Abstract
Background
Recent data suggest that an increased level of high-density lipoprotein cholesterol (HDL-C) is not causally protective against heart disease, shifting focus to other sub-phenotypes of HDL. Prior work on the effects of dietary intakes has focused largely on HDL-C. The goal of this study was to identify the dietary intakes that affect HDL-related measures: HDL-C, HDL-2, HDL-3, and apoA1 using data from a carotid artery disease case–control cohort.
Methods
A subset of 1,566 participants with extensive lipid phenotype data completed the Harvard Standardized Food Frequency Questionnaire to determine their daily micronutrient intake over the past year. Stepwise linear regression was used to separately evaluate the effects of dietary covariates on adjusted levels of HDL-C, HDL-2, HDL-3, and apoA1.
Results
Dietary folate intake was positively associated with HDL-C (p = 0.007), HDL-2 (p = 0.0011), HDL-3 (p = 0.0022), and apoA1 (p = 0.001). Alcohol intake and myristic acid (14:0), a saturated fat, were each significantly associated with increased levels of all HDL-related measures studied. Dietary carbohydrate and iron intake were significantly associated with decreased levels of all HDL-related measures. Magnesium intake was positively associated with HDL-C, HDL-2, and HDL-3 levels, but not apoA1 levels, while vitamin C was only associated with apoA1 levels. Dietary fiber and protein intake were both associated with HDL-3 levels alone.
Conclusions
This study is the first to report that dietary folate intake is associated with HDL-C, HDL-2, HDL-3, and apoA1 levels in humans. We further identify numerous dietary intake associations with apoA1, HDL-2, and HDL-3 levels. Given the shifting focus away from HDL-C, these data will prove valuable for future epidemiologic investigation of the role of diet and multiple HDL phenotypes in heart disease.
Similar content being viewed by others
Background
The strong inverse association between measures of high density lipoprotein cholesterol (HDL-C) and cardiovascular disease risk [1] has recently prompted several studies to establish the role of HDL-C in the causal pathway of atherosclerosis and its resulting end-organ damage. However, in both clinical trials [2, 3] and Mendelian randomization analyses [4], increasing HDL-C levels has failed to demonstrate a significant decrease in cardiovascular disease, raising doubts as to the cardioprotective nature of the HDL-C and the high density lipoprotein particle (HDL-P) in its entirety.
However, recent evidence from the Multi Ethnic Study of Atherosclerosis (MESA) suggests that aspects of the HDL-P not measured by HDL-C may be responsible for the cardioprotective effects of HDL [5]. In this work, Mackey et al. studied a cohort of 5,598 participants, measured both HDL-P (which reflects the total quantity of HDL and its associated proteins) and HDL-C, and performed multivariate regression on the outcomes of incident coronary heart disease (CHD) and carotid intima media thickening (cIMT). From these analyses, Mackey et al. found that when HDL-P was already included in the model, HDL-C measures no longer were protective against cardiovascular disease risk. This finding suggested that there were elements of HDL likely responsible for its cardioprotective nature that were better reflected by HDL-P than HDL-C.
HDL is broadly composed of two sub-species, HDL-2 and HDL-3, each of which have distinct biochemical, physiologic, and metabolic functions [6]. HDL-2 has a much higher density of apolipoprotein A1 (apoA1), whose levels have been consistently associated with cardioprotection [7, 8]. HDL-3 is the smaller, denser, and more lipid-poor of the two sub-fractions of HDL. However, HDL-3 is strongly antioxidant [9] and also is closely associated with the glycoprotein enzyme, paraoxonase-1 (PON1) [10]. PON1 is itself atheroprotective [11–14] and can prevent LDL [15, 16] and HDL oxidation [17] (other functions of PON1 are summarized in a recent review article [18]).
We have previously determined that HDL-3 was a superior predictor of carotid artery disease (CAAD) compared with HDL-C, HDL-2 or apoA1, considering 1,725 participants in a CAAD case–control cohort [19]. When HDL-3 was included in the model, none of the other measures of HDL were significantly associated with CAAD [19]. Moreover, the CAAD-protective effects of HDL-3 were independent of its closely associated enzyme, (PON1) [9] which is itself inversely associated with CAAD [14, 20, 21], suggesting that unmeasured elements of HDL-3 may be responsible for cardioprotective effects in our data [6].
Diet is one of the key behavioral targets for preventing cardiovascular disease [22, 23]. Specific diets, such as the Mediterranean [24] and DASH [25] diets have been associated with lower incidence of CHD. These favorable results are largely attributed to improved biomarker profiles, including increases in HDL-C [26]. However, studies have not generally analyzed the effects of diet on the more specific measures of HDL [24–26], such as HDL-2, HDL-3, and apoA1, all of which may be more closely associated with the cardioprotective elements of HDL [19].
We previously have used food frequency questionnaire data in our data to identify novel dietary micronutrient intakes that affect PON1 enzyme activity [27–29]. In this work we have leveraged our large and well-characterized Carotid Lesion Epidemiology And Risk (CLEAR) study in conjunction with thorough dietary intake data to determine the micronutrient determinants of each specific measure of HDL. Specifically, we sought to determine what specific dietary micronutrients are associated with HDL-C, HDL-2, HDL-3, and apoA1, and how these dietary associations differ across the various measures of HDL.
Methods
Ethics statement
Institutional review boards at the University of Washington, Virginia Mason Medical Center, and Veterans Affairs Puget Sound approved the CLEAR study. Written, informed consent was obtained from each participant of the study.
Sample
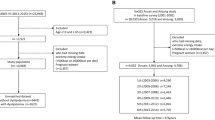
The CLEAR study is a Seattle-based prevalent CAAD case–control study, composed primarily of veterans, with controls matched by age at diagnosis (for CAAD cases) and current unaffected age (for controls). Exclusion criteria included familial hypercholesterolemia, total fasting cholesterol greater than 400 mg/dl, hypocoagulable state and/or the use of anticoagulant medication, post-organ transplant, or the inability to consent. The study population for this analysis was a subset (n = 1,566) of the previously described CLEAR study [14, 20] with both dietary intake and HDL data. All participants in the studied subset presented had complete covariate data. The studied subset consisted of 433 participants with CAAD as determined by ultrasound (>50% stenosis in either carotid artery), 70 participants with moderate obstruction (15-49% obstruction in at least one carotid artery), 3 subjects with other phenotypes, including peripheral artery disease (PAD) and coronary artery disease (CHD), and 1060 controls (<15% carotid stenosis bilaterally and absence of PAD and CHD). Of the 1,566 outpatients enrolled in this subset of the CLEAR study, 60% were recruited from Veterans Affairs Puget Sound, 22% from the University of Washington, and 18% from Virginia Mason Medical Center. Current smoking status and reported ancestry were obtained by self-report. For the purposes of our analyses, diabetes was defined by hemoglobin A1C ≥ 6.5% and/or hypoglycemic medication or insulin use, which was determined via self-report matched to hospital pharmacy records.
Lipid measurements
Standard methods were used to determine total cholesterol, triglycerides, and HDL in fasting whole plasma using an Abbott Spectrum analyzer. HDL fractions 2 and 3 were determined by precipitating HDL-2 from isolated total HDL, measuring HDL-3 in the supernatant, and subtracting this from total HDL to obtain HDL-2 [30]. Apolipoprotein A-I was measured as previously described [31]. All lipid measurements had approximate standard distributions.
Food-frequency questionnaire
At enrollment, participants were asked to complete the standardized Harvard food frequency questionnaire (FFQ) developed by the Health Professionals Follow-Up Study (https://regepi.bwh.harvard.edu/health/nutrition.html). The FFQ asked about i) the average frequency of intake over the previous year of specified portions of 131 foods and ii) the use of vitamins and mineral supplements, including the dose and duration of use. Questions regarding brand of multivitamins and cereal used were asked to clarify the quantities of specific vitamin supplementation. All vitamin usage was energy-adjusted to 2,000 kcal/day. The surveys were then returned to Harvard School of Public Health and the Brigham and Women’s Hospital, where they underwent quantitative analysis to return the inferred average intake of 162 specific and unique dietary nutrient intakes. Of the 162 returned dietary nutrient intakes, 53 non-redundant intakes with complete data across all participants with FFQ diet and plasma lipid data were carried forward to analysis. The Harvard Food Frequency Questionnaire has been validated against two, in-depth, 1-week diet records taken approximately six months apart [32]. Additionally, the inferred intake of dietary fatty acids and cholesterol have been validated against plasma lipid measurements [33, 34].
Statistical methods
Natural log transformation was performed for each of the 53 specific dietary micronutrient intake variables. Extreme observations were Winsorized [35] to 3 standard deviations from the mean before natural log transformation. For food frequency data, participants were excluded if their caloric intake was <800 calories/day or >4000 calories/day. Additionally, participants were excluded if the returned survey had ≥70 missing items.
We performed separate stepwise linear regression models on the individual lipid phenotypes of apo-A1, HDL-C, HDL-2, and HDL-3, with all 53 natural-log transformed dietary intakes included in the model. Model comparison was performed using Akaike’s information criterion (AIC), beginning with a base model comprised of age, sex, diabetes status, current smoking, and dummy variables for self-reported African, Asian, or Hispanic ancestry, with genetically-confirmed [36] European ancestry participants (the largest subgroup) serving as the reference group. Only measurements that improved model prediction of the specific lipid phenotype were retained in the final model.
Due to the high degree of correlation among the dietary fatty acid (DFA) measures, only those we had previously determined [29] to not be highly correlated with each other (pairwise correlation coefficient < 0.8) were included in this study. These DFA measures were: myristic acid (14:0), oleic acid (18:1), gadoleic acid (20:1), linolenic acid (18:3, a ω-3 DFA), arachidonic acid (20:4, an ω-6 DFA), and eicosapentaenoic acid (20:5, an ω-3 DFA).
Alcohol has previously been reported to increase HDL-C and its associated proteins; however, heavy alcohol use has also been reported to have the opposite effect [37]. Therefore, we created five groups of alcohol intake: 0 g/day, 0–12 g/day, 12–24 g/day, 24–60 g/day, and >60 g/day, as previously described by Framingham Heart Study investigators using the same food frequency questionnaire [38, 39]. Alcohol intake was then treated as a dummy variable compared to the reference of non-drinkers for stepwise linear regression analyses.
Results
Demographic, clinical, selected dietary intakes, and lipid values are presented in Table 1. Participants of European ancestry comprised the majority of the cohort (77.1%), while participants of Asian (12.6%), African (8.2%), and Hispanic (2.1%) comprised the remainder of the selected subset of the CLEAR study. Males accounted for approximately two-thirds (63.3%) of the population. Of the studied subset, 32.9% were taking statins, 17.6% were diabetic, and 10.3% were current smokers. The average age of all participants was 65.3 years. All lipid phenotypes (apoA1, HDL-C, HDL-2, and HDL-3) showed approximate standard distributions.
In addition to the demographic and clinical variables included in the base model, numerous dietary intakes increased total HDL-C variance explained in a stepwise linear regression model (see Table 2). All alcohol intake levels were positively associated with HDL-C. In addition, magnesium, folate, and the saturated fat, myristic acid (14:0), were all positively and independently associated with HDL-C. Carbohydrate intake, iron, and % of fat derived from animal sources were each negatively additive for HDL-C.
Similar effects from dietary intakes were observed for HDL-2: all alcohol intakes, magnesium, folate, and myristic acid (14:0) were each positively and independently associated with HDL-2 levels, while carbohydrate and iron intakes were both negatively associated with HDL-2 (see Table 3). Unique to HDL-2, arachidonic acid (20:4, an ω-6 DFA) was also negatively associated with HDL-2, while eicosapentaenoic acid (20:5, a ω-3 DFA) was positively associated with HDL-2.
In the dietary models explaining HDL-3 variance, all alcohol intakes, magnesium, folate, and myristic acid (14:0) were positively associated with HDL-3 levels (see Table 4). Similar to HDL-C and HDL-2, HDL-3 levels decreased with increasing carbohydrate and iron intakes. Unique to HDL-3, increasing protein intake was associated with decreased HDL-3 levels, while dietary fiber was associated with increased HDL-3 levels.
Finally, stepwise linear regression models considering dietary covariates to explain apoA1 variance identified similar trends: all alcohol intakes, folate, and myristic acid (14:0) were each positively and additively associated, while carbohydrate and iron intakes were negatively associated with apoA1 levels (see Table 5). Unique to apoA1, vitamin C intake was both positively associated with apoA1 levels. In addition to the aforementioned micronutrient intakes, increasing the percentage of fat derived from animal sources was associated with decreased apoA1 levels.
A summary table of all the identified dietary micronutrient intakes and their respective associations to the studied lipid phenotypes (HDL-C, HDL-2, HDL-3, and apoA1) is presented in Table 6. All alcohol intakes, myristic acid (14:0), and folate were each positively and independently associated with all of the studied lipid phenotypes. Carbohydrate and iron intakes were both negatively and additively associated with all of the studied lipid phenotypes. Magnesium was positively associated with all HDL-specific (HDL-C, HDL-2, and HDL-3) measures. Percentage of fat from animal sources was negatively associated with both HDL-C and apoA1 levels. Unique to HDL-2, arachidonic acid (20:4, an ω-6 DFA) was negatively associated, while eicosapentaenoic acid (20:5, an ω-3 DFA) was positively associated with HDL-2 levels. For HDL-3 alone, dietary protein and dietary fiber intake were negatively associated. Finally, for apoA1 only, vitamin C was positively associated with apoA1 levels.
Sensitivity analyses by CAAD status and gender showed consistent effects of all the dietary covariates for all studied lipid phenotypes, suggesting that these factors were not affecting the relationship between the identified dietary covariates and their respective lipid measures.
Discussion
Prior nutritional studies have generally focused on HDL-C alone. However, as measured HDL-C does not appear to be in the causative pathway for atherosclerotic disease [2–4], there is a growing recognition that any cardioprotective elements of HDL may be better captured by different measures of HDL [5]. In the current study, we have leveraged extensive dietary intake data within a large and well-characterized CAAD case–control cohort to identify the previously unreported predictors of HDL-2, HDL-3 and apoA1, and also validate numerous past associations with HDL-C. All of the HDL-related measures are highly correlated; thus, trends are seen in the dietary micronutrients and their associations with each of the lipid phenotypes. However, unique dietary associations were also elucidated for HDL-2, HDL-3, and apoA1 measures, which may reflect the differences among these HDL measures.
Low intakes of folate have previously been reported to be associated with higher incidence of CHD [40] and stroke [41] in prospective studies. One postulated mechanism for the observed cardioprotective properties of folate is through the folate-mediated lowering of homocysteine levels [42]. High levels of homocysteine are considered a modest and independent risk factor for heart disease and stroke [43]. In this study, we have what is, to the best of our knowledge, a novel finding: that dietary folate intake is also associated with favorable increases in HDL-related measures in humans. Prior epidemiologic evidence has positively linked plasma measures of folate with HDL-C [44] and apoA1 [45] levels. Although the increases in HDL-C, HDL-2, HDL-3, and apoA1 by folate intake are modest, they do represent a significant and consistent trend in our data and may represent a separate or complementary pathway through which folate mediates its cardioprotective effects.
All alcohol intake categories relative to non-drinkers were associated with an increase in all HDL-related measures. Gaziano et al. were one of the first to convincingly demonstrate that alcohol intake increased HDL-C, HDL-2, and HDL-3 levels, which they posited as a possible mechanism for decreased myocardial infarction among drinkers [46]. In addition, Gaziano et al. also reported that moderate (between 13.2 and 39.6 g/day) alcohol intake caused the greatest increase in HDL-2, while at heavy drinking (>39.6 g/day) HDL-2 levels decreased; HDL-C and HDL-3 continued to rise with increasing intake of alcohol [46]. We observed similar trends in our present work, as HDL-C and HDL-2 levels both decreased with heavy alcohol intake (>60 g/day); similarly, though HDL-3 and apoA1 increased at the highest levels of alcohol consumption, the gains were more modest in comparison to the subgroups with lower levels of alcohol intake. Interestingly, in a prospective study of 80,082 women, Rimm et al. reported that the cardioprotective effects of folate were most pronounced in women who consumed alcohol [40]. Though underpowered, we did not find evidence of an interaction between alcohol and folate intake on any measured HDL phenotype in our data (data not shown).
Both dietary carbohydrate and iron intakes were negatively associated with all measured HDL phenotypes. Dietary carbohydrates have previously been reported to decrease HDL-C levels [47, 48]. However, many of the studies were small (n = 10 and n = 8, respectively for the cited works), did not measure HDL-2 or HDL-3, and were short-term dietary intervention studies. In the work presented here, we have identified a strong, consistent, and negative association between carbohydrate intake and all HDL-measures (HDL-C, HDL-2, HDL-3, and apoA1) that reflects dietary intake over the past year, rather than a period of days. Similarly, high body stores of iron and dietary iron intake have been associated with increased risk of CHD in 1,931 Finnish men [49]. In addition, ferritin levels were negatively correlated with HDL-2 levels in that cohort of men [49]. Our findings are consistent with past reports of a negative association between HDL-2 and iron levels, and also show a consistent and negative effect of dietary iron on all HDL-related measures.
Dietary magnesium intake was positively associated with HDL-C, HDL-2, and HDL-3 levels, but not associated with apoA1 in our data. Singh et al. first reported from a randomized clinical trial of 430 patients that a magnesium-rich diet increased HDL-C levels at 12 weeks of follow-up [50]. Although the mechanism through which magnesium influences HDL-C levels is yet unknown, the positive association of dietary magnesium intake and HDL-C has been validated in a subsequent prospective study of 4,637 participants [51]. Here we expand on these findings and report that magnesium also increases levels of HDL-2 and HDL-3, but not apoA1. Further work will be needed to determine why magnesium affects levels of HDL, but not its closely related protein, apoA1.
Prior work on DFAs and lipid profiles have largely focused on the ω-3 polyunsaturated fatty acids commonly found in fish [23, 52, 53]. In this work we have found that the most significant and consistent DFA intake impacting HDL-related measures was that of the saturated fat, myristic acid (14:0). Saturated fats, including myristic acid, have been previously been reported to increase HDL-C [54]; however, they also have been reported to simultaneously increase low-density lipoprotein cholesterol levels [54, 55]. We did observe potentially protective effects of an ω-3 fatty acid, eicosapentaenoic acid (20:5), through its positive association with HDL-2 levels. We also noted a negative association of HDL-2 and arachidonic acid (20:4, ω-6). Arachidonic acid is most commonly found in meat and dairy.
Several limitations of this study should be considered. First, this cohort was composed of a majority of European Ancestry and participants selected for the presence or absence of CAAD, limiting the generalizability of our findings. Second, trans-fatty acids could not be included in analyses due to a high proportion of missing data. Unsaturated trans fatty acids have previously been associated with higher risk of CHD [56]; therefore, future analyses should consider the effect of trans fatty acids on the HDL proteome. Third, the dietary data analyzed in this study was limited to what was measured by the Harvard Standardized FFQ. As a result, it was not possible to evaluate other micronutrients, such as the flavonoids (e.g., quercetin), which have also been linked to favorable alterations to cardiovascular and neurodegenerative risk factors [57, 58]. Strengths of this study include the large sample size, extensive lipid phenotyping, and detailed demographic, clinical, and pharmacologic information coupled with a well-validated food frequency questionnaire.
In conclusion, we present the first known report of dietary folate intake affecting HDL-related phenotypes (HDL-C, HDL-2, HDL-3, and apoA1) in humans, thereby offering another potential mechanism independent of homocysteine through which folate mediates its cardioprotective effects. This study is also the first to report and validate numerous associations of dietary micronutrients with the more specific measures of HDL: HDL-2 and HDL-3. Given the recent failures of HDL-C to demonstrate cardioprotection, this work improves knowledge of how dietary factors influence the more specific measures of HDL (HDL-2, HDL-3, and apoA1). Future work should investigate the biologic mechanisms and pathways through which these micronutrients affect HDL and how specific micronutrients, such as vitamin C, might affect apoA1 but not any of the other measures of HDL.
References
Castelli WP: Cardiovascular disease and multifactorial risk: challenge of the 1980s. Am Heart J. 1983, 106: 1191-1200. 10.1016/0002-8703(83)90174-6.
Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W, AIM-HIGH Investigators: Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011, 365: 2255-2267.
Barter PJ, Barter PJ, Caulfield M, Caulfield M, Eriksson M, Eriksson M, Grundy SM, Grundy SM, Kastelein JJP, Kastelein JJP, Komajda M, Komajda M, Lopez-Sendon J, Lopez-Sendon J, Mosca L, Mosca L, Tardif J-C, Tardif J-C, Waters DD, Waters DD, Shear CL, Shear CL, Revkin JH, Revkin JH, Buhr KA, Buhr KA, Fisher MR, Fisher MR, Tall AR, Tall AR: Effects of Torcetrapib in Patients at High Risk for Coronary Events. N Engl J Med. 2007, 357: 2109-2122. 10.1056/NEJMoa0706628.
Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, Hindy G, Hólm H, Ding EL, Johnson T, Schunkert H, Samani NJ, Clarke R, Hopewell JC, Thompson JF, Li M, Thorleifsson G, Newton-Cheh C, Musunuru K, Pirruccello JP, Saleheen D, Chen L, Stewart AF, Schillert A, Thorsteinsdottir U, Thorgeirsson G, Anand S, Engert JC, Morgan T, Spertus J: Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. The Lancet. 2012, 380: 572-580. 10.1016/S0140-6736(12)60312-2.
Mackey RH, Greenland P, Goff DC, Lloyd-Jones D, Sibley CT, Mora S: High-density lipoprotein cholesterol and particle concentrations, carotid atherosclerosis, and coronary events: MESA (multi-ethnic study of atherosclerosis). J Am Coll Cardiol. 2012, 60: 508-516. 10.1016/j.jacc.2012.03.060.
Asztalos BF, Tani M, Schaefer EJ: Metabolic and functional relevance of HDL subspecies. Curr Opin Lipidol. 2011, 22: 176-185. 10.1097/MOL.0b013e3283468061.
Asztalos BF, Roheim PS, Milani RL, Lefevre M, McNamara JR, Horvath KV, Schaefer EJ: Distribution of ApoA-I-Containing HDL Subpopulations in Patients With Coronary Heart Disease. Arterioscler Thromb Vasc Biol. 2000, 20: 2670-2676. 10.1161/01.ATV.20.12.2670.
Asztalos BF, Batista M, Horvath KV, Cox CE, Dallal GE, Morse JS, Brown GB, Schaefer EJ: Change in alpha1 HDL concentration predicts progression in coronary artery stenosis. Arterioscler Thromb Vasc Biol. 2003, 23: 847-852. 10.1161/01.ATV.0000066133.32063.BB.
Kontush A, Chantepie S, Chapman MJ: Small, dense HDL particles exert potent protection of atherogenic LDL against oxidative stress. Arterioscler Thromb Vasc Biol. 2003, 23: 1881-1888. 10.1161/01.ATV.0000091338.93223.E8.
Bergmeier C, Siekmeier R, Gross W: Distribution spectrum of paraoxonase activity in HDL fractions. Clin Chem. 2004, 50: 2309-2315. 10.1373/clinchem.2004.034439.
Mackness MI, Harty D, Bhatnagar D, Winocour PH, Arrol S, Ishola M, Durrington PN: Serum paraoxonase activity in familial hypercholesterolaemia and insulin-dependent diabetes mellitus. Atherosclerosis. 1991, 86: 193-199. 10.1016/0021-9150(91)90215-O.
Shih DM, Gu L, Xia Y-R, Navab M, Li W-F, Hama S, Castellani LW, Furlong CE, Costa LG, Fogelman AM, Lusis AJ: Mice lacking serum paraoxonase are susceptible to organophosphate toxicity and atherosclerosis. Nature. 1998, 394: 284-287. 10.1038/28406.
Bhattacharyya T, Nicholls SJ, Topol EJ, Zhang R, Yang X, Schmitt D, Fu X, Shao M, Brennan DM, Ellis SG: Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. JAMA. 2008, 299: 1265-1276. 10.1001/jama.299.11.1265.
Jarvik GP, Rozek LS, Brophy VH, Hatsukami TS, Richter RJ, Schellenberg GD, Furlong CE: Paraoxonase (PON1) Phenotype Is a Better Predictor of Vascular Disease Than Is PON1192 or PON155 Genotype. Arterioscler Thromb Vasc Biol. 2000, 20: 2441-2447. 10.1161/01.ATV.20.11.2441.
Mackness MI, Arrol S, Abbott C, Durrington PN: Protection of low-density lipoprotein against oxidative modification by high-density lipoprotein associated paraoxonase. Atherosclerosis. 1993, 104: 129-135. 10.1016/0021-9150(93)90183-U.
Mackness MI, Arrol S, Durrington PN: Paraoxonase prevents accumulation of lipoperoxides in low-density lipoprotein. FEBS Lett. 1991, 286: 152-154. 10.1016/0014-5793(91)80962-3.
Aviram M, Rosenblat M, Bisgaier CL, Newton RS, Primo-Parmo SL, La Du BN: Paraoxonase inhibits high-density lipoprotein oxidation and preserves its functions. A possible peroxidative role for paraoxonase. J Clin Invest. 1998, 101: 1581-1590. 10.1172/JCI1649.
Kim DS, Marsillach J, Furlong CE, Jarvik GP: Pharmacogenetics of paraoxonase activity: elucidating the role of high-density lipoprotein in disease. Pharmacogenomics. 2013, 14: 1495-1515. 10.2217/pgs.13.147.
Kim DS, Burt AA, Rosenthal EA, Ranchalis JE, Eintracht JF, Hatsukami TS, Furlong CE, Marcovina S, Albers JJ, Jarvik GP: HDL-3 is a Superior Predictor of Carotid Artery Disease in a Case–control Cohort of 1725 Participants. J Am Heart Assoc. 2014, 3 (3): e000902-10.1161/JAHA.114.000902. doi:10.1161/JAHA.114.000902
Jarvik GP, Hatsukami TS, Carlson C, Richter RJ, Jampsa R, Brophy VH, Margolin S, Rieder M, Nickerson D, Schellenberg GD: Paraoxonase activity, but not haplotype utilizing the linkage disequilibrium structure, predicts vascular disease. Arterioscler Thromb Vasc Biol. 2003, 23: 1465-1471. 10.1161/01.ATV.0000081635.96290.D3.
Kim DS, Burt AA, Ranchalis JE, Richter RJ, Marshall JK, Eintracht JF, Rosenthal EA, Furlong CE, Jarvik GP: Additional Common Polymorphisms in the PON Gene Cluster Predict PON1 Activity but Not Vascular Disease. J Lipids. 2012, 2012: 476316-
Krauss RM, Eckel RH, Howard B, Appel LJ, Daniels SR, Deckelbaum RJ, Erdman JW, Kris-Etherton P, Goldberg IJ, Kotchen TA, Lichtenstein AH, Mitch WE, Mullis R, Robinson K, Wylie-Rosett J, St Jeor S, Suttie J, Tribble DL, Bazzarre TL: AHA Dietary Guidelines: Revision 2000: A Statement for Healthcare Professionals From the Nutrition Committee of the American Heart Association. Circulation. 2000, 102: 2284-2299. 10.1161/01.CIR.102.18.2284.
Lichtenstein AH: Diet and Lifestyle Recommendations Revision 2006: A Scientific Statement From the American Heart Association Nutrition Committee. Circulation. 2006, 114: 82-96. 10.1161/CIRCULATIONAHA.106.176158.
de Lorgeril M, Renaud S, Mamelle N, Salen P, Martin JL, Monjaud I, Guidollet J, Touboul P, Delaye J: Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. The Lancet. 1994, 343: 1454-1459. 10.1016/S0140-6736(94)92580-1.
Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB: Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med. 2008, 168: 713-720. 10.1001/archinte.168.7.713.
Carter SJ, Roberts MB, Salter J, Eaton CB: Relationship between Mediterranean Diet Score and atherothrombotic risk: findings from the Third National Health and Nutrition Examination Survey (NHANES III), 1988–1994. Atherosclerosis. 2010, 210: 630-636. 10.1016/j.atherosclerosis.2009.12.035.
Jarvik GP, Tsai NT, McKinstry LA, Wani R, Brophy VH, Richter RJ, Schellenberg GD, Heagerty PJ, Hatsukami TS, Furlong CE: Vitamin C and E intake is associated with increased paraoxonase activity. Arterioscler Thromb Vasc Biol. 2002, 22: 1329-1333. 10.1161/01.ATV.0000027101.40323.3A.
Kim DS, Burt AA, Ranchalis JE, Richter RJ, Marshall JK, Nakayama KS, Jarvik ER, Eintracht JF, Rosenthal EA, Furlong CE, Jarvik GP: Dietary cholesterol increases paraoxonase 1 enzyme activity. J Lipid Res. 2012, 53: 2450-2458. 10.1194/jlr.P030601.
Kim DS, Maden SK, Burt AA, Ranchalis JE, Furlong CE, Jarvik GP: Dietary fatty acid intake is associated with paraoxonase 1 activity in a cohort-based analysis of 1,548 subjects. Lipids Health Dis. 2013, 12: 183-10.1186/1476-511X-12-183.
Bachorik PS, Albers JJ: Precipitation methods for quantification of lipoproteins. Methods Enzymol. 1986, 129: 78-100.
Marcovina SM, Albers JJ, Henderson LO, Hannon WH: International Federation of Clinical Chemistry standardization project for measurements of apolipoproteins A-I and B. III. Comparability of apolipoprotein A-I values by use of international reference material. Clin Chem. 1993, 39: 773-781.
Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC: Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992, 135: 1114-1126. discussion 1127–36
Hunter DJ, Hunter DJ, Rimm EB, Rimm EB, Sacks FM, Sacks FM, Stampfer MJ, Stampfer MJ, Colditz GA, Colditz GA, Litin LB, Litin LB, Willett WC, Willett WC: Comparison of measures of fatty acid intake by subcutaneous fat aspirate, food frequency questionnaire, and diet records in a free-living population of US men. Am J Epidemiol. 1992, 135: 418-427.
Willett W, Stampfer M, Chu NF, Spiegelman D, Holmes M, Rimm E: Assessment of questionnaire validity for measuring total fat intake using plasma lipid levels as criteria. Am J Epidemiol. 2001, 154: 1107-1112. 10.1093/aje/154.12.1107.
Dixon WJ, Tukey JW: Approximate behavior of the distribution of Winsorized t (Trimming/Winsorization 2). Technometrics. 1968, 10: 83-98.
Pritchard JK, Stephens M, Donnelly P: Inference of population structure using multilocus genotype data. Genetics. 2000, 155: 945-959.
Rao MN, Marmillot P, Gong M, Palmer DA, Seeff LB, Strader DB, Lakshman MR: Light, but not heavy alcohol drinking, stimulates paraoxonase by upregulating liver mRNA in rats and humans. Metabolism. 2003, 52: 1287-1294. 10.1016/S0026-0495(03)00191-4.
Djousse L, Ellison RC, Beiser A, Scaramucci A, D’Agostino RB, Wolf PA: Alcohol Consumption and Risk of Ischemic Stroke: The Framingham Study. Stroke. 2002, 33: 907-912. 10.1161/hs0402.105245.
Djoussé L, Dorgan JF, Zhang Y, Schatzkin A, Hood M, D’Agostino RB, Copenhafer DL, Kreger BE, Ellison RC: Alcohol consumption and risk of lung cancer: the Framingham Study. J Natl Cancer Inst. 2002, 94: 1877-1882. 10.1093/jnci/94.24.1877.
Rimm EB, Willett WC, Hu FB, Sampson L, Colditz GA, Manson JE, Hennekens C, Stampfer MJ: Folate and vitamin B6 from diet and supplements in relation to risk of coronary heart disease among women. JAMA. 1998, 279: 359-364. 10.1001/jama.279.5.359.
Bazzano LA, He J, Ogden LG, Loria C, Vupputuri S, Myers L, Whelton PK, Kasner SE: Dietary Intake of Folate and Risk of Stroke in US Men and Women: NHANES I Epidemiologic Follow-Up Study * Editorial Comment: NHANES I Epidemiologic Follow-Up Study. Stroke. 2002, 33: 1183-1189. 10.1161/01.STR.0000014607.90464.88.
Lowering blood homocysteine with folic acid based supplements: meta-analysis of randomised trials. Homocysteine Lowering Trialists’ Collaboration. BMJ. 1998, 316: 894-898. 10.1136/bmj.316.7135.894.
Homocysteine Studies Collaboration: Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. JAMA. 2002, 288: 2015-2022. 10.1001/jama.288.16.2015.
Semmler A, Moskau S, Grigull A, Farmand S, Klockgether T, Smulders Y, Blom H, Zur B, Stoffel-Wagner B, Linnebank M: Plasma folate levels are associated with the lipoprotein profile: a retrospective database analysis. Nutr J. 2010, 9: 31-10.1186/1475-2891-9-31.
Söderström E, Eliasson M, Johnson O, Hallmans G, Weinehall L, Jansson J-H, Hultdin J: Plasma folate, but not homocysteine, is associated with Apolipoprotein A1 levels in a non-fortified population. Lipids Health Dis. 2013, 12: 74-10.1186/1476-511X-12-74.
Gaziano JM, Buring JE, Breslow JL, Goldhaber SZ, Rosner B, VanDenburgh M, Willett W, Hennekens CH: Moderate alcohol intake, increased levels of high-density lipoprotein and its subfractions, and decreased risk of myocardial infarction. N Engl J Med. 1993, 329: 1829-1834. 10.1056/NEJM199312163292501.
Jeppesen J, Schaaf P, Jones C, Zhou MY, Chen YD, Reaven GM: Effects of low-fat, high-carbohydrate diets on risk factors for ischemic heart disease in postmenopausal women. Am J Clin Nutr. 1997, 65: 1027-1033.
Abbasi F, McLaughlin T, Lamendola C, Kim HS, Tanaka A, Wang T, Nakajima K, Reaven GM: High carbohydrate diets, triglyceride-rich lipoproteins, and coronary heart disease risk. Am J Cardiol. 2000, 85: 45-48. 10.1016/S0002-9149(99)00604-9.
Salonen JT, Nyyssonen K, Korpela H, Tuomilehto J, Seppanen R, Salonen R: High stored iron levels are associated with excess risk of myocardial infarction in eastern Finnish men. Circulation. 1992, 86: 803-811. 10.1161/01.CIR.86.3.803.
Singh RB, Rastogi SS, Sharma VK, Saharia RB, Kulshretha SK: Can dietary magnesium modulate lipoprotein metabolism?. Magnes Trace Elem. 1990, 9: 255-264.
He K: Magnesium Intake and Incidence of Metabolic Syndrome Among Young Adults. Circulation. 2006, 113: 1675-1682. 10.1161/CIRCULATIONAHA.105.588327.
Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC: Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med. 2000, 343: 16-22. 10.1056/NEJM200007063430103.
Kromhout D: Prevention of Coronary Heart Disease by Diet and Lifestyle: Evidence From Prospective Cross-Cultural, Cohort, and Intervention Studies. Circulation. 2002, 105: 893-898. 10.1161/hc0702.103728.
Zock PL, de Vries JH, Katan MB: Impact of myristic acid versus palmitic acid on serum lipid and lipoprotein levels in healthy women and men. Arterioscler Thromb Vasc Biol. 1994, 14: 567-575. 10.1161/01.ATV.14.4.567.
Müller H, Lindman AS, Brantsaeter AL, Pedersen JI: The serum LDL/HDL cholesterol ratio is influenced more favorably by exchanging saturated with unsaturated fat than by reducing saturated fat in the diet of women. J Nutr. 2003, 133: 78-83.
Hu FB, Stampfer MJ, Manson JE, Rimm E, Colditz GA, Rosner BA, Hennekens CH, Willett WC: Dietary fat intake and the risk of coronary heart disease in women. N Engl J Med. 1997, 337: 1491-1499. 10.1056/NEJM199711203372102.
Hooper L, Kroon PA, Rimm EB, Cohn JS, Harvey I, Le Cornu KA, Ryder JJ, Hall WL, Cassidy A: Flavonoids, flavonoid-rich foods, and cardiovascular risk: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2008, 88: 38-50.
Costa LG, Tait L, Laat R, Dao K, Giordano G, Pellacani C, Cole TB, Furlong CE: Modulation of Paraoxonase 2 (PON2) in Mouse Brain by the Polyphenol Quercetin: A Mechanism of Neuroprotection?. Neurochem Res. 2013, 38: 1809-1818. 10.1007/s11064-013-1085-1.
Acknowledgements
We would like to thank all CLEAR participants. This work was funded in part by National Institutes of Health RO1 HL67406 and a State of Washington Life Sciences Discovery Award (265508) to the Northwest Institute of Genetic Medicine. DSK was supported in part by the Benjamin and Margaret Hall Endowed Fellowship in Genome Sciences, a Markey Foundation award, and National Institutes of Health 5T31HG000035-18 and 1F31MH101905-01.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
JFE, CEF, and GPJ enrolled participants into the study. JER and LEJ processed the data and performed assays on participant blood samples. DSK and AAB analyzed the data and GPJ supervised this analysis. DSK and GPJ wrote the manuscript. All authors reviewed and edited the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Kim, D.S., Burt, A.A., Ranchalis, J.E. et al. Effects of dietary components on high-density lipoprotein measures in a cohort of 1,566 participants. Nutr Metab (Lond) 11, 44 (2014). https://doi.org/10.1186/1743-7075-11-44
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1743-7075-11-44