Abstract
Background
Both exercise and vitamin E supplementation have been shown to reduce oxidative stress and cardiovascular disease risk in older adults, and when combined there is evidence suggesting that they act synergistically. The currently recommended amount of exercise for older adults is 150 min/week of moderate-intensity exercise; however, the minimum amount of exercise necessary to achieve health benefits is not known. The purpose of this study was to investigate the effects of 12 weeks of participation in a low-volume walking exercise programme (i.e. 90 min/week) combined with daily vitamin E supplementation on thiobarbituric acid reactive substances (TBARS) and oxidised low-density lipoprotein (LDL) concentrations in older adults.
Methods
The participants were recruited from the following four groups separately: 1) control (CG, n = 14), 2) vitamin E supplementation (SG, n = 10), 3) walking (WG, n = 7), or 4) walking + supplementation (WSG, n = 7). In the CG, participants were advised to maintain their normal lifestyle during the study. Participants in both the SG and WSG received 450 IU (300 mg) /day of α-tocopherol for 12 weeks. The exercise programme for the WG and WSG consisted of two 30–60 minute sessions weekly for 12 weeks (average walking time was 44.5 ± 1.6 min/session). Blood samples were collected at baseline and at 12 weeks.
Results
Delta plasma oxidised LDL concentrations did not differ among four groups (One-factor ANOVA, P = 0.116). However, negative delta plasma TBARS, a marker of oxidative damage, concentrations were observed in the WG, WSG and SG relative to the CG at the end of the study period (One-factor ANOVA, P = 0.001; post hoc tests; CG compared with WG, WSG and SG, P = 0.005; P = 0.021; P = 0.024, respectively).
Conclusion
These findings suggest that a low-volume of physical activity and/or vitamin E supplementation may be an effective intervention strategy for reducing TBARS concentrations of older adults.
Trial registration
Similar content being viewed by others
Background
Oxidative stress leads to vascular dysfunction and has been implicated in the pathogenesis of aging and atherosclerosis [1]. The oxidative modification of low-density lipoprotein (LDL) is a key step in the initiation and progression of atherosclerosis [2]. Uptake of oxidised LDL by macrophages via scavenger receptors leads to lipid-laden foam cell formation and fatty streak development in the arterial wall [3]. In addition, oxidised LDL, oxidative stress, and markers of chronic inflammation increase with advancing age [4, 5]. Identifying effective strategies for preventing the oxidative modification of LDL and oxidative stress in older adults may reduce cardiovascular disease risk, and thus is an important public health issue.
It has been previously reported that plasma concentrations of oxidised LDL and triacylglycerol were reduced after high intensity training (66% of heart rate reserve, 60 minutes/session, 3 times/week for 10 weeks) in older adults [6]. However, it is important to note that it is difficult to achieve compliance when recommending high-intensity physical activity to older adults. In addition, the optimal amount of exercise for achieving health benefits is still unknown, and may be lower than the currently recommended level of 150 minutes/week of moderate-intensity exercise [7]. To develop effective physical activity strategies to reduce cardiovascular disease risks in older adults, it is important to further identify the effects of lower amounts of moderate-intensity physical activity (such as walking) on markers of cardiovascular disease risk, including insulin, glucose and high-density lipoprotein (HDL)-cholesterol.
There is an inverse relationship between vitamin E intake and the prevalence of coronary heart disease [8, 9]. In particular, α-tocopherol (an active form of vitamin E), exhibits antioxidant and anti-inflammatory effects and reduces the risk of atherosclerosis and cardiovascular disease [10, 11]. However, there is inconsistency in the literature. A previous study has demonstrated that 3 years of vitamin E supplementation (i.e. 136 IU of dl-α-tocopherol (91 mg)) did not influence oxidative stress in hypercholesterolaemic women patients [12]. Whereas another previous study has indicated that 16 weeks of moderate-intensity endurance exercise training with high dose of vitamin E (i.e. 800 IU of dl-α-tocopherol (534 mg)) daily intake reduces lipid hydroperoxide (a marker of oxidative stress) and resting blood pressure in older adults [13]. Contrastively, a study of Bobeuf et al. investigated that the combination of 6 month of resistance training and antioxidant supplementation (i.e. 1000 mg of vitamin C and 400 IU of vitamin E (267 mg)) had a positive effect on the plasma antioxidant profile but not on the oxidative stress in older adults [14]. These previous studies remain unclear whether exercise programme and/or vitamin E supplementation in the older adults has a positive effect on the oxidative stress and oxidised LDL concentrations.
Therefore, the main purpose of this study was to examine the effects of 12 weeks of participation in a low-volume walking programme combined with vitamin E supplementation on concentrations of thiobarbituric acid reactive substances (TBARS, a marker of oxidative damage) and oxidised LDL in older adults.
Methods
Participants
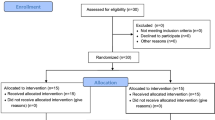
Participants aged 60–81 years were recruited from the local communities. For the baseline evaluation, we used a simple lifestyle-related questionnaire (physical activity, medication, sleep, alcohol intake, and smoking). Patients with history of cardiovascular disease, physically active lifestyle, those who used lipid- and/or glucose-lowering medication, were smokers, or <60 years of age were excluded from the study. A physically active lifestyle was defined as any form of planned, structured, and repetitive physical activity undertaken a minimum of 3 times per week, lasting at least 30 min per session, for the purpose of improving or maintaining one or more components of physical fitness described by Butcher et al. [15]. Consequently, none of the study participants were trained athletes in any sporting events but some participants were recreationally active. We recruited forty-two participants (10 males and 32 females) who initially met the criteria for the enrollment in the present study. From an ethical point of view, we have considered that we should not randomly assign the participants to each group, particularly those who do not wish to increase/decrease their activities of daily living or receive dietary supplements - we have recruited the participants of each group at the same time and thus each participant knows what they are volunteering for. Fourteen older adults were recruited for the control group (CG). Ten older adults were recruited for the vitamin E supplementation group (SG). Seven older adults were recruited for the walking group (WG). Seven older adults were recruited for the walking and vitamin E supplementation group (WSG). Four participants (1 male and 3 females) in the WG could not complete the walking program of the present study because of injury or disease (i.e. not related to the present study). Consequently, thirty-eight participants (9 males and 29 females) were included in the analysis. Each participant was instructed not to change their lifestyle, including dietary habits, throughout the study period. Participants of the control group were advised to maintain their normal lifestyle during the study. The physical characteristics of the participants are shown in Table 1. This study was approved by the Waseda University Ethical Advisory Committee (2011–061). Informed consent was obtained from all participants.
Walking programme
All participants in the WG and WSG underwent a 12-week, supervised exercise programme that consisted of 2 walking exercise sessions per week. Each 30–60 minute walking session was done in the morning, between 10:00 and 11:00, and included 5 minutes of warm-up activities and 5 minutes of cool-down activities. During weeks 1–5, participants walked 2.6–3.5 km in 30–40 minute walking sessions. During weeks 6–12, participants completed 40–60 minute walking sessions covering distances of 3.5–4.9 km. All walking sessions were performed outdoors and were supervised by experienced assistants. The participants were asked to indicate their perceived exertion during each walking session using the Borg scale [16], and heart rate was measured using short-range telemetry (Polar RS400, Polar Electro Oy, Finaland). Participants in all groups were advised to maintain their usual daily living activities during the study.
Vitamin E supplementation
Participants in the SG and WSG were issued a 12-week supply of 150 IU (100 mg) vitamin E (RRR α-tocopherol) (Juvelux 300, Eisai Co., Ltd., Japan) capsules and instructed to take one-capsule thrice daily (RRR α-tocopherol, 450 IU (300 mg) /day) with meals for the entire 12 week study period.
Physical activity measurement
To determine the general physical activity level of the participants, all of the participants were asked to wear a uniaxial accelerometer (Lifecoder-EX, Suzuken Co. Ltd., Nagoya, Japan) for the 12 week study period. This device determines the level of activity intensity by measuring the magnitude and frequency of accelerations every 4 seconds. Activity intensity is classified into one of eleven levels (0, 0.5, 1–9), with 0 indicating the lowest and 9 indicating the highest level of activity. Participants were contacted by phone at the beginning of each week to assess compliance and any device problems, and all reported that there were no problems with the use of the accelerometer. Our data analysis confirmed that all participants wore the accelerometer every day during the data collection period. Since we collected accelerometer data from each group simultaneously, seasonality was not an issue in the present study. Data from participants who had worn the accelerometer for at least 10 hours a day for at least 4 weekdays and 1 weekend day after calculation of wear time were considered valid [17, 18]. The main physical activity variable used in this study was the time spent in moderate to vigorous physical activity (MVPA). The number of minutes spent performing MVPA was calculated on a daily basis, and then used to estimate weekly activity by taking a weighted average of daily weekday and weekend activity (i.e., weekly MVPA = (average daily weekday MVPA × 5) + (average daily weekend MVPA × 2)). All minutes of recording with a total of ≥ 4 activity levels were classified as MVPA. The activity level score of 4 was utilised as a threshold for MVPA based on a previous calibration study [19] and corresponded to approximately 3 metabolic equivalents.
Anthropometric and blood pressure measurements
Anthropometric variables were measured at baseline and after 12 weeks in each group. Body mass was measured to the nearest 0.05 kg using a digital scale (Inner Scan 50, Tanita Corporation, Tokyo, Japan). Height was measured to the nearest 0.1 cm using a wall-mounted stadiometer (YS-OA, As One Corporation, Osaka, Japan). Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. Waist circumference was measured to the nearest 0.1 cm at the level of the umbilicus using a flexible plastic tape while the participants were in a standing position. Arterial blood pressure was measured using a mercury sphygmomanometer (605P, Yagami Co. Ltd., Nagoya, Japan) after participants had been seated at rest for 5 minutes. For each variable, two measurements were taken at each time point and the mean of these values was recorded.
Dietary assessment
To examine whether dietary intake of antioxidants influenced outcome factors, diet recall questions were conducted at baseline and during week 10 of the study period. Diet records were analysed using a computerised nutritional analysis system (Excel Eiyokun, Kenpakusha, Japan). Dietary assessments were conducted by a registered dietitian.
Blood collection and laboratory assays
Fasting venous blood samples were taken from an antecubital vein at baseline and at 12 weeks. Participants were asked to avoid physical activity for 48 hours, and to fast overnight for at least 10 hours, before the blood samples were taken. Blood concentrations of oxidised LDL, thiobarbituric acid reactive substances (TBARS), α-tocopherol, glucose, insulin, HbA1c, soluble E-selectin (sE-selectin), soluble vascular cell adhesion molecule (sVCAM-1), C-peptide, triacylglycerol, total cholesterol (TC), HDL-cholesterol and low-density lipoprotein (LDL)-cholesterol were measured. For α-tocopherol, triacylglycerol, TC, HDL-cholesterol and LDL-cholesterol, the blood samples were collected into tubes containing clotting activators to facilitate separation of serum. The samples were allowed to clot for 45 minutes at room temperature and were then centrifuged at 3000 rpm for 10 minutes at 4°C. After separation, serum was aliquoted into plain micro tubes and stored at −80°C for later analysis. For HbA1c, oxidised LDL, TBARS, insulin, sE-selectin, sVCAM-1 and C-peptide, venous blood samples were collected into tubes containing disodium salt-EDTA. The samples were immediately centrifuged and the plasma separated and aliquoted as described above. For glucose measurements, venous blood samples were collected into tubes containing sodium fluoride-EDTA, immediately centrifuged, and aliquoted as described above.
Plasma concentrations of oxidised LDL (Mercodia AB, Uppsala, Sweden), insulin (Mercodia AB, Uppsala, Sweden), sE-selectin (eBioscience, Inc., San Diego, USA), sVCAM-1 (eBioscience, Inc., San Diego, USA) and C-peptide (Mercodia AB, Uppsala, Sweden) were measured by enzyme-linked immunosorbent assay using commercially available kits. Concentrations of serum triacylglycerol, TC, HDL-cholesterol, LDL-cholesterol, plasma glucose and HbA1c were determined using standard laboratory methods. Plasma TBARS was measured using an assay kit from Cayman Chemicals (Cayman Chemicals, Michigan, USA). The concentration of serum α-tocopherol was measured using high performance liquid chromatography (HPLC) by the method of Talwar et al. [20]. Then, serum α-tocopherol concentrations were normalised to serum triacylglycerol and TC concentrations.
Statistical analysis
Data were analysed using Predictive Analytics Software (PASW) version 18.0 for Windows (SPSS Japan Inc., Tokyo, Japan). The Shapiro-Wilk test was used to check for normality of distribution. One-factor analysis of variance (ANOVA) was used to examine the differences in the baseline and delta values of physical and physiological variables, dietary and physical activity data, and blood markers among the 4 groups. A two-factor ANOVA was used to determine differences among groups over time for all outcome variables. Where significant effects were found, post hoc pair-wise comparisons were performed using the Bonferroni method. Paired Student’s t-tests were used to assess group differences between pre- and post-intervention data. Statistical significance was accepted at the 5% level. Results are presented as means ± SEM.
Results
Physical characteristics and dietary data
There were no between-group differences in physical and physiological characteristics at baseline, with the exception of systolic blood pressure (SBP) (Table 1). One-factor ANOVA revealed a significant baseline difference in SBP among groups (P = 0.004). SBP was significantly lower in the SG (120.3 ± 3.1 mm Hg) than in the CG (135.7 ± 3.4 mm Hg, P = 0.016), WG (136.4 ±4.2 mm Hg, P = 0.045), and WSG (139.7 ± 4.2 mm Hg, P = 0.010). Within-group analyses showed that body mass increased significantly in the SG (paired t-test, P = 0.008) and WG (paired t-test, P = 0.045) after 12 weeks relative to their baseline values, while BMI increased only in the SG (paired t-test, P = 0.006) and waist circumference increased only in the WG (paired t-test, P = 0.035) (Table 1). Figure 1 shows the MVPA levels measured during the 12 weeks period among the four groups. One-factor ANOVA revealed a significant difference in MVPA measured during the 12 weeks period among groups (P = 0.001). Post hoc tests showed that MVPA measured during the 12 week study period was significantly higher in the WG (225.1±19.7 min/week) and WSG (228.2± 22.8 min/week) than in the CG (132.7±18.2 min/week) and SG (134.0± 11.3 min/week). Analysis of dietary data revealed that dietary intake (excluding supplementation) of the antioxidants vitamin C, vitamin E and β-carotene did not differ between or within groups at baseline or during the study.
Exercise compliance and exercise amount
The mean duration of the walking sessions was 44.5 ± 1.6 min/session. The mean heart rate and perceived exertion during the walking sessions was 113 ± 3 beats/min and 10.6 ± 0.3 (i.e. light), respectively. This corresponded to an exercise intensity of 48 ± 4% of heart rate reserve.
Serum α-tocopherol concentrations
Figure 2 shows the serum α-tocopherol concentrations measured at baseline and at 12 weeks in the CG, SG, WG and WSG. At baseline, there were no differences in serum α-tocopherol concentrations among groups. Two-factor ANOVA revealed that there were main effects of time (P = 0.001) and time by group interactions (P = 0.001) for serum α-tocopherol concentrations.
The serum α-tocopherol concentrations (normalised for triacylglycerol and total cholesterol concentrations) measured at baseline and at 12 weeks in the control group (CG), vitamin E supplementation group (SG), walking group (WG) and vitamin E plus walking group (WSG). Data are means ± SEM and time-group interactions are the main influencing factors (P = 0.001, Two-factor ANOVA). **Significantly different from the baseline value in the same group (paired Student’s t test, P < 0.01).
Plasma oxidised LDL and TBARS responses
Two-factor ANOVA revealed that there were no main effects of time (P = 0.172) and no time by group interactions (P = 0.116) for plasma oxidised LDL concentrations. One-factor ANOVA revealed that changes in plasma oxidised LDL concentrations did not differ among groups (P = 0.116) (Figure 3). However, within-group analysis showed that plasma oxidised LDL concentrations significantly increased in the CG (P= 0.019) over the 12 week study period. For plasma TBARS concentrations, two-factor ANOVA revealed that there was no main effect of time (P = 0.083), but there was a significant time by group interaction (P = 0.007). Plasma TBARS concentration was significantly greater at 12 weeks in the CG compared with the WG, WSG and SG (post hoc tests; CG compared with WG, WSG and SG, P = 0.005; P = 0.021; P = 0.024, respectively) (Figure 4). Within-group analyses showed that plasma TBARS concentrations significantly decreased over the study period only in the WG (P = 0.038), and increased only in the CG (P = 0.042), relative to baseline values.
Delta value of plasma thiobarbituric acid reactive substances (TBARS) measured at baseline and at 12 weeks in the control group (CG), vitamin E supplementation group (SG), walking group (WG) and vitamin E plus walking group (WSG). Data are means ± SEM. Data were analysed using one-factor ANOVA followed by a Bonferroni multiple comparisons test (P = 0.001, One-factor ANOVA). ** Significantly different from the control group (P ≤ 0.024).
Delta value of oxidised low-density lipoprotein (LDL) concentrations measured at baseline and 12 weeks in the control group (CG), vitamin E supplementation group (SG), walking group (WG) and vitamin E plus walking group (WSG). Data are means ± SEM. Data were analysed using one-factor ANOVA followed by a Bonferroni multiple comparisons test (P = 0.116, One-factor ANOVA). There were no significant differences among group.
Biochemical and hormonal parameters
Biochemical and hormonal parameters at baseline and after 12 weeks are presented in Table 2. For serum TC concentrations, two-factor ANOVA revealed that there were main effects of time (P = 0.001) and time by group interactions (P = 0.012). Within-group analyses showed that serum TC concentrations were significantly increased in the WG, WSG and SG (paired t-test, P = 0.004; P = 0.048; P = 0.043, respectively) after 12 weeks relative to baseline values. Two-factor ANOVA revealed that there were no significant time by group interactions (P ≥ 0.060) for serum HDL-cholesterol, plasma HbA1c, sE-selectin, sVCAM-1 and C-peptide concentrations. Within-group analyses showed that serum HDL-cholesterol concentrations significantly increased in the WG (paired t-test, P = 0.038), plasma HbA1c concentrations significantly decreased in both the WSG and SG (paired t-test, P = 0.001; P = 0.023, respectively), and plasma glucose, sE-selectin, sVCAM-1, and C-peptide concentrations significantly increased in the CG (paired t-test, P = 0.045; P = 0.004; P = 0.002; P = 0.001, respectively) over the 12 week study period (Table 2). No significant changes were observed in serum triacylglycerol, serum LDL-cholesterol and plasma insulin concentrations between or within the groups.
Discussion
The main purpose of this study was to examine the effects of 12 weeks of a low-volume walking exercise programme (< 150 minutes/week) combined with vitamin E supplementation on plasma TBARS and oxidised LDL concentrations in older adults. We found that plasma oxidised LDL concentrations did not decrease over the study period, but lower TBARS concentrations were observed in all intervention groups (WG, WSG, SG) relative to the control group at the end of the study period. These results suggest that 90 minutes of walking per week and/or increased vitamin E intake may protect against oxidative damage in older adults.
Oxidative stress is associated with the development of chronic disease processes such as atherosclerosis and cardiovascular disease [21, 22]. The oxidative state is dependent on the equilibrium between the formation of free radicals and the action of antioxidant systems [23, 24]. However, free radicals increase with advancing age [25]. Our results demonstrate that a low-volume walking exercise programme decreased plasma concentrations of TBARS (a marker of oxidative damage). This has important for public health implications, and determining the minimum activity level that is beneficial could provide practical guidance to the general public and facilitate compliance with exercise recommendations. A number of studies have reported that the majority of individuals in many countries engage in insufficient amounts of physical activity and fail to meet current physical activity recommendations [26]. Although the clinical relevance of our findings is not yet known, elevated concentrations of oxidative stress markers increase with aging and are associated with an increased risk of conditions such as cancer, cardiovascular disease and metabolic syndrome [27, 28]. A recent prospective study demonstrated that insufficient physical activity was related to higher mortality rates and shorter life expectancies in both men and women [29]. Thus, a small change in plasma TBARS concentrations brought about by walking could have important health benefits.
We found that plasma TBARS concentrations were lower in the SG, WG and WSG compared with the CG at the end of the study period. Vitamin E is believed to be a beneficial for reducing oxidative stress and much attention has been paid to whether vitamin E improves the oxidative status in older adults [13]. Moreover, Vitamin E, exhibits antioxidant and anti-inflammatory effects and is able to modulate immune function [30]. The Recommended Daily Allowance for vitamin E is 15 mg (35 μmol/L) /day, which is required to maintain normal plasma α-tocopherol concentrations [31]. In Japan, adequate intake is 7.0 and 6.5 mg/day of vitamin E for men and women, respectively, to maintain the normal plasma α-tocopherol concentration of 12 μmol/L (Dietary Reference Intakes for Japanese, 2010). In our study, participants maintained their normal diet during the intervention period, and the SG and WSG received additional supplementation of 450 IU (300 mg) /day of vitamin E. The Tolerable Upper Intake Level for adults is set at 1,000 mg (2,325 μmol) /day of vitamin E; this limit is based on the adverse effect of increased tendency to haemorrhage when higher doses are taken [28]. However, the present study did not provide placebo tablets for the CG and WG. Therefore, compared with the results of other placebo-controlled studies, our results should be interpreted with caution [13, 32]. It has been previously reported that both regular physical activity and vitamin E supplementation are useful for reducing lipid hydroperoxide concentrations and resting blood pressure in older adults [13]. In this previous study, the exercise intervention consisted of exercising at 50–75% of the predicted maximum heart rate for a minimum of 30 min twice weekly combined with 400 IU (267 mg) vitamin E supplementation. Our findings emphasise the potential health benefits of low-volume walking programme (90 minutes/week) and encouraging vitamin E supplementation (450 IU (300 mg) /day) for older adults. Low-volume physical activity may act similarly to, or synergistically with, vitamin E to reduce markers of oxidative damage in this population.
We found no significant changes in plasma oxidised LDL concentrations in the intervention groups over the study period. These findings are inconsistent with those of a previous study [33], which reported that long term (3 years) supplementation with 400 IU (267 mg) of vitamin E daily in healthy individuals significantly reduced circulating oxidised LDL. Whereas many studies have reported that high dose of vitamin E are poorly effective at decreasing levels of lipid peroxidation in humans [34, 35]. Therefore, the shorter duration of our study might explain this difference in results [36]. Another study previously reported that high intensity training (66% of heart rate reserve, 60 minute/session, 3 times/week for 10 weeks) significantly reduced plasma oxidised LDL concentrations in older adults [6]. However, we chose to utilise a low-volume walking exercise programme because it is difficult to achieve compliance with high intensity physical activity recommendations, particularly in older adults. It would be of interest to examine a more long term exercise programme and vitamin E supplementation in a similar population to see whether this influences oxidised LDL concentrations.
Previous studies have examined the combined effects of healthy life style choices on mortality risk to assess the additive effects of such behaviours [37, 38]. It is important to address this issue because combining physical activity with other healthy lifestyle behaviours (such as a healthy diet, not smoking, and only moderate alcohol consumption) appears to provide greater protection from disease than physical activity alone. In the present study, our results showed that cardiovascular disease risk markers (i.e. plasma glucose, sE-selectin, sVCAM-1, C-peptide concentrations) were significantly increased in the CG after 12 weeks compared with the baseline values; but no significant changes occurred in the WG, SG and WSG. On the other hand, the present study found that a low-volume walking exercise programme increases serum HDL-C concentrations. These observations are consistent with data from the meta-analyses [39], indicating physically active individuals have significantly higher concentrations of HDL-C compared with sedentary counterparts. Also, a meta-analysis of previous studies has suggested that higher training volume (i.e. duration × intensity) was necessary to increase HDL-C [40]. Since increased circulating concentrations of oxidised LDL and other cardiovascular disease risk markers observed in the CG these results may suggest that a sedentary lifestyle lead to accelerated atherosclerotic lesion formation. However, this is only speculation. Thus, it would be interesting to assess whether increased or decreased these parameters during a long-term period influences by their sedentary lifestyle.
The present study had some limitations. First, this study was a non-randomised trial and thus we have recruited the participants of each group at the same time. Thus, we could not strictly match the baseline value among groups. Consequently, there were significant differences in SBP among the four groups. Furthermore, the non-randomisation of participants to groups may negatively impact the significance of the present study due to the sample selection bias. Second, we are not able to speculate increased concentrations of glucose, sE-selectin, VCAM and C-peptide in the CG. In addition, intra-group analyses revealed that body mass in the S and Ex groups and BMI in the S group significantly increased after the intervention. It is possible to speculate that this may due in part to the dietary control as we did not fully control the participants’ diet throughout the study.
Conclusion
In conclusion, this study demonstrated that 12 weeks of a low-volume walking programme (< 150 min/week) combined with daily vitamin E supplementation did not reduce plasma oxidised LDL concentrations and cardiovascular disease risk markers, but did reduce plasma TBARS concentrations in older adults.
References
Nedeljkovic ZS, Gokce N, Loscalzo J: Mechanisms of oxidative stress and vascular dysfunction. Postgrad Med J. 2003, 79 (930): 195-199. 10.1136/pmj.79.930.195.
Stocker R, Keaney JF: Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004, 84 (4): 1381-1478. 10.1152/physrev.00047.2003.
Hulthe J, Fagerberg B: Circulating oxidized LDL is associated with subclinical atherosclerosis development and inflammatory cytokines (AIR Study). ArteriosclerThrombVascBiol. 2002, 22 (7): 1162-1167.
Cakatay U, Kayali R, Uzun H: Relation of plasma protein oxidation parameters and paraoxonase activity in the ageing population. ClinExp Med. 2008, 8 (1): 51-57. 10.1007/s10238-008-0156-0.
Kasapoglu M, Ozben T: Alterations of antioxidant enzymes and oxidative stress markers in aging. Exp Gerontol. 2001, 36 (2): 209-220. 10.1016/S0531-5565(00)00198-4.
Cornelissen VA, Arnout J, Holvoet P, Fagard RH: Influence of exercise at lower and higher intensity on blood pressure and cardiovascular risk factors at older age. J Hypertens. 2009, 27 (4): 753-762. 10.1097/HJH.0b013e328322cf60.
Tully MA, Cupples ME, Hart ND, McEneny J, McGlade KJ, Chan WS, Young IS: Rondomised controlled trial of home-based walking programmes at and below current recommended levels of exercise in sedentary adults. J Epidemiol Community Health. 2007, 61 (9): 778-783. 10.1136/jech.2006.053058.
Rimm EB, Stampfer MJ, Ascherio A, Giovannucci E, Colditz GA, Willett WC: Vitamin E consumption and the risk of coronary heart disease in men. N Engl J Med. 1993, 328 (20): 1450-1456. 10.1056/NEJM199305203282004.
Stampfer MJ, Hennekens CH, Manson JE, Colditz GA, Rosner B, Willett WC: Vitamin E consumption and the risk of coronary disease in women. N Engl J Med. 1993, 328 (20): 1444-1449. 10.1056/NEJM199305203282003.
Devaraj S, Tang R, Adams-Huet B, Harris A, Seenivasan T, de Lemos JA, Jialal I: Effect of high-dose alpha-tocopherol supplementation on biomarkers of oxidative stress and inflammation and carotid atherosclerosis in patients with coronary artery disease. Am J Clin Nutr. 2007, 86 (5): 1392-1398.
Traber MG, Atkinson J: Vitamin E, antioxidant and nothing more. Free Radic Biol Med. 2007, 43 (1): 4-15. 10.1016/j.freeradbiomed.2007.03.024.
Salonen RM, Nyyssönen K, Kaikkonen J, Porkkala-Sarataho E, Voutilainen S, Rissanen TH, Tuomainen TP, Valkonen VP, Ristonmaa U, Lakka HM, Vanharanta M, Salonen JT, Poulsen HE, Antioxidant Supplementation in Atherosclerosis Prevention Study: Six-year effect of combined vitamin C and E supplementation on atherosclerotic progression: the Antioxidant Supplementation in Atherosclerosis Prevention (ASAP) Study. Circulation. 2003, 107 (7): 947-953. 10.1161/01.CIR.0000050626.25057.51.
Jessup JV, Horne C, Yarandi H, Quindry J: The Effects of Endurance Exercise and Vitamin E on Oxidative Stress in the Elderly. Biol Res Nurs. 2003, 5 (1): 47-55. 10.1177/1099800403005001005.
Bobeuf F, Labonte M, Dionne IJ, Khalil A: Combined effect of antioxidant supplementation and resistance training on oxidative stress markers, muscle and body composition in an elderly population. J Nutr Health Aging. 2011, 15 (10): 883-889. 10.1007/s12603-011-0097-2.
Butcher LR, Thomas A, Backx K, Roberts A, Webb R, Morris K: Low-intensity exercise exerts beneficial effects on plasma lipids via PPARgamma. Med Sci Sports Exerc. 2008, 40 (7): 1263-1270. 10.1249/MSS.0b013e31816c091d.
Borg GA: Perceived exertion: a note on “history” and methods. Med Sci Sports. 1973, 5 (2): 90-93.
Chen C, Jerome GJ, Laferriere D, Young DR, Vollmer WM: Procedures used to standardize data collected by RT3 triaxial accelerometers in a large-scale weight-loss trial. J Phys Act Health. 2009, 6 (3): 354-359.
Matthews CE, Chen KY, Freedson PS, Buchowski MS, Beech BM, Pate RR, Troiano RP: Amount of time spent in sedentary behaviors in the United States, 2003–2004. Am J Epidemiol. 2008, 167 (7): 875-881. 10.1093/aje/kwm390.
Kumahara H, Schutz Y, Ayabe M, Yoshioka M, Yoshitake Y, Shindo M, Ishii K, Tanaka H: The use of uniaxial accelerometry for the assessment of physical-activity-related energy expenditure: a validation study against whole-body indirect calorimetry. Br J Nutr. 2004, 91 (2): 235-243. 10.1079/BJN20031033.
Talwar D, Ha TK, Cooney J, Brownlee C, O’Reilly DS: A routine method for the simultaneous measurement of retinol, alpha-tocopherol and five carotenoids in human plasma by reverse phase HPLC. Clin Chim Acta. 1998, 270 (2): 85-100. 10.1016/S0009-8981(97)00224-6.
Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA: The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011, 11 (9): 607-615. 10.1038/nri3041.
Patel RS, Al Mheid I, Morris AA, Ahmed Y, Kavtaradze N, Ali S, Dabhadkar K, Brigham K, Hooper WC, Alexander RW, Jones DP, Quyyumi AA: Oxidative stress is associated with impaired arterial elasticity. Atherosclerosis. 2011, 218 (1): 90-95. 10.1016/j.atherosclerosis.2011.04.033.
Elosua R, Arquer A, Marrugat J: Physical activity and lipid oxidation. Apunts Med Esport. 2009, 45: 30-39.
Witzum JL: The oxidation hypothesis of atherosclerosis. Lancet. 1994, 344 (8925): 793-795. 10.1016/S0140-6736(94)92346-9.
Harman D: Origin and evolution of the free radical theory of aging: a brief personal history, 1954–2009. Biogerontology. 2009, 10 (6): 773-781. 10.1007/s10522-009-9234-2.
Wai JP, Wen CP, Chan HT, Tsai MK, Tsai SP, Chang HY: Assessing physical activity in an Asian country: low energy expenditure and exercise frequency among adults in Taiwan. Asia Pac J Clin Nutr. 2008, 17 (2): 297-308.
Ferrucci L, Corsi A, Lauretani F, Bandinelli S, Bartali B, Taub DD, Guralnik JM, Longo DL: The origins of age-related proinflammatory state. Blood. 2005, 105 (6): 2294-2299. 10.1182/blood-2004-07-2599.
Ji LL: Antioxidant enzyme response to exercise and aging. Medicine & Science in Sports & Exercise. 1993, 25 (2): 225-231.
Wen CP, Wai JP, Tsai MK, Yang YC, Cheng TY, Lee MC, Chan HT, Tsao CK, Tsai SP, Wu X: Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet. 2011, 378 (9798): 1244-1253. 10.1016/S0140-6736(11)60749-6.
Capuron L, Moranis A, Combe N, Cousson-Gélie F, Fuchs D, De S-PV, Barberger-Gateau P, Layé S: Vitamin E status and quality of life in the elderly: influence of inflammatory processes. Br J Nutr. 2009, 102 (10): 1390-1394. 10.1017/S0007114509990493.
Institute of Medicine: Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids: Washington. 2000, DC: National Academics Press
Rokitzki L, Logemann E, Huber G, Keck E, Keul J: alpha-Tocopherol supplementation in racing cyclists during extreme endurance training. Int J Sport Nutr. 1994, 4 (3): 253-264.
Hodis HN, Mack WJ, LaBree L, Mahrer PR, Sevanian A, Liu CR, Liu CH, Hwang J, Selzer RH, Azen SP: Alpha-tocopherol supplementation in healthy individuals reduces low-density lipoprotein oxidation but not atherosclerosis: the Vitamin E Atherosclerosis Prevention Study (VEAPS). Circulation. 2002, 106 (12): 1453-1459. 10.1161/01.CIR.0000029092.99946.08.
Meagher EA, Barry OP, Lawson JA, Rokach J, FitzGerald GA: Effects of vitamin E on lipid peroxidation in healthy persons. JAMA. 2001, 285 (9): 1178-1182. 10.1001/jama.285.9.1178.
Morrow JD: Quantification of isoprostanes as indices of oxidant stress and the risk of atherosclerosis in humans. Arterioscler Thromb Vasc Biol. 2005, 25 (2): 279-286.
Steinhubl SR: Why have antioxidants failed in clinical trials?. Am J Cardiol. 2008, 101 (10A): 14D-19D.
Knoops KT, de Groot LC, Kromhout D, Perrin AE, Moreiras-Varela O, Menotti A, van Staveren WA: Mediterranean diet, lifestyle factors, and 10-year mortality in elderly European men and women: the HALE project. JAMA. 2001, 292 (12): 1433-1439.
Khaw KT, Wareham N, Bingham S, Welch A, Luben R, Day N: Combined impact of health behaviours and mortality in men and women: the EPIC-Norfolk prospective population study. PLoS Med. 2008, 5 (1): e12. 10.1371/journal.pmed.0050012.
Hata Y, Nakajima K: Life-style and serum lipids and lipoproteins. J Atheroscler Thromb. 2007, 7 (4): 177-197.
Kodama S, Tanaka S, Saito K, Shu M, Sone Y, Onitake F, Suzuki E, Shimano H, Yamamoto S, Kondo K, Ohashi Y, Yamada N, Sone H: Effect of aerobic exercise training on serum levels of high-density lipoprotein cholesterol: a meta-analysis. Arch Inern Med. 2007, 167 (10): 999-1008. 10.1001/archinte.167.10.999.
Acknowledgements
This study was supported by a grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan and Grants-in-Aid for the Global COE program “Sport Science for the Promotion of Active life” (2011–2012).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
All authors declare that they have no competing interests.
Authors’ contributions
JHP supervised data collection, performed data analysis, wrote the first draft of the manuscript, and supervised the walking programme. MM conceived the study, obtained funding, recruited participants, assisted JHP with data collection and blood analysis, edited the manuscript, and supervised the walking programme. MT was involved in the recruitment of participants, assisted JHP with data collection, and supervised the walking programme. KN, SRB, and SHK were involved in the recruitment of participants, assisted JHP with data collection, and supervised the walking programme. KS performed venous blood collection and provided guidance and assistance to JHP during the study. YN conceived the study and provided guidance and assistance to JHP during the study. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Park, JH., Miyashita, M., Takahashi, M. et al. Effects of low-volume walking programme and vitamin E supplementation on oxidative damage and health-related variables in healthy older adults. Nutr Metab (Lond) 10, 38 (2013). https://doi.org/10.1186/1743-7075-10-38
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1743-7075-10-38