Abstract
Background
S100B is a calcium-binding protein that is produced primarily by astrocytes. Increased serum S100B protein levels reflect neurological damage. Autoimmunity may have a role in the pathogenesis of autism in some patients. Autoantibodies may cross the blood-brain barrier and combine with brain tissue antigens, forming immune complexes and resulting in neurological damage. We are the first to investigate the relationship between serum levels of S100B protein, a marker of neuronal damage, and antiribosomal P protein antibodies in autistic children.
Methods
Serum S100B protein and antiribosomal P antibodies were measured in 64 autistic children in comparison to 46 matched healthy children.
Results
Autistic children had significantly higher serum S100B protein levels than healthy controls (P < 0.001). Children with severe autism had significantly higher serum S100B protein than patients with mild to moderate autism (P = 0.01). Increased serum levels of antiribosomal P antibodies were found in 40.6% of autistic children. There were no significant correlations between serum levels of S100B protein and antiribosomal P antibodies (P = 0.29).
Conclusions
S100B protein levels were elevated in autistic children and significantly correlated to autistic severity. This may indicate the presence of an underlying neuropathological condition in autistic patients. Antiribosomal P antibodies may not be a possible contributing factor to the elevated serum levels of S100B protein in some autistic children. However, further research is warranted to investigate the possible link between serum S100B protein levels and other autoantibodies, which are possible indicators of autoimmunity to central nervous system in autism.
Similar content being viewed by others
Introduction
S100 proteins comprise a multitude of low-molecular-weight, calcium-binding proteins that interact with other proteins to modulate biological processes [1]. They have been named "S100" because of their biochemical property of remaining soluble after precipitation with 100% ammonium sulfate [2]. S100B protein is characterized by the presence of two calcium binding sites of the EF-hand type (helix-loop-helix), one of which is located in the NH2 terminus and is noncanonical, whereas the other binding site is located in the COOH terminus and is canonical. This configuration enables S100 protein to respond to a calcium stimulus induced by cell signaling [3]. S100B protein is chiefly found in glial cells and Schwann cells in the central nervous system (CNS) [4]. The clinical significance of S100B protein has substantially increased throughout several areas of clinical neuroscience as it can be used as a reliable and early predictor of poor physiological and cognitive neurological outcomes [5]. Serum and cerebrospinal fluid (CSF) levels of S100B protein levels are raised in some autoimmune neuropsychiatric disorders, reflecting the presence of glial cell pathology and continuing neurological damage [6–8].
Autoimmunity may play a role in autism in a subgroup of patients [9, 10], as indicated by the presence of brain-specific autoantibodies in some autistic children [11–17]. These autoantibodies may cross the blood-brain barrier (BBB) and combine with brain tissue antigens, forming immune complexes that result in damage of the neurological tissue [10]. Also, there is an increase in the frequency of autoimmune disorders within autistic families [18–23]. In spite of the fact that the origins of autoimmunity in autism are unknown, in some autistic children there is an imbalance of T helper 1 (Th1)/Th2 subsets toward Th2, which are responsible for allergic response and production of antibodies [9]. Moreover, there is a strong association between autism and the major histocompatibility complex for the null allele of C4B in the class III region. This results in low production of C4B protein, leading to repeated infections, which play an important role in the development of autoimmunity [21, 24, 25].
Various antibodies against neuronal tissues have been discovered in immune-mediated neurological disorders. Some of these antibodies have been found to correlate with the pathomechanism of these diseases [26]. Antiribosomal P protein antibodies are one group of potentially pathogenic autoantibodies that have a specificity for the functional center of the ribosomal P proteins. These proteins are a family of highly conserved acidic phosphoproteins located primarily on the stalk of the large (60s) ribosomal subunit [27]. They bind three ribosomal proteins, identified as P0, P1 and P2 (38, 19 and 17 kDa, respectively) by recognizing a certain epitope found in those three proteins. Several possible pathogenic mechanisms for these antibodies in some autoimmune diseases include their binding to epitopes on the cell membrane surface, intracellular penetration, inhibition of protein synthesis, production of proinflammatory cytokines and induction of cellular apoptosis [28].
In this study, we aimed to investigate the relationship between serum levels of S100B protein, a marker of neuronal damage, and antiribosomal P protein antibodies as indicators of the presence of autoimmunity in a group of autistic children.
Methods
Study population
This cross-sectional study was conducted on 64 children with autism. They were recruited from the Autism Research and Treatment Center, Faculty of Medicine, King Saud University, Riyadh, Saudi Arabia. Patients were fulfilling the criteria of the diagnosis of autism according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition [29]. The autistic group comprised 50 males and 14 females. Their ages ranged from 5 to 12 years (mean ± SD = 8.4 ± 2.5 years).
Exclusions criteria
The exclusion criteria were (1) patients who had associated neurological diseases (such as cerebral palsy and tuberous sclerosis) and metabolic disorders (such as phenylketonuria); (2) patients with associated allergic, inflammatory or autoimmune disorders; and (3) patients who were receiving any medications.
The control group comprised 46 age- and sex-matched, apparently healthy children (34 males and 12 females). They were the healthy older siblings of the healthy infants who attend the Well Baby Clinic, King Khalid University Hospital, Faculty of Medicine, King Saud University, Riyadh, Saudi Arabia, for routine follow-up of their growth parameters. The control children were not related to the children with autism and demonstrated no clinical findings suggestive of immunological or neuropsychiatric disorders. Their ages ranged from 6 to 12 years (mean ± SD = 9.1 ± 2.4 years). The local ethical committee of the Faculty of Medicine, King Saud University, Riyadh, Saudi Arabia, approved this study. In addition, an informed written consent statement of participation in the study was signed by the parents or the legal guardians of the studied subjects.
Study measurements
Clinical evaluation of autistic patients
The evaluation of patients was based on clinical history taken from caregivers, clinical examination and neuropsychiatric assessment. In addition, the degree of the disease severity was assessed by using the Childhood Autism Rating Scale (CARS) [30], which rates the child on a scale from 1 to 4 in each of 15 areas (relating to people; emotional response; imitation; body use; object use; listening response; fear or nervousness; verbal communication; nonverbal communication; activity level; level and consistency of intellectual response; adaptation to change; visual response; taste, smell and touch responses; and general impressions). According to this scale, children who score 30 to 36 have mild to moderate autism (n = 30) and those with scores ranging from 37 to 60 have a severe degree of autism (n = 34).
Serum assessment of S100B protein
Serum levels of S100B protein were evaluated using an ELISA kit [31]. To increase accuracy, all samples were analyzed twice in two independent experiments to assess the interassay variations and to ensure reproducibility of the observed results (P > 0.05). No significant cross-reactivity or interference was observed.
Measurement of serum anti-ribosomal P protein antibodies
Serum total immunoglobulin G (IgG) and IgM antiribosomal P protein antibodies were measured by ELISA using ribosomal P peptide-BSA conjugate as an antigen (Nunc-Immuno Module F8 MaxiSorp; Nunc, Roskilde, Denmark). To increase accuracy, all samples were analyzed twice in two independent experiments to assess the interassay variations and to ensure reproducibility of the observed results (P > 0.05). No significant cross-reactivity or interference was observed.
Statistical analysis
The results were analyzed by using a commercially available software package (StatView; Abacus Concepts, Inc, Berkeley, CA, USA). The data are presented as means ± 2 SD in addition to medians and IQRs, which are between the 25th and 75th percentiles, for parametric and nonparametric data, respectively. Student's t-test and the Mann-Whitney U test were used for comparisons between parametric and nonparametric data, respectively. A χ 2 test was used for comparison between qualitative variables of the studied groups. Spearman's ρ correlation coefficient r was used to determine the relationship between different variables. For all tests, P < 0.05 was considered significant. Patients were considered to have elevated serum S100B protein or antiribosomal P protein antibodies if their levels were above the highest cutoff values (223.3 pg/ml and 101.3 U/ml, respectively), which were the means ± 2 SD and 95th percentiles of serum S100B protein and antiribosomal P protein levels, respectively, of healthy controls.
Results
Serum S100B protein levels in autistic children and their relation to the degree of the severity of autism
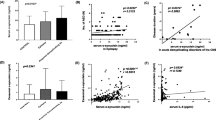
Autistic children had significantly higher serum S100B protein levels (207.97 ± 52.6 pg/ml) than healthy controls (171.33 ± 34.65 pg/ml) (P < 0.001) (Figure 1). Increased serum S100B protein levels were found in 23 (35.9%) of 64 autistic patients.
Patients with severe autism had significantly higher serum S100B protein levels (222.47 ± 52.95 pg/ml) than children with mild to moderate autism did (191.53 ± 47.89 pg/ml) (P = 0.01). Although the frequency of increased serum S100B protein levels was higher in children with severe autism (44.1%) than in patients with mild to moderate autism (26.7%), this difference did not reach statistical significance (P = 0.1) (Table 1). In spite of the presence of positive correlations between serum levels of S100B protein and CARS in autistic patients, these correlations did not reach statistical significance (P = 0.055).
Relationship between elevated serum levels of S100B protein and antiribosomal P protein antibodies in autistic children
Increased serum levels of antiribosomal P protein antibodies were found in 26 (40.6%) of 64 autistic patients. Patients with severe autism had significantly higher serum antiribosomal P protein antibodies [median (IQR) = 400 (459) U/ml] than children with mild to moderate autism [median (IQR) = 9 (23) U/ml] (P = 0.01) (Figure 2). Also, the frequency of increased serum antiribosomal P protein antibodies was significantly higher in children with severe autism (64.7%) than in patients with mild to moderate autism (13.3%) (P < 0.001) (Table 1). Moreover, there were significant positive correlations between serum levels of antiribosomal P protein antibodies and CARS in autistic patients (P < 0.001) (Figure 3).
Although patients with elevated serum S100B protein levels had a higher frequency of positivity of serum antiribosomal P protein antibodies (47.8%) than patients with normal serum S100B protein levels did (36.6%), this difference did not reach statistical significance (P = 0.27) (Table 2). In addition, serum S100B protein levels had no significant correlations with serum levels of antiribosomal P protein antibodies (P = 0.29).
Discussion
S100B is an astrocytic calcium-binding protein that has been proposed as a biochemical marker of brain damage or dysfunction in acute and chronic neurological diseases [6]. In our series, autistic children had significantly higher serum S100B protein levels than healthy controls (P < 0.001). Increased serum S100B protein levels were found in 35.9% of autistic patients. S100B protein is a marker of neuronal damage that was first isolated from the CNS in vertebrates [2], and it is chiefly found in glial cells and Schwann cells [4]. Researchers in a previous study reported that risperidone, a drug used to improve autism symptoms, induced a statistically significant increment of about 80% of S100B protein secretion. These data contribute to the proposal that glial cells are targets of risperidone [32].
S100B is a protein produced primarily by brain astrocytes, and it is an established peripheral biomarker of altered BBB permeability associated with various CNS diseases [32, 33]. Elevated S100B protein levels accurately reflect the presence of neuropathological conditions, including traumatic head injuries [33–35], psychiatric disorders [36], cerebrovascular insults [37] and neurodegenerative diseases [38], whereas normal levels reliably exclude major CNS pathology [35, 39, 40]. Thus the increase of serum S100B protein levels in autistic patients may indicate the presence of an underlying neuropathological condition.
The potential clinical use of measurement of serum S100B protein levels in the therapeutic decision-making process is substantiated by a vast body of literature validating variations in serum S100B levels with standard modalities for prognosticating the extent of CNS damage, alterations in neuroimaging, cerebrospinal pressure and other brain molecular markers (neuron-specific enolase, glial fibrillary acidic protein) [41, 42]. The major advantage of using S100B levels is that elevations in serum can be measured easily, providing a sensitive tool with which to help rule out major CNS dysfunction. Moreover, as serum S100B levels reflect BBB permeability changes even in the absence of neuronal injury [33–39, 43, 44], they may increase prior to a significant change in neurological function or neuronal cell death. This is an important clinical finding, as the normal range of S100B levels rules out cerebrovascular damage and injury to the CNS in nearly 99% of patients by neurological imaging [40, 45].
S100B protein is implicated in intracellular and extracellular regulatory activities. Intracellularly, it exhibits regulatory effects on cell growth, differentiation and shape, as well as energy metabolism. Extracellularly, S100B protein stimulates neuronal survival, differentiation, astrocytic proliferation, neuronal death via apoptosis and regulation of the activity of inflammatory cells [46]. S100 proteins are key mediators in polymorphonuclear neutrophil migration [47]. The degree of systemic inflammation is associated with S100B protein concentration in acute ischemic stroke [48]. Several studies have suggested that S100B protein has a role in the pathogenesis of some autoimmune neuropsychiatric diseases, such as multiple sclerosis (MS) and neuropsychiatric systemic lupus erythematosus (NPSLE) [6–8]. Phenotypically and functionally, S100B-specific T cells can be recovered from the peripheral blood of patients with MS, making S100B a potential candidate autoantigen in MS [49]. Furthermore, S100B protein may act as a cytokine [46, 50, 51], and in vitro studies have shown that, at high levels, S100B protein can induce the neuronal expression and secretion of proinflammatory IL-6. Elevated levels of S100B have been detected in the CSF of MS patients during acute phases or exacerbations of the disease [50], and it has therefore been proposed that elevated S100B protein may be indicative of active cell injury [51] and can reflect an axonal and glial pathology. Measurement of serum concentrations of S100B protein may be useful for monitoring immunosuppressive therapy and may support clinical assessment of patients with MS [8]. Serum and CSF S100B protein levels were raised in patients with NPSLE, especially among patients with organic brain syndrome, seizures, cerebral vascular accident and psychosis. This may imply that S100B protein might serve as an available and complementary biochemical marker within evaluations of NPSLE. The association of anti-double-stranded DNA (anti-dsDNA) antibodies with higher S100B protein levels may indicate that raised serum levels of S100B protein may reflect continuing neurological damage [6, 7, 52].
In the present work, patients with severe autism had significantly higher serum S100B protein levels than children with mild to moderate autism (P = 0.01). This may indicate that the extent of the elevation of serum S100B protein levels was closely linked to the degree of the severity of autism. This may be explained by the increment in the degree of neurological damage with autistic severity, resulting in more production of S100B protein. However, it is not easy to determine whether the increase in serum S100B protein levels is a mere consequence of autism or has a pathogenic role in the disease.
A possible role of abnormalities in the immune system in the pathogenesis of autism was previously postulated [9, 10, 53, 54]. Autoimmunity to the CNS is the commonest of these abnormalities in autism. This may be indicated by the presence of brain-specific autoantibodies in some autistic children [9–17]. Immune system dysfunction may represent a novel target for treatment in autism [55]. In our series, increased serum levels of antiribosomal P protein antibodies were found in 40.6% of autistic patients. Autoantibodies are the hallmark of autoimmune diseases. We recently reported increased serum levels of antiribosomal P protein antibodies in 44.3% of another group of 70 autistic children between ages 6 and 11 years [56]. This was the only study that in which the serum levels of these antibodies in autism were investigated.
Antiribosomal P protein antibodies are highly specific for SLE, especially for the neuropsychiatric manifestations, including psychosis, mood disorders, anxiety, cognitive dysfunction and delirium [57]. A recent study has demonstrated a strong association between the seropositivity of antiribosomal P protein antibodies and the presence of neuropsychiatric manifestations in a group of children with SLE [58]. Some studies in the literature have related antiribosomal P protein antibodies to the pathogenesis of organ damage in SLE. The main pathways described are cross-reaction with anti-dsDNA antibodies, a cytotoxic effect on mesangium cell proliferation, invasion into living cells and initiation of apoptosis, a defect in the synthesis of apolipoprotein B resulting in accumulation of lipids inside the cell, and downregulation of total protein synthesis. P proteins are posttranslationally modified (dephosphorylated) during apoptosis, and a dysregulation in the normal clearance of apoptotic cells leads to aberrant exposure of the immune system to modified non-self-antigens. This could be one of the triggering events for the development of anti-P protein autoimmune response in some autoimmune diseases [57].
Moreover, in an experimental study, mice that received intracerebroventricular injections of antiribosomal P protein antibodies developed depression-like behaviors, which seems to be mediated by specific binding of these antibodies to limbic system brain areas, such as the hippocampus and the cingulate cortex. It has been proposed that antiribosomal P protein antibodies directly and/or indirectly affect the CNS and produce a cytotoxic effect on neuronal cells. The mechanism by which these antibodies cross the BBB is unknown [59].
In this work, patients with severe autism had significantly higher serum antiribosomal P protein antibodies than did children with mild to moderate autism (P = 0.01). Also, the frequency of increased serum antiribosomal P protein antibodies was significantly higher in children with severe autism (64.7%) than in patients with mild to moderate autism (13.3%) (P < 0.001). Moreover, there were significant positive correlations between serum levels of antiribosomal P protein antibodies and CARS in autistic patients (P < 0.001). This might indicate that the extent of the elevation of serum antiribosomal P protein antibodies was linked to the degree of severity of autism as assessed by CARS. The relationship between antiribosomal P protein antibodies and the severity of autism might be a causal one in which these autoantibodies might play a role in the pathogenesis of brain damage, the extent of which may determine the clinical severity of autism. This warrants other studies to reveal the pathogenic role of these antibodies in autism.
The reason underlying the formation of some autoantibodies in some patients with autism is not fully understood. It is speculated that an autoimmune reaction might be trigged by cross-reacting antigens in the environment, resulting in the release of some self-antigens. These antigens may result in the induction of autoimmune reactions through the activation of inflammatory cells in genetically susceptible individuals [9, 10]. The involvement of the cellular redox state in the pathogenesis of autoimmune diseases, including autism, has been extensively demonstrated. Protein peroxidation confers alterations in protein structure with impairment of their functional properties. Proteins with a substantial conformational loss are potential sources of neoantigens, leading to induction of autoimmunity in a variety of autoimmune diseases [60, 61]. Researchers in a recent study reported that many autistic children have increased oxidative stress resulting from enhanced lipid peroxidation and/or a decrease in the level of glutathione peroxidase, which is an important antioxidant. The same researchers in that study also reported a possible role of oxidative stress in the induction of autoimmunity in some autistic patients [62]. Moreover, one study found that CD4+CD25high regulatory T cells were deficient in 73.3% of a group of 30 autistic children [23]. These cells play an active part in the establishment and maintenance of immunological self-tolerance and thereby prevent autoimmunity [63, 64]. Thus a deficiency of these cells may contribute to autoimmunity in a subgroup of autistic children [23].
Serum S100B protein levels have been reported to be increased in some autoimmune neuropsychiatric diseases, indicating the presence of underlying neurological damage [6–8]. Because autoantibodies can cross the BBB and combine with brain tissue antigens to form immune complexes that result in neurological damage [10], we have tried to find a possible link between the elevated serum levels of S100B protein and antiribosomal P protein antibodies in autism. In this work, although patients with elevated serum S100B protein levels had a higher frequency of positivity of serum antiribosomal P protein antibodies (47.8%) than patients with normal serum S100B protein levels did (36.6%), this difference did not reach statistical significance (P = 0.27).
In addition, serum S100B protein levels had no significant correlations with serum levels of antiribosomal P protein antibodies (P = 0.29). We could not trace data in the literature to compare our results concerning the relationship between serum levels of S100B protein and autoantibodies in autistic patients. This study is the first to explore such a relationship.
The results of this study may indicate that the presence of antiribosomal P protein antibodies in some autistic children may not be a possible contributing factor to their elevated serum levels of S100B protein. The increased serum levels of S100B protein may be attributable to other factors that may participate in neurological damage in autism. However, this is an initial report that warrants further research to determine the possible link between the elevated serum levels of S100B protein and antiribosomal P protein antibodies in autistic children.
Conclusions
S100B protein levels were elevated in autistic children, and they were significantly correlated with the degree of the severity of autism. This may indicate the presence of an underlying neuropathological condition in autistic patients. Antiribosomal P protein antibodies might not be a contributing factor to the elevated serum levels of S100B protein in some autistic children. However, further research is warranted to investigate the possible link between serum S100B protein levels and other autoantibodies, which are possible indicators of autoimmunity to CNS, in autism.
Abbreviations
- BSA:
-
bovine serum albumin
- CARS:
-
Childhood Autism Rating Scale
- CNS:
-
central nervous system
- ELISA:
-
enzyme-linked immunosorbent assay
- IL:
-
interleukin
- kDa:
-
kilodalton
- Th:
-
T helper
- SLE:
-
systemic lupus erythematosus
References
Tu CL, Chang W, Bikle DD: The extracellular calcium-sensing receptor is required for calcium-induced differentiation in human keratinocytes. J Biol Chem 2001, 276:41079–41085.
Smit LH, Korse CM, Hart AA, Bonfrer JM, Haanen JB, Kerst JM, Nieweg OE, de Gast GC: Normal values of serum S-100B predict prolonged survival for stage IV melanoma patients. Eur J Cancer 2005, 41:386–392.
Donato R: Intracellular and extracellular roles of S100 proteins. Microsc Res Tech 2003, 60:540–551.
Jackel A, Deichmann M, Waldmann V, Bock M, Näher H: S-100β protein in serum, a tumor marker in malignant melanoma--current state of knowledge and clinical experience. Hautarzt 1999, 50:250–256.
Lippi G, Aloe R, Numeroso F, Cervellin G: The significance of protein S-100B testing in cardiac arrest patients. Clin Biochem 2011, 44:567–575.
Portela LV, Brenol JC, Walz R, Bianchin M, Tort AB, Canabarro UP, Beheregaray S, Marasca JA, Xavier RM, Neto EC, Gonçalves CA, Souza DO: Serum S100B levels in patients with lupus erythematosus: preliminary observation. Clin Diagn Lab Immunol 2002, 9:164–166.
Schenatto CB, Xavier RM, Bredemeier M, Portela LV, Tort AB, Dedavid e Silva TL, Souza DO, Brenol JC: Raised serum S100B protein levels in neuropsychiatric lupus. Ann Rheum Dis 2006, 65:829–831.
Bartosik-Psujek H, Psujek M, Jaworski J, Stelmasiak Z: Total tau and S100b proteins in different types of multiple sclerosis and during immunosuppressive treatment with mitoxantrone. Acta Neurol Scand 2011, 123:252–256.
Cohly HH, Panja A: Immunological findings in autism. Int Rev Neurobiol 2005, 71:317–341.
Vojdani A, Pangborn JB, Vojdani E, Cooper EL: Infections, toxic chemicals and dietary peptides binding to lymphocyte receptors and tissue enzymes are major investigators of autoimmunity in autism. Int J Immunopathol Pharmacol 2003, 16:189–199.
Singh VK, Warren RP, Odell JD, Warren WL, Cole P: Antibodies to myelin basic protein in children with autistic behavior. Brain Behav Immun 1993, 7:97–103.
Mostafa GA, Al-Ayadhi LY: A lack of association between hyperserotonemia and the increased frequency of serum anti-myelin basic protein auto-antibodies in autistic children. J Neuroinflammation 2011, 8:71.
Singh VK, Lin SX, Yang VC: Serological association of measles virus and human herpesvirus-6 with brain autoantibodies in autism. Clin Immunol Immunopathol 1998, 89:105–108.
Singh VK, Warren RP, Averett R, Ghaziuddin M: Circulating autoantibodies to neuronal and glial filament proteins in autism. Pediatr Neurol 1997, 17:88–90.
Singh VK, Rivas WH: Prevalence of serum antibodies to caudate nucleus in autistic children. Neurosci Lett 2004, 355:53–56.
Mostafa GA, El-Sayed ZA, Abd El Aziz MM, El-Sayed MF: Serum anti-myelin-associated glycoprotein antibodies in Egyptian autistic children. J Child Neurol 2008, 23:1413–1418.
Mostafa GA, Al-Ayadhi LY: Increased serum levels of anti-ganglioside M1 auto-antibodies in autistic children: relation to the disease severity. J Neuroinflammation 2011, 8:39.
Comi AM, Zimmerman AW, Frye VH, Law PA, Peeden JN: Familial clustering of autoimmune disorders and evaluation of medical risk factors in autism. J Child Neurol 1999, 14:388–394.
Sweeten TL, Bowyer SL, Posey DJ, Halberstadt GM, McDougle CJ: Increased prevalence of familial autoimmunity in probands with pervasive developmental disorders. Pediatrics 2003, 112:420–424.
Mostafa GA, Kitchener N: Serum anti-nuclear antibodies as a marker of autoimmunity in Egyptian autistic children. Pediatr Neurol 2009, 40:107–112.
Mostafa GA, Shehab A: The link of C4B null allele to autism and to a family history of autoimmunity in Egyptian autistic children. J Neuroimmunol 2010, 223:115–119.
Crespi BJ, Thiselton DL: Comparative immunogenetics of autism and schizophrenia. Genes Brain Behav 2011, 10:689–701.
Mostafa GA, Al Shehab A, Fouad NR: Frequency of CD4 + CD25 high regulatory T cells in the peripheral blood of Egyptian children with autism. J Child Neurol 2010, 25:328–335.
Warren RP, Singh VK, Cole P, Odell JD, Pingree CB, Warren WL, White E: Increased frequency of the null allele at the complement C4b locus in autism. Clin Exp Immunol 1991, 83:438–440.
Odell D, Maciulis A, Cutler A, Warren L, McMahon WM, Coon H, Stubbs G, Henley K, Torres A: Confirmation of the association of C4B null allele in autism. Hum Immunol 2005, 66:140–145.
Greenwood DL, Gitlits VM, Alderuccio F, Sentry JW, Toh BH: Auto-antibodies in neuropsychiatric lupus. Autoimmunity 2002, 35:79–86.
Gerli R, Caponi L: Anti-ribosomal P protein antibodies. Autoimmunity 2005, 38:85–92.
Toubi E, Shoenfeld Y: Clinical and biological aspects of anti-P-ribosomal protein autoantibodies. Autoimmun Rev 2007, 3:119–125.
American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders. 4th edition. Washington, DC: American Psychiatric Association; 1994.
Schopler E, Reichler RJ, Renner BR: The Childhood Autism Rating Scale (CARS) for Diagnostic Screening and Classification of Autism. New York: Irvington; 1986.
Green AJ, Keir G, Thompson EJ: A specific and sensitive ELISA for measuring S-100b in cerebrospinal fluid. J Immunol Methods 1997, 205:35–41.
Quincozes-Santos A, Abib RT, Leite MC, Bobermin D, Bambini-Junior V, Gonçalves CA, Riesgo R, Gottfried C: Effect of the atypical neuroleptic risperidone on morphology and S100B secretion in C6 astroglial lineage cells. Mol Cell Biochem 2008, 314:59–63.
Marchi N, Rasmussen P, Kapural M, Fazio V, Kight K, Mayberg MR, Kanner A, Ayumar B, Albensi B, Cavaglia M, Janigro D: Peripheral markers of brain damage and blood-brain barrier dysfunction. Restor Neurol Neurosci 2003, 21:109–121.
Blyth BJ, Farhavar A, Gee C, Hawthorn B, He H, Nayak A, Stöcklein V, Bazarian JJ: Validation of serum markers for blood-brain barrier disruption in traumatic brain injury. J Neurotrauma 2009, 26:1497–1507.
Biberthaler P, Linsenmeier U, Pfeifer KJ, Kroetz M, Mussack T, Kanz KG, Hoecherl EF, Jonas F, Marzi I, Leucht P, Jochum M, Mutschler W: Serum S-100B concentration provides additional information for the indication of computed tomography in patients after minor head injury: a prospective multicenter study. Shock 2006, 25:446–453.
Rothermundt M, Ponath G, Arolt V: S100B in schizophrenic psychosis. Int Rev Neurobiol 2004, 59:445–470.
Missler U, Wiesmann M, Friedrich C, Kaps M: S-100 protein and neuron-specific enolase concentrations in blood as indicators of infarction volume and prognosis in acute ischemic stroke. Stroke 1997, 28:1956–1960.
Griffin WS, Stanley LC, Ling C, White L, MacLeod V, Perrot LJ, White CL, Araoz C: Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci USA 1989, 86:7611–7615.
Ingebrigtsen T, Waterloo K, Jacobsen EA, Langbakk B, Romner B: Traumatic brain damage in minor head injury: relation of serum S-100 protein measurements to magnetic resonance imaging and neurobehavioral outcome. Neurosurgery 1999, 45:468–475.
Biberthaler P, Mussack T, Wiedemann E, Kanz KG, Koelsch M, Gippner-Steppert C, Jochum M: Evaluation of S-100b as a specific marker for neuronal damage due to minor head trauma. World J Surg 2001, 25:93–97.
Herrmann M, Curio N, Jost S, Wunderlich MT, Synowitz H, Wallesch CW: Protein S-100B and neuron specific enolase as early neurobiochemical markers of the severity of traumatic brain injury. Restor Neurol Neurosci 1999, 14:109–114.
Aurell A, Rosengren LE, Karlsson B, Olsson JE, Zbornikova V, Haglid KG: Determination of S-100 and glial fibrillary acidic protein concentrations in cerebrospinal fluid after brain infarction. Stroke 1991, 22:1254–1258.
Kanner AA, Marchi N, Fazio V, Mayberg MR, Koltz MT, Siomin V, Stevens GH, Masaryk T, Aumayr B, Vogelbaum MA, Barnett GH, Janigro D: Serum S100β: a noninvasive marker of blood-brain barrier function and brain lesions. Cancer 2003, 97:2806–2813.
Ruan S, Noyes K, Bazarian JJ: The economic impact of S-100B as a pre-head CT screening test on emergency department management of adult patients with mild traumatic brain injury. J Neurotrauma 2009, 26:1655–1664.
Gonçalves CA, Leite MC, Nardin P: Biological and methodological features of the measurement of S100B, a putative marker of brain injury. Clin Biochem 2008, 41:755–763.
Donato R: S100: a multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int J Biochem Cell Biol 2001, 33:637–668.
Yano J, Lilly E, Barousse M, Fidel PL Jr: Epithelial cell-derived S100 calcium-binding proteins as key mediators in the hallmark acute neutrophil response during Candid vaginitis. Infect Immun 2010, 78:5126–5137.
Beer C, Blacker D, Bynevelt M, Hankey GJ, Puddey IB: Systemic markers of inflammation are independently associated with S100B concentration: results of an observational study in subjects with acute ischaemic stroke. J Neuroinflammation 2010, 29:7–71.
Schmidt S: S100B: pathogenetic and pathophysiologic significance in neurology. Nervenarzt 1998, 69:639–646.
Massaro AR, Michetti F, Laudisio A, Bergonzi P: Myelin basic protein and S-100 antigen in cerebrospinal fluid of patients with multiple sclerosis in the acute phase. Ital J Neurol Sci 1985, 6:53–56.
Michetti F, Massaro A, Russo G, Rigon G: The S-100 antigen in cerebrospinal fluid as a possible index of cell injury in the nervous system. J Neurol Sci 1980, 44:259–263.
Yang XY, Lin J, Lu XY: Expression of S100B protein levels in serum and cerebrospinal fluid with different forms of neuropsychiatric systemic lupus erythematosus. Clin Rheumatol 2008, 27:353–357.
Al-Ayadhi LY, Mostafa GA: Increased serum osteopontin levels in autistic children: relation to the disease severity. Brain Behav Immun 2011, 25:1393–1398.
Al-Ayadhi LY, Mostafa GA: Low plasma progranulin levels in children with autism. J Neuroinflammation 2011, 8:111.
Enstrom AM, Van de Water JA, Ashwood P: Autoimmunity in autism. Curr Opin Investig Drugs 2009, 10:463–473.
Mostafa GA, Al-Ayadhi LY: The possible link between the elevated serum levels of neurokinin A and anti-ribosomal P protein antibodies in children with autism. J Neuroinflammation 2011, 8:180.
Ben-Ami SD, Blank M, Altman A: The clinical importance of anti-ribosomal-P antibodies. Harefuah 2010, 149:794–797.
Mostafa GA, Ibrahim DH, Shehab AA, Mohammed AK: The role of measurement of serum autoantibodies in prediction of pediatric neuropsychiatric systemic lupus erythematosus. J Neuroimmunol 2010, 227:195–201.
Katzav A, Solodeev I, Brodsky O, Chapman J, Pick CG, Blank M, Zhang W, Reichlin M, Shoenfeld Y: Induction of autoimmune depression in mice by anti-ribosomal P antibodies via the limbic system. Arthritis Rheum 2007, 56:938–948.
Sheikh Z, Ahmad R, Sheikh N, Ali R: Enhanced recognition of reactive oxygen species damaged human serum albumin by circulating systemic lupus erythematosus autoantibodies. Autoimmunity 2007, 40:512–520.
Wang G, König R, Ansari GA, Khan MF: Lipid peroxidation-derived aldehyde-protein adducts contribute to trichloroethene-mediated autoimmunity via activation of CD4 + T cells. Free Radic Biol Med 2008, 44:1475–1482.
Mostafa GA, El-Hadidi ES, Hewedi DH, Abdou MM: Oxidative stress in Egyptian children with autism: relation to autoimmunity. J Neuroimmunol 2010, 219:114–118.
Javeed A, Zhao Y: The effects of immunosuppression on regulatory CD4 + CD25 + T cells: impact on immunosuppression selection in transplantation. Mol Diagn Ther 2008, 12:171–181.
Vignali DA, Collison LW, Workman CJ: How regulatory T cells work. Nat Rev Immunol 2008, 8:523–532.
Acknowledgements
This work was financially supported by the King Abdulaziz City for Science and Technology, Riyadh, Saudi Arabia. It was also supported by NPST, Health Research and Studies program at Kind Saud University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
Both authors designed, performed and wrote the research. In addition, both authors read and approved the final manuscript.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Al-Ayadhi, L.Y., Mostafa, G.A. A lack of association between elevated serum levels of S100B protein and autoimmunity in autistic children. J Neuroinflammation 9, 54 (2012). https://doi.org/10.1186/1742-2094-9-54
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1742-2094-9-54