accuracy. Int J Radiat Oncol Biol Phys 2011, in press.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
JJ gathered the data, delineated the contours in collaboration with KS, created software needed for the study and drafted the manuscript. PB and AG aided in the creation of the registration software. MK participated in the design and coordination of the study. TN conceived the study and helped draft the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Jonsson, J.H., Brynolfsson, P., Garpebring, A. et al. Registration accuracy for MR images of the prostate using a subvolume based registration protocol. Radiat Oncol 6, 73 (2011). https://doi.org/10.1186/1748-717X-6-73
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1748-717X-6-73