Abstract
Background
Neoadjuvant chemoradiotherapy is the treatment of choice in advanced rectal cancer, even though there are many patients who will not benefit from it. There are still no effective methods for predicting which patients will respond or not. The present study aimed to define the genomic profile of rectal tumors and to identify alterations that are predictive of response in order to optimize therapeutic strategies.
Methods
Forty-eight candidates for neoadjuvant chemoradiotherapy were recruited and their pretherapy biopsies analyzed by array Comparative Genomic Hybridization (aCGH). Pathologic response was evaluated by tumor regression grade.
Results
Both Hidden Markov Model and Smoothing approaches identified similar alterations, with a prevalence of DNA gains. Non responsive patients had a different alteration profile from responsive ones, with a higher number of genome changes mainly located on 2q21, 3q29, 7p22-21, 7q21, 7q36, 8q23-24, 10p14-13, 13q12, 13q31-34, 16p13, 17p13-12 and 18q23 chromosomal regions.
Conclusions
This exploratory study suggests that an in depth characterization of chromosomal alterations by aCGH would provide useful predictive information on response to neoadjuvant chemoradiotherapy and could help to optimize therapy in rectal cancer patients.
The data discussed in this study are available on the NCBI Gene Expression Omnibus [GEO: GSE25885].
Similar content being viewed by others
Background
The benefits of neoadjuvant chemoradiotherapy (NCRT) in rectal cancer are well documented. In particular, preoperative treatment is indicated to downsize tumors in order to achieve tumor-free margins, reduce tumor burden and increase the possibility of conservative surgery, which results in a high rate of sphincter preservation and significant improvement in local disease control and survival [1, 2]. However, although complete pathologic response rates of 10-25% can be achieved, more than one third of patients either do not respond or show only modest response to treatment [3].
Whilst numerous studies have analyzed the correlation between expression levels of candidate genes and response to therapies [4, 5], the predictive role of such genes is controversial and there is still no firm evidence upon which to base treatment strategies [6]. The gene expression profile evaluated by cDNA microarray has recently been found to provide indications about response of rectal tumors to NCRT [7–9], but such preliminary findings require confirmation in larger patient cohorts.
It is well known that the altered transcription of genes frequently depends on genomic copy number changes, such as deletion of one or both alleles of tumor suppressor genes, amplification of oncogenes or other rearrangements [10, 11]. Although several basic research studies have highlighted the presence of non random patterns of DNA alterations in colorectal cancer [12–15], almost none of these alterations have been analyzed as predictive markers of response to clinical treatment, especially in rectal cancer. It was established only recently that genomic imbalances detected by metaphase Comparative Genomic Hybridization (CGH) could be of value for response prediction [16]. With respect to this technique, higher resolution mapping of chromosomal copy number changes can be achieved by array CGH (aCGH), a technique capably of accurately identifying even small variations in genomic DNA sequence [17, 18].
The main objective of the present study was to define the molecular profile of rectal cancers in order to identify markers that are predictive of response to NCRT. The acquisition of more detailed genomic information would optimize treatment planning and lead to improved clinical and cost benefits.
Methods
Patients, samples and treatment
A series of 51 consecutive patients with a confirmed diagnosis of rectal adenocarcinoma localized in the mid-low rectum (up to 12 cm from the anal verge) and who were candidates for NCRT were considered eligible. The study was approved by the Local Ethics Committee, in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. All patients gave their written informed consent.
After pretherapeutic staging with a computerised tomography scan and also, in the majority of cases (> 80%), with endorectal ultrasonography, all patients were treated with a total dose of 50.4 Gy for 5-6 weeks with conventional fractionation. A daily dose of 225 mg/m2 of 5-fluorouracil was infused by central catheter during radiotherapy. Surgery was planned 6-8 weeks after completion of chemoradiotherapy. The median duration of the interval between the day after the end of therapy and surgery was 52.3 ± 10.9 days (range 40-91). Compliance to treatment was good as only 6% (n = 3) of enrolled patients were excluded because of high toxicity.
Blood samples and tumor biopsies were collected from patients before therapy. Two biopsies from tumor areas were taken from each patient; the first was used to obtain histopathologic confirmation of tumor diagnosis, while the second was immediately stored at -80°C and, after microscopic verification of the presence of > 70% of tumor cells in the former, used for genomic profile determination. The pathologic response to NCRT was evaluated using the tumor regression grade (TRG) classification, according to the criteria proposed by Dworak [19].
Immunohistochemistry
In parallel, two conventional markers of proliferative (Ki67) and apoptotic (p21WAF1) processes were determined by immunohistochemical (IHC) methods using the following primary antibodies: anti-Ki67 (clone MM1; Leica Microsystems, Heidelberg, Germany; working dilution 1:100) and anti-p21WAF1 (clone DC-60.2; Neomarkers, Fremont, CA, USA; working dilution 1:50). For antigen retrieval, sections were treated with 10 mM citrate (pH 6.0) at 98°C for 40 min and were then immunostained with LSAB+ System-HRP Kit (Dako, Carpinteria, CA, USA) according to the manufacturer's specifications. For Ki67, all washes were performed with TBS rather than PBS. Both antibodies were visualized by diaminobenzidine. Two independent observers with no prior knowledge of clinicopathologic data performed blinded immunohistochemical analysis. At least 500 cells were evaluated in representative microscopic fields and results were expressed as a percentage of cell showing nuclear Ki67 or p21WAF1 staining.
Array comparative genomic hybridization (aCGH)
Whole genome CGH arrays (Cytochip, Bluegnome, Cambridge, UK), which cover the entire human genome at a 1-Mb resolution and, in subtelomeric regions, at a median 250-Kb resolution, were used for the analysis. Each clone in the array was spotted in quadruplicate. Genomic DNA was isolated from tumor tissue using QIAamp DNA MiniKit (Qiagen, Hilden, Germany). A pool of normal female or male genomic DNA from healthy individuals was used as a reference, depending on patient gender. Approximately 1.2 μg of DNA was labelled by random priming (BioPrime Labeling System, Invitrogen, Milan, Italy), using 1.4 μl of 1 mmol/L Cy3-dCTP or Cy5-dCTP (Perkin-Elmer Life Sciences, Waltham, MA, USA) according to the manufacturer's instructions. Equal amounts of labelled tumor and reference DNA were mixed and unincorporated fluorochromes were removed using Bioprime Array-CGH Purification Module (Invitrogen). DNA was then precipitated with 140 μl of blocking mix (TechnoGenetics-Bouty, Milan, Italy), 6 μl of yeast tRNA 10 mg/ml (Sigma-Aldrich, St. Louis, MO, USA), 0.1 V/V of 3 M sodium acetate pH 5.2 and 2 V/V of absolute ethanol. The DNA pellet was dissolved in 10 μl of distilled water and 30 μl of hybridization solution (TechnoGenetics-Bouty). After denaturation at 72°C for 10 min and incubation at 37°C for 30 min, the solution was applied to the array. Hybridization was done in a Hyb Chamber (Biorad, Hercules, CA, USA), which was then incubated at 42°C for 40 h. Finally, the slides were washed according to the supplier's instructions, dried by spinning in a centrifuge for 5 min at 1000 rpm and scanned on a VersaArray ChipReader scanner (Biorad).
Image and data analysis
Images were analyzed using BlueFuse version 3.5 software (BlueGnome), permitting an automated approach to aCGH. Spots were excluded when the quality flag was < 1, the confidence value < 0.3 or the standard deviation of quadruplicate > 0.2. Log2 ratios of spots that were not excluded were normalized using block median or block lowess approaches. Alteration analysis was performed using two different approaches: (1) aCGH-Smooth approach [20], implemented in BlueFuse version 3.5 software; (2) Hidden Markov Model (HMM) approach, implemented in the aCGH package [21–23]. Molecular analyses were performed in a blinded manner.
Statistics
Statistical analyses were carried out using SPSS (SPSS Inc, Chicago, IL, USA) and SAS (release 9.1, SAS Institute, Cary, NC, USA) statistical software. The Kruskal-Wallis non parametric test was used to assess the predictive relevance of genomic instability, expressed as the fraction of genome altered (FGA) in relation to response to therapy in different stage and age subgroups. The Pearson chi-square test and Fisher's exact test were used to analyze the relation between patient characteristics and response to NCRT, defined according to TRG criteria, and the difference in the frequency of altered regions among response groups, respectively.
Results
Clinical parameters and pathologic response to NCRT
Information on patient and tumor characteristics and on response to NCRT is shown in Table 1. On the basis of TRG criteria proposed by Dworak, 13 (27%) patients reached TRG4, ie, complete tumor regression; 8 (17%), 17 (35%) and 9 (19%) reached TRG3, TRG2, and TRG1, respectively. Only 1 (2%) case was classified as TRG0, ie, did not show any pathologic response. To further simplify the analysis and summarize clinically relevant results, patients were grouped into two subsets, as already done by other authors [7]: those who achieved TRG0-2 (56%) were defined as non responders, while patients who obtained TRG3-4 (44%) were considered responders. Analysis of the relationship between clinical pretreatment parameters and pathologic response did not reveal any significant association. However, a significant correlation (p = 0.0004) was found between TRG and ypT, with 57% of responsive patients who were ypT0 and 52% of non responsive patients who were ypT3. Conversely, no association was found between ypN and TRG.
Ki67 and p21WAF1 expression and pathologic response to NCRT
Ki67 and p21WAF1 expression was determined in 35/48 (73%) and 34/48 (71%) of cases, respectively, due to insufficient bioptic material. Median Ki67 index was 69% in the overall series (range 23-93) and was similar in responsive (69%; range 23-85) and non-responsive (69.5%; range 29-93) patients. With regard to p21WAF1, the median value of positive cells was 4% (range 0-46), specifically 5.5% (range 0-17) in the responsive group and 2.5% (range 0-46) in the non responsive group. No correlations were observed between pathologic response and Ki67 or p21WAF1 expression.
Genomic profile and pathologic response to NCRT
Validation of aCGH approach
The performance and reproducibility of our aCGH approach was validated by dye swap and normal/normal experiments (data not shown). Specifically, we performed three array CGH analyses of normal female (XX) DNA against normal male (XY) DNA extracted from blood samples. No copy number changes were detected apart from sexual chromosomes. As expected, the log2 values of the average normalized fluorescence ratios for all the autosomal loci showed two copies, whereas the fluorescence ratios for the chromosomes X and Y loci indicated the gain and loss of one DNA copy, respectively. These experiments also permitted us to establish the best thresholds for significant DNA alterations (loss: log2 ratios < - 0.3219; gain: log2 ratios > 0.2630).
HMM analysis and unsupervised hierarchical clustering
The FGA identified by HMM analysis was 4.19% ± 7% (range 1.7-29%), with FGA-gain of 2.4% ± 4.4% (range 0.47-18.1%) and FGA-loss of 1.8% ± 3.2% (range 0.2 -15.6%). No significant associations were observed between FGA and pathologic response, other clinical parameters, or Ki67/p21WAF1 expression.
The loss of genomic copies was more frequent on chromosome arms 1p, 8p, 17p, 18p and 18q, and 22q, while chromosome regions on 7p and 7q, 8q,13q, and 20p and 20q were frequently amplified (Figure 1). Small regions of relevant copy number changes (log2 ratio > 0.9 for high-level gain and log2 ratio < -0.75 for high-magnitude deletions) were also observed. High-level gain was observed in 10 tumors, 9 of which were non responsive according to TRG classification, and occurred most frequently in chromosome 7 (4/10), 13 (4/10), 17 (3/10) and 20 (5/10).
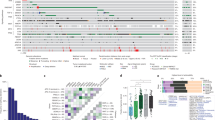
Unsupervised hierarchical clustering of the 48 tumors showed molecular heterogeneity and 5 well-defined clusters (1-5) were identified (Figure 2). The noteworthy peculiarity among the clusters was the difference in response to NCRT. The three main clusters (1, 2 and 4) included 83% of cases. In cluster 1 (21% of cases), 70% of patients were responders on the basis of TRG, while in clusters 2 (33%) and 4 (29%), 43% and 29% of patients responded to NCRT, respectively (p < 0.0001). If only patients with extreme differences in response to NCRT (TRG4 versus TRG0-1) were considered, for clusters 1, 2 and 4 the total number of patients that reached TRG4 were 40%, 25% and 21%, respectively (p = 0.0005). We also performed supervised clustering but did not identify any specific signature, possibly because of the small number of cases (data not shown).
Unsupervised hierarchical clustering of 48 rectal cancers analyzed by HMM. Clones are shown in chromosomal order from 1ptel to 22qtel (red, gain; green, loss; yellow spots, high level gain). Tumor regression grade (TRG) classification: responders (TRG3-4) yellow; non responders (TRG0-2) black. Extreme response classes are reported in the upper line: TRG4 yellow and TRG0-1 black. ypT status: ypT0-1green; ypT2 orange and ypT3 red. ypN status: ypN0 green, ypN1 red and ypNx white.
Smoothing analysis
Smoothing analysis of all DNA copy number changes showed that the FGA was 1.33% ± 4% (range 0-14.6%). The most frequent alteration was the gain of genomic regions. In our tumor series, a total of 3888 clones were gained (FGA-gain 1.17% ± 3.7%; range 0-14.6%) and 517 were lost (FGA-loss 0.16% ± 0.8%; range 0-3%). Some alterations involved entire or extensive portions of chromosome arms and mainly affected the long arm of chromosomes 13 and 20, with a 31% and 38% gain frequency, respectively. Other common alterations were detected on specific genomic regions, e.g. on 7p22-21 and 8q12, which were amplified in about 13% and 16% of patients, respectively. An average of 80 clones showed high-level alterations, mainly gain, with a high percentage (49%) located on chromosome 20q.
The analysis of the relationship between FGA and response to NCRT according to TRG did not reach statistical significance. However, the evaluation of tumor aCGH profiles clearly showed differences between the two response groups with regard to type and/or frequency of DNA alterations at specific chromosomal regions (2q21, 3q29, 7p22-21, 7q21, 7q36, 8q23-24, 10p14-13, 13q12, 13q31-34, 16p13, 17p13-12, 18q23) (Table 2). Among the most altered clones were those mapping in 2q21.1, which were amplified in non responsive patients and deleted in responsive ones. A number of clones were altered in only responsive or non responsive patients, such as clones in 7q21.11, amplified in 10% of responsive patients, or clones in 7q36.1-3 or 16p13.3 regions, amplified in 15% and 11% of non responsive patients, respectively. Finally, high-level alterations were distributed differently with respect to response to NCRT, with 85% of patients who showed this kind of alteration in the group of non responsive cases.
Genomic profile and pathologic TNM after NCRT
Information on pathologic stage and lymph node status after NCRT (ypT and ypN) was available for 47/48 patients. Among these, 30% had ypT0 while 13%, 23% and 34% had ypT1, ypT2, ypT3 respectively. With regard to ypN, 78% of patients were ypN0, whereas 22% had a lymph node involvement at the time of surgery.
FGA was significantly different in tumors that showed different pathologic stages after NCRT, with a higher frequency of alterations in tumors that had an advanced T stage (p < 0.05). With respect to ypT stage, the five groups identified by hierarchical clustering showed a different frequency of ypT0-1, ypT2 and ypT3 tumors. In particular, grouping together clusters 1 and 2 (left side of Figure 2), and clusters 3, 4 and 5 (right side of Figure 2), the percentage of different ypT-stage tumors was as follows: 56% and 27% of ypT0-1, 16% and 32% of ypT2, and 28% and 41% of ypT3, respectively. This difference was statistically significant (p < 0.0001). No association was observed between FGA and ypN, and no differences were noted between clusters with regard to ypN status.
Discussion
Neoadjuvant chemoradiotherapy followed by surgery is accepted as the therapy of choice in rectal cancer. However, although good results have been obtained with current treatment strategies, clinically identical tumors sometimes differ in their response, and more personalized treatments would undoubtedly yield greater benefits [24].
The two most widely adopted systems of assessing response are tumor regression grade (TRG), according to Mandard or Dworak criteria, and downstaging. However, there is still no general consensus as to which one is the most accurate and the most clinically relevant for patient prognosis [7, 25–28]. In our study, we adopted the TRG system proposed by Dworak [19] (the response classification system used by our pathologists), grouping TRG 0-2 tumors as non responders and TRG 3-4 tumors as responders. This type of division has also been proposed by numerous other authors [19, 7, 25] and has been acknowledged as having a prognostic impact [25].
We did not find any significant correlation between pathologic response to NCRT and clinical pretreatment parameters. In addition, the small number of patients evaluated prevents us from drawing any definitive conclusions about the correlation between Ki67 and p21WAF1 expression (markers involved in cell cycle control and proliferation) and TRG. We did, however, observe a higher median value of p21WAF1 protein expression in responsive tumors.
Although several studies have investigated colorectal cancer by the aCGH approach to better characterize the alterations of this tumor [29–31], few have focused on rectal cancer [32]. Furthermore, to our knowledge, the only study analyzing rectal cancer genomic imbalances in relation to response used metaphase CGH and concluded that the probability of detecting copy number changes by chance was high [16]. In our case series, analysis of tumor DNA using higher resolution scanning with aCGH showed that the fraction of altered genome obtained using the HMM approach (4.19%) was slightly higher than that obtained by Smoothing analysis (1.33%); this is to be expected as the latter considers only larger alterations. However, both approaches appear to identify similar alterations, with a prevalence of DNA gains.
According to the literature [12–14, 16, 29–32], the most frequent DNA alterations specific for this tumor were gains of chromosome arms 7p and 7q, 8q, 13q, 20p and 20q, and losses in 1p, 8p, 17p, 18p and 18q and 22q. In our case-series, 38% of tumors showed a gain at 20q chromosome arm, where multiple candidate oncogenes associated with increased proliferative activity, reduced survival and progression of colorectal cancer (e.g. ZNF217, CYP24 and AURKA), are mapped [12, 33].
With regard to differences in genomic alterations between responsive and non responsive tumors, whether revealed by HMM or Smoothing analyses, our results showed that a higher number of genome changes was associated, albeit not significantly, with resistance to treatment, in contrast to findings published by Grade and colleagues [16].
The overall difference in genomic instability between responders and non responders could be due to the fact that gene drivers of genetic alterations, i.e. genes involved in DNA repair, mitotic checkpoints and carcinogen detoxification, are frequently also involved in the response to therapies which act by targeting proliferating cells and damaging DNA. In our case series, however, the association between FGA and pathologic response was not significant, probably owing to the small number of patients enrolled and to the complex pathways involved in genomic stability which may themselves influence response to therapy.
Our clustering analysis indicates a difference in response to NCRT among patients with a specific DNA alteration pattern. In particular, in cluster 1 we observed 70% of responders, whereas in cluster 4 more than 70% of patients were non responders. Even considering only the extreme response classes (TRG4 as responders versus TRG0-1 as non responders), there is a similar distribution of TRG4 in clusters 1, 2 and 4. Moreover, in view of the important role played by final pathologic stage in determining prognosis, we also took into account the relationships between ypTNM, response to therapy and genomic alterations. A significant correlation was found between TRG and ypT, as well as a significant association between HMM-detected FGA and ypT stage. In fact, a higher number of DNA alterations in pretreatment tumors was associated with higher pathologic tumor stage obtained after NCRT. In the hierarchical clustering was confirmed a statistically different frequency of ypT: in particular, in clusters 1 and 2 (46% of non responders), the vast majority of cases were ypT0-T1, whereas in clusters 3-5 (68% of non responders), ypT3 tumors were predominant (p < 0.0001).
In our case series, specific chromosomal regions presenting alterations capable of distinguishing between responsive and non responsive tumors, e.g. 2q21, 3q29, 7p22-21, 7q21, 7q36, 8q23-24, 10p14-13, 13q12, 13q31-34, 16p13, 17p13-12 and 18q23 were observed. Several alterations, such as that in the 7q36.1-7q36.3 region (sometimes amplified in non responsive patients), appear interesting as a number of important genes map there; these genes are mainly involved in transcription, cell-cell signalling, chromosome stability and DNA repair, cell growth, differentiation and oncogenic transformation, e.g. MLL3 and XRCC2, known to be altered in colorectal cancer [34, 35]. We also observed fairly marked differences in chromosome 13 alterations between tumors from patients who benefited from NCRT and those from patients who did not. In fact, both HMM and Smoothing approaches identified a gain of several clones spanning the 13q31-34 region in numerous patients, the vast majority of whom were not responsive to treatment.
The preferential gain of 13q regions in non responders is of particular interest because of the relevance of a number of genes located within these regions, e.g. Wave3, which contributes to tumor cell invasion and metastasis [36]; XPO4, an exportin that plays a primary role in the nucleocytoplasmic shuttling of Smad3 [37]; and APC6/CDC16, a subunit of the anaphase-promoting complex in which amplification and overexpression may lead to chromosomal instability [38].
Furthermore, genes that play a role in the control of cell division and growth regulation, migration and chromosomal instability (GPC5, GPC6, HS6ST3) map in these chromosomal bands [39, 40]. Two other interesting genes, CLDN10 and ABCC4, localized in 13q31-34, may be involved in determining resistance to therapy. The former encodes a member of the claudin family, integral membrane proteins and components of tight junction strands associated with cell invasion and migration [41]. The latter gene, ABCC4/MRP4, belongs to the ABC-type multidrug transporter family that mediates drug resistance by energy-dependent drug efflux from cells and seems to be implicated in resistance to purine analogs and to other nucleoside-based antiviral drugs [42, 43].
Another interesting region, deleted in some non responsive patients of our case series, is localized at 17p12.1. LLGL1, a candidate tumor suppressor gene involved in the maintenance of epithelial integrity through its cytoskeletal interactions, maps in this chromosomal band. As highlighted in some studies, LLGL1 is deleted preferentially in CIN-type tumors [30], and the downregulation of this gene would seem to contribute to colorectal cancer progression [44].
It is known that there is often a correlation between genomic copy number and gene expression levels as DNA imbalances seem to have a direct effect on the deregulation of the transcriptional profile of cancer cells [45]. Gene expression signature studies in rectal cancer patients subjected to neoadjuvant therapy have shown that different approaches yield different gene sets which may be predictive of response. Genes involved in common molecular pathways and cellular processes have been identified, including those involved in DNA damage repair, microtubule organization, apoptosis, transcription, cell growth, signal transduction, drug metabolism, and transport functions [7–9, 46]. Our results are in line with such findings as the main differences in DNA copy number between responsive and non responsive patients were observed in genes involved in DNA damage response, transcription, cell cycle and mitosis regulation, excretion, intercellular junction assembly and calcium-independent cell-cell adhesion pathways.
Conclusions
The results from the present study indicate that the aCGH technique could be a potentially useful tool to better characterize rectal cancer. Our findings also suggest that genome-wide profiling could be used to distinguish between patients who respond to NCRT and those who do not, although further genomic studies in larger patient cohorts are needed to better classify the differences in DNA copy number changes between responsive and non responsive patients. Research is ongoing to confirm these preliminary data.
Abbreviations
- NCRT:
-
neoadjuvant chemoradiotherapy
- aCGH:
-
array comparative genomic hybridization
- TRG:
-
tumor regression grade
- HMM:
-
Hidden Markov Model
- FGA:
-
fraction of genome altered.
References
Bosset JF, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A, Briffaux A, Collette L: Enhanced tumorocidal effect of chemotherapy with preoperative radiotherapy for rectal cancer: preliminary results--EORTC 22921. J Clin Oncol 2005, 23: 5620-5627. 10.1200/JCO.2005.02.113
Roh MS, Colangelo LH, O'Connell MJ, Yothers G, Deutsch M, Allegra CJ, Kahlenberg MS, Baez-Diaz L, Ursiny CS, Petrelli NJ, Wolmark N: Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol 2009, 27: 5124-5130. 10.1200/JCO.2009.22.0467
Balch GC, De Meo A, Guillem JG: Modern management of rectal cancer: a 2006 update. World J Gastroenterol 2006, 12: 3186-3195.
Jakob C, Aust DE, Meyer W, Baretton GB, Schwabe W, Häusler P, Becker H, Liersch T: Thymidylate synthase, thymidine phosphorylase, dihydropyrimidine dehydrogenase expression, and histological tumour regression after 5-FU-based neo-adjuvant chemoradiotherapy in rectal cancer. J Pathol 2004, 204: 562-568. 10.1002/path.1663
Jakob C, Liersch T, Meyer W, Becker H, Baretton GB, Aust DE: Predictive value of Ki67 and p53 in locally advanced rectal cancer: correlation with thymidylate synthase and histopathological tumor regression after neoadjuvant 5-FU-based chemoradiotherapy. World J Gastroenterol 2008, 14: 1060-1066. 10.3748/wjg.14.1060
Kuremsky JG, Tepper JE, McLeod HL: Biomarkers for response to neoadjuvant chemoradiation for rectal cancer. Int J Radiat Oncol Biol Phys 2009, 74: 673-688. 10.1016/j.ijrobp.2009.03.003
Ghadimi BM, Grade M, Difilippantonio MJ, Varma S, Simon R, Montagna C, Füzesi L, Langer C, Becker H, Liersch T, Ried T: Effectiveness of gene expression profiling for response prediction of rectal adenocarcinomas to preoperative chemoradiotherapy. J Clin Oncol 2005, 23: 1826-1838. 10.1200/JCO.2005.00.406
Kim IJ, Lim SB, Kang HC, Chang HJ, Ahn SA, Park HW, Jang SG, Park JH, Kim DY, Jung KH, Choi HS, Jeong SY, Sohn DK, Kim DW, Park JG: Microarray gene expression profiling for predicting complete response to preoperative chemoradiotherapy in patients with advanced rectal cancer. Dis Colon Rectum 2007, 50: 1342-1353. 10.1007/s10350-007-277-7
Rimkus C, Friederichs J, Boulesteix AL, Theisen J, Mages J, Becker K, Nekarda H, Rosenberg R, Janssen KP, Siewert JR: Microarray-based prediction of tumor response to neoadjuvant radiochemotherapy of patients with locally advanced rectal cancer. Clin Gastroenterol Hepatol 2008, 6: 53-61. 10.1016/j.cgh.2007.10.022
Albertson DG: Gene amplification in cancer. Trends Genet 2006, 22: 447-455. 10.1016/j.tig.2006.06.007
Grade M, Ghadimi BM, Varma S, Simon R, Wangsa D, Barenboim-Stapleton L, Liersch T, Becker H, Ried T, Difilippantonio MJ: Aneuploidy-dependent massive deregulation of the cellular transcriptome and apparent divergence of the Wnt/beta-catenin signaling pathway in human rectal carcinomas. Cancer Res 2006, 66: 267-282. 10.1158/0008-5472.CAN-05-2533
Diep CB, Kleivi K, Ribeiro FR, Teixeira MR, Lindgjaerde OC, Lothe RA: The order of genetic events associated with colorectal cancer progression inferred from meta-analysis of copy number changes. Genes Chromosomes Cancer 2006, 45: 31-41. 10.1002/gcc.20261
Douglas EJ, Fiegler H, Rowan A, Halford S, Bicknell DC, Bodmer W, Tomlinson IP, Carter NP: Array comparative genomic hybridization analysis of colorectal cancer cell lines and primary carcinomas. Cancer Res 2004, 64: 4817-4825. 10.1158/0008-5472.CAN-04-0328
Gaasenbeek M, Howarth K, Rowan AJ, Gorman PA, Jones A, Chaplin T, Liu Y, Bicknell D, Davison EJ, Fiegler H, Carter NP, Roylance RR, Tomlinson IP: Combined array-comparative genomic hybridization and single-nucleotide polymorphism-loss of heterozygosity analysis reveals complex changes and multiple forms of chromosomal instability in colorectal cancers. Cancer Res 2006, 66: 3471-3479. 10.1158/0008-5472.CAN-05-3285
Sugai T, Takahashi H, Habano W, Nakamura S, Sato K, Orii S, Suzuki K: Analysis of genetic alterations, classified according to their DNA ploidy pattern, in the progression of colorectal adenomas and early colorectal carcinomas. J Pathol 2003, 200: 168-176. 10.1002/path.1340
Grade M, Gaedcke J, Wangsa D, Varma S, Beckmann J, Liersch T, Hess C, Becker H, Difilippantonio MJ, Ried T, Ghadimi BM: Chromosomal copy number changes of locally advanced rectal cancers treated with preoperative chemoradiotherapy. Cancer Genet Cytogenet 2009, 193: 19-28. 10.1016/j.cancergencyto.2009.03.016
Lockwood WW, Chari R, Chi B, Lam WL: Recent advances in array comparative genomic hybridization technologies and their applications in human genetics. Eur J Hum Genet 2006, 14: 139-148. 10.1038/sj.ejhg.5201531
Pinkel D, Albertson DG: Array comparative genomic hybridization and its applications in cancer. Nat Genet 2005,37(Suppl):S11-17.
Dworak O, Keilholz L, Hoffmann A: Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis 1997, 12: 19-23. 10.1007/s003840050072
Jong K, Marchiori E, Meijer G, Vaart AV, Ylstra B: Breakpoint identification and smoothing of array comparative genomic hybridization data. Bioinformatics 2004, 20: 3636-3637. 10.1093/bioinformatics/bth355
Fridlyand J, Dimitrov P:Package: aCGH. Accessed 11.05.11 [http://www.bioconductor.org/packages/2.8/bioc/html/aCGH.html]
Fridlyand J, Snijders A, Pinkel D, Albertson DG, Jain AN: Hidden Markov Model approach to the analysis of array CGH data. J Multivariate Anal 2004, 90: 132-153. 10.1016/j.jmva.2004.02.008
R Development Core Team (2008): A language and environment for statistical computingR Foundation for Statistical Computing, Vienna, Austria; 2008. [http://www.R-project.org]
Weiss J, Moghanaki D, Plastaras JP, Haller DG: Improved patient and regimen selection in locally advanced rectal cancer: who, how, and what next? Clin Colorectal Cancer 2009, 8: 194-199. 10.3816/CCC.2009.n.033
Losi L, Luppi G, Gavioli M, Iachetta F, Bertolini F, D'Amico R, Jovic G, Bertoni F, Falchi AM, Conte PF: Prognostic value of Dworak grade of regression (GR) in patients with rectal carcinoma treated with preoperative radiochemotherapy. Int J Colorectal Dis 2006, 21: 645-651. 10.1007/s00384-005-0061-x
Vironen J, Juhola M, Kairaluoma M, Jantunen I, Kellokumpu I: Tumour regression grading in the evaluation of tumour response after different preoperative radiotherapy treatments for rectal carcinoma. Int J Colorectal Dis 2005, 20: 440-445. 10.1007/s00384-004-0733-y
Quah HM, Chou JF, Gonen M, Shia J, Schrag D, Saltz LB, Goodman KA, Minsky BD, Wong WD, Weiser MR: Pathologic stage is most prognostic of disease-free survival in locally advanced rectal cancer patients after preoperative chemoradiation. Cancer 2008, 113: 57-64. 10.1002/cncr.23516
Bujko K, Kolodziejczyk M, Nasierowska-Guttmejer A, Michalski W, Kepka L, Chmielik E, Wojnar A, Chwalinski M, Polish Colorectal Cancer Group: Tumour regression grading in patients with residual rectal cancer after preoperative chemoradiation. Radiother Oncol 95: 298-302.
Camps J, Grade M, Nguyen QT, Hörmann P, Becker S, Hummon AB, Rodriguez V, Chandrasekharappa S, Chen Y, Difilippantonio MJ, Becker H, Ghadimi BM, Ried T: Chromosomal breakpoints in primary colon cancer cluster at sites of structural variants in the genome. Cancer Res 2008, 68: 1284-1295. 10.1158/0008-5472.CAN-07-2864
Lassmann S, Weis R, Makowiec F, Roth J, Danciu M, Hopt U, Werner M: Array CGH identifies distinct DNA copy number profiles of oncogenes and tumor suppressor genes in chromosomal- and microsatellite-unstable sporadic colorectal carcinomas. J Mol Med 2007, 85: 293-304. 10.1007/s00109-006-0126-5
Nakao K, Mehta KR, Fridlyand J, Moore DH, Jain AN, Lafuente A, Wiencke JW, Terdiman JP, Waldman FM: High-resolution analysis of DNA copy number alterations in colorectal cancer by array-based comparative genomic hybridization. Carcinogenesis 2004, 25: 1345-1357. 10.1093/carcin/bgh134
Lips EH, de Graaf EJ, Tollenaar RA, van Eijk R, Oosting J, Szuhai K, Karsten T, Nanya Y, Ogawa S, van de Velde CJ, Eilers PH, van Wezel T, Morreau H: Single nucleotide polymorphism array analysis of chromosomal instability patterns discriminates rectal adenomas from carcinomas. J Pathol 2007, 212: 269-277. 10.1002/path.2180
Korn WM, Yasutake T, Kuo WL, Warren RS, Collins C, Tomita M, Gray J, Waldman FM: Chromosome arm 20q gains and other genomic alterations in colorectal cancer metastatic to liver, as analyzed by comparative genomic hybridization and fluorescence in situ hybridization. Genes Chromosomes Cancer 1999, 25: 82-90. 10.1002/(SICI)1098-2264(199906)25:2<82::AID-GCC2>3.0.CO;2-6
Ashktorab H, Schaffer AA, Daremipouran M, Smoot DT, Lee E, Brim H: Distinct genetic alterations in colorectal cancer. PLoS One 2010, 5: e8879. 10.1371/journal.pone.0008879
Curtin K, Lin WY, George R, Katory M, Shorto J, Cannon-Albright LA, Smith G, Bishop DT, Cox A, Camp NJ: Genetic variants in XRCC2: new insights into colorectal cancer tumorigenesis. Cancer Epidemiol Biomarkers Prev 2009, 18: 2476-2484. 10.1158/1055-9965.EPI-09-0187
Sossey-Alaoui K, Safina A, Li X, Vaughan MM, Hicks DG, Bakin AV, Cowell JK: Down-regulation of WAVE3, a metastasis promoter gene, inhibits invasion and metastasis of breast cancer cells. Am J Pathol 2007, 170: 2112-2121. 10.2353/ajpath.2007.060975
Kurisaki A, Kurisaki K, Kowanetz M, Sugino H, Yoneda Y, Heldin CH, Moustakas A: The mechanism of nuclear export of Smad3 involves exportin 4 and Ran. Mol Cell Biol 2006, 26: 1318-1332. 10.1128/MCB.26.4.1318-1332.2006
Wang Q, Moyret-Lalle C, Couzon F, Surbiguet-Clippe C, Saurin JC, Lorca T, Navarro C, Puisieux A: Alterations of anaphase-promoting complex genes in human colon cancer cells. Oncogene 2003, 22: 1486-1490. 10.1038/sj.onc.1206224
Habuchi H, Tanaka M, Habuchi O, Yoshida K, Suzuki H, Ban K, Kimata K: The occurrence of three isoforms of heparan sulfate 6-O-sulfotransferase having different specificities for hexuronic acid adjacent to the targeted N-sulfoglucosamine. J Biol Chem 2000, 275: 2859-2868. 10.1074/jbc.275.4.2859
Yu W, Inoue J, Imoto I, Matsuo Y, Karpas A, Inazawa J: GPC5 is a possible target for the 13q31-q32 amplification detected in lymphoma cell lines. J Hum Genet 2003, 48: 331-335.
Aldred MA, Huang Y, Liyanarachchi S, Pellegata NS, Gimm O, Jhiang S, Davuluri RV, de la Chapelle A, Eng C: Papillary and follicular thyroid carcinomas show distinctly different microarray expression profiles and can be distinguished by a minimum of five genes. J Clin Oncol 2004, 22: 3531-3539. 10.1200/JCO.2004.08.127
Ho LL, Kench JG, Handelsman DJ, Scheffer GL, Stricker PD, Grygiel JG, Sutherland RL, Henshall SM, Allen JD, Horvath LG: Androgen regulation of multidrug resistance-associated protein 4 (MRP4/ABCC4) in prostate cancer. Prostate 2008, 68: 1421-1429. 10.1002/pros.20809
Lee K, Klein-Szanto AJ, Kruh GD: Analysis of the MRP4 drug resistance profile in transfected NIH3T3 cells. J Natl Cancer Inst 2000, 92: 1934-1940. 10.1093/jnci/92.23.1934
Schimanski CC, Schmitz G, Kashyap A, Bosserhoff AK, Bataille F, Schäfer SC, Lehr HA, Berger MR, Galle PR, Strand S, Strand D: Reduced expression of Hugl-1, the human homologue of Drosophila tumour suppressor gene lgl, contributes to progression of colorectal cancer. Oncogene 2005, 24: 3100-3109. 10.1038/sj.onc.1208520
Lips EH, van Eijk R, de Graaf EJ, Oosting J, de Miranda NF, Karsten T, van de Velde CJ, Eilers PH, Tollenaar RA, van Wezel T, Morreau H: Integrating chromosomal aberrations and gene expression profiles to dissect rectal tumorigenesis. BMC Cancer 2008, 8: 314. 10.1186/1471-2407-8-314
Watanabe T, Komuro Y, Kiyomatsu T, Kanazawa T, Kazama Y, Tanaka J, Tanaka T, Yamamoto Y, Shirane M, Muto T, Nagawa H: Prediction of sensitivity of rectal cancer cells in response to preoperative radiotherapy by DNA microarray analysis of gene expression profiles. Cancer Res 2006, 66: 3370-3374. 10.1158/0008-5472.CAN-05-3834
Acknowledgements
This study was supported by a grant from the Emilia Romagna Region-University Research Program, Area 1a: Innovative research; Project Title: "Radiochemioterapia neoadiuvante nel carcinoma del retto: predittori biologici di risposta". The study sponsors did not have any involvement in the study design, in the collection, analysis and interpretation of data, in the writing of the manuscript, and in the decision to submit the manuscript for publication. The authors wish to thank Gráinne Tierney for editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
In keeping with the latest guidelines of the International Committee of Medical Journal Editors, each author's contribution to the paper has been quantified as follows: CM carried out the molecular studies, participated in the statistical analysis and drafted the manuscript; MB performed the statistical analysis; NT participated in the study design and in the enrollment of patients; MG partecipated in the enrollment of patients; WZ participated in the study design and in revising the manuscript; EE partecipated in the study design and in the enrollment of patients; EL performed the collection of samples; AP and PR participated in the enrollment of patients; LS carried out the histopathologic confirmation of tumor diagnosis, the determination of the pathological response to therapy and collaborate to immunohistochemical analysis; MG performed immunohistochemical analysis; DA conceived the study; DC participated in the methodology development, in the study design and in drafting the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Molinari, C., Ballardini, M., Teodorani, N. et al. Genomic alterations in rectal tumors and response to neoadjuvant chemoradiotherapy: an exploratory study. Radiat Oncol 6, 161 (2011). https://doi.org/10.1186/1748-717X-6-161
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1748-717X-6-161