Abstract
Objective
To assess the long-term response to add-on quetiapine therapy in patients with bipolar I disorder who were not adequately responding to standard medications.
Methods
Outpatients with bipolar I disorder (DSM-IV-TR) responding inadequately to standard treatment were observed before and after the addition of quetiapine. Symptom severity was evaluated using the Clinical Global Impressions scale for Bipolar Disorder (CGI-BP) each month. Relapses included hospitalization, treatment in a day hospital or clinic, scores ≥ 1 point higher than previous CGI-BP scores and/or upward titration of quetiapine or other medications.
Results
Sixty-one patients (age range of 18–68 years) were observed prospectively for an average of 7.5 months (range 3–18 months) prior to addition of quetiapine and subsequently followed for an average of 15.7 months (range 6–42 months). The final mean quetiapine dose was 537.1 ± 91.7 mg/d. Prior to quetiapine addition, an annual relapse rate of 2.09 episodes was recorded, relating to 0.94 depressive and 1.15 manic or mixed episodes. Following quetiapine addition, annual relapse rates were reduced to 0.61 episodes, representing 0.14 depressive and 0.46 manic or mixed episodes. Compared with the period of add-on quetiapine treatment, the relative risk of relapse prior to quetiapine therapy was 3.4 for all episodes (χ2 = 24.8, P < 0.001), 6.7 for depressive episodes (χ2 = 24.7, P < 0.001), and 2.5 for manic or mixed episodes (χ2 = 9.0, P < 0.05).
Conclusion
This naturalistic follow-up study provides preliminary evidence for the efficacy of long-term add-on quetiapine treatment in the prevention of relapses of manic or mixed and depressive episodes of bipolar I disorder, and particularly in the prevention of depressive episodes.
Similar content being viewed by others
Background
Bipolar I disorder is a severe and chronic illness characterized by episodes of mania and depression [1]. Two major challenges of treating bipolar I disorder are the high percentage of patients who do not respond to therapy and the high percentage of patients who relapse after initially responding. To address these challenges, patients are often given long-term treatment with combinations of drugs from different classes [2]. Quetiapine is an atypical antipsychotic with a superior tolerability profile to conventional antipsychotics. Large, placebo-controlled studies have shown the efficacy of quetiapine for treating both acute manic episodes (as monotherapy and combination therapy) and acute depressive episodes (as monotherapy) associated with bipolar disorder [3–6].
Objective
To assess the long-term response to add-on quetiapine therapy in patients with bipolar I disorder who were not adequately responding to standard medications.
Methods
Study design
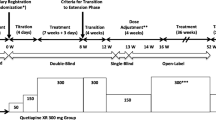
An open-label study of patients with bipolar I disorder inadequately responsive to ongoing medications who were prospectively observed for 3–18 months before receiving add-on quetiapine treatment for 6–42 months.
Study population
Adult outpatients with bipolar I disorder (based on DSM-IV-TR) [7] who had responded inadequately to prior standard treatment. Inadequate response to prior treatment was defined as a Clinical Global Impressions scale for Bipolar Disorder (CGI-BP) [8] score ≥ 3 with no improvement in score after 3 months of therapy. Patients who were pregnant or breastfeeding, or had a recent history of alcohol or drug abuse, were excluded. Written consent for the study was obtained after giving patients a complete description of the study.
Study medication
Quetiapine was added to ongoing medication at an initial dose of 25 mg/d for the first 2 days, increased to 50 mg/d for the next 2 days, and then increased by 50 mg increments every 2 days until a clinical response was observed (up to a maximum dose of 800 mg/d). This dose was then maintained throughout the remainder of the study.
Assessments
Prospective evaluations were made at least once every 2 months and no fewer than 8 times per year. Clinical response was evaluated using the CGI-BP scale [8]. The relative risk of relapse, defined as the number of relapse events per patient-year of treatment, was determined for the period before initiating quetiapine. Relapse events included hospitalization, treatment in a day hospital or clinic, or an increase of ≥ 1 in CGI-BP [8] score accompanied by a change in therapy.
Statistical methods
Mean CGI-BP scores were compared by one-way analysis of variance (ANOVA) for repeated measures. Confidence intervals for comparing the relative risks of relapse were calculated using the simplified method of Miettinen [9].
Results
Patient and treatment characteristics
Of the 61 patients, 41% were male (mean age 41.4 ± 8.2 years) and 59% were female (mean age 47.2 ± 16.9 years). Patients were prospectively observed for 3–18 months (average 7.5 months) before quetiapine therapy was added. Patients' ongoing medications are listed in Table 1. Add-on quetiapine therapy was maintained for 6–42 months (average 15.7 months) until study termination. Fourteen patients received quetiapine add-on therapy for ≥ 24 months. The final mean quetiapine dose was 537.1 ± 91.7 mg/day. Four patients discontinued the study: 1 due to adverse effects (hypotension and drowsiness) and 3 due to non-adherence after the first evaluation at 6 months.
Efficacy
Risk of relapse
The overall relapse rate decreased following the addition of quetiapine (Table 2, Figure 1). When analyzed by episode type, the relapse rates of depressive and manic/mixed episodes also decreased after adding quetiapine compared with the period before adding quetiapine (Table 2, Figure 1). Relative risks of relapse for all episodes, manic episodes, and depressive episodes prior to quetiapine treatment are shown in Table 2.
Symptom improvement
Mean change in CGI-BP score showed a significant improvement in symptoms from baseline at 6, 12, 18, and 24 months (P < 0.001; Table 3).
Tolerability
Side effects during quetiapine combination therapy (Table 4) were generally mild or moderate. Mild extrapyramidal symptoms (EPS) were reported by 4 patients (6.5%), all of whom were taking lithium or divalproex. No tardive dyskinesia was reported.
Conclusion
In patients with bipolar I disorder who had shown inadequate responses to prior standard therapy, relapse rates and symptoms were significantly improved with 6 months of add-on quetiapine therapy. These improvements were maintained in 14 patients treated for 24 months. Add-on quetiapine therapy was well tolerated, with no incidences of tardive dyskinesia reported following addition of quetiapine and only 4 patients reporting mild EPS. This naturalistic follow-up study demonstrates the efficacy of quetiapine in the prevention of relapses of manic and depressive episodes of bipolar I disorder in the long term, and particularly in the prevention of depressive episodes, which is consistent with our earlier findings [10] and with other follow-up studies concerning bipolar depression [11], bipolar depression and rapid cycling disease course [12], rapid cycling bipolar disorders [13]. These results warrant confirmation in large, randomized, placebo-controlled studies.
References
Carta MG, Angst J: Epidemiological and clinical aspects of bipolar disorders: controversies or a common need to redefine the aims and methodological aspects of surveys. Clin Pract Epidemol Ment Health. 2005, 1: 4-10.1186/1745-0179-1-4.
American Psychiatric Association: Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry. 2002, 159: 1-50. 10.1176/appi.ajp.159.1.1.
Vieta E, Mullen J, Brecher M, Paulsson B, Jones M: Quetiapine monotherapy for mania associated with bipolar disorder: combined analysis of two international, double-blind, randomized, placebo-controlled studies. Curr Med Res Opin. 2005, 21: 923-934. 10.1185/030079905X46340.
Sachs G, Chengappa K, Suppes T, et al: Quetiapine with lithium or divalproex for the treatment of bipolar mania: a randomized, double-blind, placebo-controlled study. Bipolar Disord. 2004, 6: 213-223. 10.1111/j.1399-5618.2004.00115.x.
Calabrese JR, Keck PE, Macfadden W, et al: A randomized, double-blind, placebo-controlled trial of quetiapine in the treatment of bipolar I or II depression. Am J Psychiatry. 2005, 162: 1351-1360. 10.1176/appi.ajp.162.7.1351.
Yatham LN, Paulsson B, Mullen J, Vågerö M: Quetiapine versus placebo in combination with lithium or divalproex for the treatment of bipolar mania. J Clin Psychopharmacol. 2004, 24: 599-606. 10.1097/01.jcp.0000144887.66319.2f.
American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th edn, text revision (DSM-IV-TR). 2000, Washington, DC: American Psychiatric Association
Spearing MK, Post RM, Leverich GS, Brandt D, Nolen W: Modification of the Clinical Global Impression (CGI) Scale for use in bipolar illness (BP): the CGI-BP. Psychiatry Res. 1997, 73: 159-171. 10.1016/S0165-1781(97)00123-6.
Miettinen O: Confounding and effect-modification. Am J Epidemiol. 1974, 100: 350-353.
Hardoy MC, Garofalo A, Carpiniello B, Calabrese JR, Carta MG: Combination quetiapine therapy in the long-term treatment of patients with bipolar I disorder. Clin Pract Epidemiol Ment Health. 2005, 1: 7-10.1186/1745-0179-1-7.
Milev R, Abraham G, Zaheer J: Add-on quetiapine for bipolar depression: a 12-month open-label trial. Can J Psychiatry. 2006, 51 (8): 523-30.
Vieta E, Calabrese JR, Goikolea JM, Raines S, Macfadden W, BOLDER Study Group: Quetiapine monotherapy in the treatment of patients with bipolar I or II depression and a rapid-cycling disease course: a randomized, double-blind, placebo-controlled study. Bipolar Disord. 2007, 9 (4): 413-25. 10.1111/j.1399-5618.2007.00479.x.
Goldberg JF, Kelley ME, Rosenquist KJ, Hsu DJ, Filkowski MM, Nassir Ghaemi SJ: Effectiveness of quetiapine in rapid cycling bipolar disorder: A preliminary study. J Affect Disord. 2007, 4-
Author information
Authors and Affiliations
Corresponding author
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Hardoy, M.C., Garofalo, A., Mellino, G. et al. Quetiapine as add-on treatment for bipolar I disorder: efficacy in preventing relapse of depressive episodes. Clin Pract Epidemiol Ment Health 3, 17 (2007). https://doi.org/10.1186/1745-0179-3-17
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1745-0179-3-17