Abstract
Background
Inflammatory processes are increased in the Parkinson's disease (PD) brain. The long-term use of nonsteroidal anti-inflammatory drugs has been associated, in retrospective studies, with decreased risk for PD, suggesting that inflammation may contribute to development of this disorder. The objective of this study was to determine the extent of complement activation, a major inflammatory mechanism, in PD.
Methods
Substantia nigra specimens from young normal subjects (n = 11–13), aged normal subjects (n = 24–28), and subjects with PD (n = 19–20), Alzheimer's disease (AD; n = 12–13), and dementia with Lewy bodies (DLB; n = 9) were stained for iC3b and C9, representing early- and late-stage complement activation, respectively. Numbers of iC3b+, C9+, and total melanized neurons in each section were counted in a blinded fashion. Nonparametric analyses were used to evaluate differences between groups and to evaluate correlations between complement staining, numbers of melanized neurons, and the duration of PD.
Results
Lewy bodies in both PD and DLB specimens stained for iC3b and C9. Staining was also prominent on melanized neurons. The percentage of iC3b+ neurons was significantly increased in PD vs. aged normal and AD specimens, and in young normal vs. aged normal specimens. C9 immunoreactivity was significantly increased in PD vs. AD specimens, but unlike iC3b, the increased C9 staining in PD and young normal specimens did not achieve statistical significance vs. aged normal specimens. iC3b and C9 staining in PD specimens was not correlated with the numbers of remaining melanized neurons, nor with the duration of PD.
Conclusion
Complement activation occurs on Lewy bodies and melanized neurons in the PD substantia nigra. Early complement activation (iC3b) is increased on melanized neurons in PD vs. aged normal specimens, and late-stage complement activation (C9) also tends to increase. This latter finding suggests that complement activation may contribute to loss of dopaminergic neurons in some individuals with PD. Complement activation on melanized neurons appears to decrease with normal aging, suggesting a possible neuroprotective role for this process in the normal substantia nigra.
Similar content being viewed by others
Background
Multiple neurotoxic processes have been described in the Parkinson's disease (PD) brain including inflammation, oxidative stress, excitotoxicity, and mitochondrial dysfunction [1]. The evidence for inflammation in PD includes gliosis [2, 3], increased major histocompatibility complex expression on microglia [2, 4], microglial phagocytosis of degenerating neuromelanin-containing neurons [5], and increased inflammatory cytokines [6, 7]. Inflammation has also been reported in some animal models of PD [8, 9]. The significance of inflammation in PD is unclear. Two retrospective studies indicated an association between the long-term use of nonsteroidal anti-inflammatory drugs (NSAIDs) and decreased risk for PD [10, 11], suggesting that inflammation may be important in the development of this disorder; however, a third retrospective study found no evidence for protective effects of NSAIDs against PD [12].
Complement activation is a major inflammatory process which promotes the removal of microorganisms and cell debris, and the processing of immune complexes. Three interrelated pathways, the classical, alternative, and mannan binding lectin-mediated cascades, have been described. Proteins generated early in this process function as chemotactic factors [13, 14], opsonins [15, 16], and anaphylatoxins [17]. Full activation of any of these pathways results in the generation of C5b-9, the membrane attack complex (MAC), which is neurotoxic [18]. In contrast to Alzheimer's disease (AD), in which complement activation has been extensively investigated [reviewed by McGeer and McGeer [19], 2002, and Shen and Meri [20], 2003], few studies have addressed this issue in PD. Yamada et al. [21] reported staining of Lewy bodies in the PD substantia nigra for both early-stage (C3d and C4d) and late-stage (C7 and C9) complement proteins, and C3d and C4d staining on Lewy bodies was subsequently reported in the brain stem from subjects with dementia with Lewy bodies (DLB) [22]. However, a third study found no complement reactivity on Lewy bodies in the cingulate gyrus in either PD or DLB [23]. Because of these conflicting results, the extent of complement activation in PD is unclear. The objective of the present study was to further examine this issue.
Methods
Brain specimens
Paraffin-embedded, formalin-fixed substantia nigra specimens were obtained from young normal (YN) subjects (n = 11–13), aged normal (AN) subjects (n = 24–28), and subjects with PD (n = 19–20), AD (n = 12–13), and DLB (n = 9). These specimens were obtained from the Harvard Brain Tissue Resource Center (McLean Hospital, Belmont, MA), the University of California at Irvine Institute for Brain Aging and Dementia (Irvine, CA), the Massachusetts General Hospital Alzheimer Disease Research Center (Charlestown, MA), and the University of California School of Medicine (Department of Medical Pathology, Sacramento, CA). Each group (YN, AN, PD, AD, and DLB) included specimens from all four brain banks. Means (± SEM) and ranges for subject ages and post-mortem intervals (PMI) are shown in Table 1. PMI means were similar between groups, and subject ages differed only between YN and the other groups.
Immunocytochemical staining for iC3b and C9
Formalin-fixed, paraffin-embedded sections of 6 – 8 μm thickness were placed on Superfrost Plus slides (Cardinal Health, McGaw Park, IL) and heated for 1 hr at 56°C. The sections were subsequently deparaffinized and rehydrated through graded ethanol baths, then rinsed in Tris buffered saline (TBS; 0.1 M Tris, 0.85% NaCl, pH 7.6). (This and all subsequent rinses were performed three times at five min intervals.) They were treated for 4 min with 88% formic acid (Fisher Scientific, Fair Lawn, NJ), then boiled for 5 min in citrate buffer, pH 6.0 (Antigen Unmasking Solution, Vector Laboratories, Burlingame, CA). After rinsing in TBS, the sections were treated with 3% H2O2/10% methanol in TBS for 30 min to eliminate endogenous peroxidase activity, rinsed in TBS with 0.1% Triton X-100 (hereafter, TBS-T), then treated with TBS-T with 1% bovine serum albumin (TBS-T-BSA) and 10% normal horse serum (Vector) for 30 min. The specimens were then incubated overnight at room temperature with mouse monoclonal anti-human iC3b (Quidel Corp., San Diego, CA; 1:200 dilution, final concentration 5.5 μg/ml) or goat anti-human C9 (Quidel; 1:5000 dilution, final concentration 11 μg/ml). Negative controls, performed for each specimen, consisted of substituting the nonsecreting mouse hybridoma MOPC-21 (mouse IgG1-kappa; Sigma-Aldrich, St. Louis, MO) (1:164 dilution, final concentration 5.5 μg/ml) for anti-iC3b serum, and normal goat serum (Vector; 1:5000 dilution) for goat anti-C9. After rinsing in TBS-T, biotinylated horse anti-mouse IgG (for iC3b staining) or biotinylated horse anti-goat IgG (for C9 staining) (both from Vector; 1:200 dilution in TBS-T-BSA) was applied at room temperature for one hr (for iC3b) or 90 min (for C9), followed by rinsing in TBS and then avidin-biotin-horseradish peroxidase conjugate (ABC reagent, Vector; 1:100 dilution in TBS-BSA) for 1 hr. Sections were developed with 3,3'-diaminobenzidine (DAB)/H2O2 with nickel enhancement (DAB Peroxidase Substrate Kit, Vector), then dehydrated in ethanol baths to xylene and coverslipped with Cytoseal-60 Mounting Medium (Richard-Allan Scientific, Kalamazoo, MI). AD hippocampus specimens from the University of California at Irvine Institute for Brain Aging and Dementia were included as positive controls in each experiment.
Statistical analyses
The number of neuromelanin-containing neurons (hereafter, "melanized neurons") in each substantia nigra section (one side only), and the number of these neurons immunoreactive for iC3b or C9, were counted by one observer (D.L.) in a blinded fashion with the 40× objective. (Neuromelanin, a by-product of dopamine metabolism [24], is considered to be a marker for dopaminergic neurons in the substantia nigra, although some dopamine-containing neurons in this region are non-melanized [25]).
The percentage of iC3b+ or C9+melanized neurons and number of melanized neurons in each specimen were compared between groups via the Kruskal-Wallis test and subject ages and PMI were compared between groups by a one-way ANOVA. When significant differences between groups were detected, pairwise comparisons were then performed to determine the location(s) of these differences. Data from iC3b+, C9+, and total melanized neuron counts were analyzed with a Wilcoxon Rank Sum test, with the p-values adjusted for multiplicity of testing via Hochberg's procedure [26]. The two demographic factors, subject age and PMI, were compared between groups in a pair-wise fashion via the Tukey-Kramer HSD. Correlations between variables (percentages of C3b+ and C9+melanized neurons, PMI, age, number of melanized neurons, and duration of PD) were determined by Spearman's rank correlation coefficient. The overall level of statistical significance for all tests was 0.05.
Results
Lewy bodies were immunoreactive for both iC3b (7 of 20 PD specimens, 6 of 9 DLB specimens) and C9 (11 of 19 PD specimens, 9 of 9 DLB specimens). Staining was also detected on melanized neurons (cell bodies, axons, and melanin fragments), occasional non-melanized neurons, glia, and, in AD specimens, senile plaques. In PD specimens, many of the iC3b+ and C9+ melanized neurons had few remaining melanin granules. No cellular staining was present in negative controls, although faint vascular staining was observed in a few specimens. Staining for iC3b and C9 is shown in Figs. 1 and 2, respectively. There was marked variation in the percentages of immunoreactive melanized neurons for different specimens within each group, with little or no staining in some specimens and more than 25% staining in others; staining even exceeded 50% of melanized neurons in a few specimens. Complement immunoreactivity of melanized neurons generally was not localized to a particular sector (lateral, middle, or medial) of the substantia nigra.
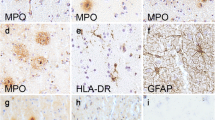
iC3b staining in substantia nigra specimens. Fig. 1A: Immunoreactive Lewy bodies in a PD substantia nigra specimen; Fig. 1B: Staining of melanized neurons (arrows) in a different PD specimen; Fig. 1C: Immunoreactive neuron with little melanin remaining, same PD specimen as Fig. 1B; Fig. 1D: iC3b staining of melanized neurons (arrows) in a young normal specimen; compare with unstained neurons in lower part of field; Fig. 1E: similar staining pattern in an AD specimen; two prominently stained melanized neurons are seen (arrows) among several unstained neurons; Fig. 1F: iC3b-stained senile plaques in a different AD substantia nigra specimen. (Figs. 1A and 1C, bar = 10 μm; Figs. 1B and 1D–F, bar = 50 μm; immunoreactive structures are dark blue or gray, in contrast to brown melanin and yellow background).
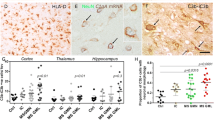
C9 staining in substantia nigra specimens. Fig. 2A: Staining of multiple Lewy bodies within a melanized neuron in a PD specimen; adjacent melanized neuron (arrow) and its axon are also C9-positive; Fig. 2B: immunoreactivity for C9 in a Lewy body (arrowhead) and in melanin-depleted neurons (arrows) in a different PD specimen; Fig. 2C: staining of melanized neuron (arrow) and its processes in a DLB specimen; Fig 2D: multiple immunoreactive melanized neurons in an aged normal specimen. (Fig. 2A, bar = 10 μm; Figs. 2B–D, bar = 50 μm; immunoreactive structures are dark blue or gray, in contrast to brown melanin and yellow background).
Statistical analysis of iC3b staining revealed significant differences among groups (p = 0.003), and pairwise comparisons indicated that the percentage of iC3b+ melanized neurons was significantly increased in PD vs. both AN and AD specimens (p = 0.0011 and 0.0099, respectively), and in YN vs. AN specimens (p = 0.0146) (Fig. 3). Total numbers of melanized neurons were significantly decreased in PD vs. AN, YN, and AD specimens, and in DLB vs. AN and AD specimens (Fig. 4). iC3b immunoreactivity was significantly correlated with numbers of melanized neurons only in YN specimens (r = 0.63, p = 0.016). There was no correlation in PD specimens between the percentage of iC3b+ melanized neurons and the duration of PD (r = 0.09), and no gender differences were detected on pooled data from all groups for iC3b staining.
Percentages of iC3b-positive melanized neurons in different groups of substantia nigra specimens. The percentage of iC3b+ melanized neurons was significantly increased in PD vs. both aged normal and AD specimens, and in young normal vs. aged normal specimens. Data are expressed as means ± SEM. (ap < 0.05 vs. PD; bp < 0.05 vs. young normal specimens; abbreviations: AD, Alzheimer's disease; AN, aged normal; DLB, dementia with Lewy bodies; PD, Parkinson's disease; YN, young normal)
Numbers of melanized neurons in different groups of substantia nigra specimens. Total numbers of melanized neurons were significantly decreased in PD vs. aged normal, young normal, and AD specimens, and in DLB vs. aged normal and AD specimens. Data (means ± SEM) are shown for slides from specimens in which iC3b immunoreactivity was assessed; essentially similar results were obtained for slides from specimens in which C9 staining was evaluated. (ap < 0.05 vs. PD; bp < 0.05 vs. DLB; abbreviations: AD, Alzheimer's disease; AN, aged normal; DLB, dementia with Lewy bodies; PD, Parkinson's disease; YN, young normal)
C9 staining yielded generally similar results to those for iC3b. This was reflected by significant correlations between the percentages of C9+ and iC3b+ melanized neurons in all groups (r values ranging from 0.67 to 0.82, all p < 0.02) except for DLB (r = 0.35, p = 0.40). C9 staining was increased in PD vs. AD specimens (p = 0.0048; Fig. 5). Unlike iC3b, however, the trends towards increased C9 staining in PD vs. AN specimens, and in YN vs. AN specimens, were not statistically significant (p = 0.04 [not significant after adjustment for multiple comparisons] and p = 0.08, respectively). The percentage of C9+ melanized neurons was not correlated with the number of melanized neurons per specimen in any of the groups. As with iC3b, neuronal C9 staining was not correlated with the duration of PD, and there were no gender differences within groups for C9 staining.
Percentages of C9-positive melanized neurons in different groups of substantia nigra specimens. C9 staining was significantly increased in PD vs. AD specimens. The percentages of C9+ melanized neurons in PD and young normal specimens tended to be increased vs. aged normal specimens, but these differences were not significant (p = 0.04 [not significant after adjustment for multiple comparisons] and 0.08, respectively). Data are expressed as means ± SEM. (ap < 0.05 vs. PD; abbreviations: AD, Alzheimer's disease; AN, aged normal; DLB, dementia with Lewy bodies; PD, Parkinson's disease; YN, young normal)
Discussion
This study confirmed the presence of both early- and late-stage complement proteins on Lewy bodies in the PD substantia nigra, as reported by Yamada et al. [21]. In contrast to that study, however, complement activation was also detected on melanized neuron cell bodies and axons. These differences may be due to technical factors; the present study used on-slide staining of formalin-fixed sections and included antigen retrieval pretreatment of sections with formic acid and citric acid, whereas the earlier study used free-floating staining, primarily of paraformaldehyde-fixed sections, without antigen retrieval.
The antibody used to detect iC3b in this study is iC3b-specific and does not recognize the native complement protein C3 from which iC3b is generated. iC3b staining of melanized neurons is therefore evidence for early complement activation, i.e., cleavage of C3, on these cells. iC3b and its active form, C3b, are opsonins, promoting phagocytosis of foreign antigens and cell debris. Deposition of iC3b on melanized neurons could facilitate binding of these cells by activated microglia, known to be present in increased numbers in the PD substantia nigra [3]. C3a, the other major C3 cleavage protein, is an anaphylatoxin, increasing vascular permeability. Though C3a is generally considered to be pro-inflammatory [27–29] because it attracts and activates eosinophils, basophils, and mast cells, few of these cells are present in the brain. C3a may, in fact, limit brain inflammation, by decreasing the production of inflammatory cytokines and inducing the production of immunosuppressive ones [30]. It exerts neuroprotective and (indirectly) neurotrophic effects, protecting neurons against excitotoxins [31] and inducing production of microglial neuronal growth factor (NGF) [32]. iC3b staining of melanized neurons was greater in YN than in AN specimens, and was positively correlated with the numbers of melanized neurons in YN specimens (r = 0.63, p = 0.016). These results suggest that early complement activation might play a protective role for melanized neurons in the young normal brain; if so, a decrease in early complement activation on melanized neurons during normal aging could leave these cells more susceptible to oxidative and/or inflammatory damage. The decrease in iC3b staining of melanized neurons which occurred with normal aging was not detected when PD was present. The significance of this finding is unclear. The lack of correlation in PD specimens between the numbers of remaining melanized neurons and the percentage of these neurons that were iC3b+ suggests that, even if early complement activation is primarily neuroprotective, this process fails to protect melanized neurons from whatever insults cause them to be lost in the PD brain.
Goat anti-C9 was used rather than monoclonal anti-C5b-9 for assessment of late-stage complement activation because, in preliminary studies, more consistent staining of senile plaques in AD hippocampus sections was obtained with the anti-C9 antibody. (AD brain was the appropriate positive control for these studies because extensive deposition of C5b-9 has been reported in the AD brain [33]). Although staining for C9 was also used in the study by Yamada et al. [21] and has been used by others to detect the MAC [34–36], C9 immunoreactivity on melanized neurons could indicate late-stage complement activation, upregulation of neuronal C9 synthesis, or both. C9 staining on melanized neurons tended to increase in PD vs. AN specimens (60% increase), although this increase was not statistically significant. Detection of C9 on degenerating melanized neurons suggests that deposition of the MAC on dopamine neurons may reach lytic levels in PD and contribute to the loss of these neurons. The mechanism by which complement is activated on PD melanized neurons is unknown; one possibility may be surface immunoglobulin G (IgG), which was recently reported by Orr et al. [37] to be present on 30% of dopamine neurons in the PD substantia nigra. Alternatively, complement activation on melanized neurons could occur secondary to cell injury, triggered by newly exposed tissue antigens and/or byproducts of damaged tissue, although this would not explain the apparent activation of complement on melanized neurons in the YN substantia nigra specimens.
The increase in iC3b immunoreactivity on melanized neurons in YN substantia nigra specimens in comparison with AN specimens was an unexpected finding. A similar trend was present for C9, although it was not statistically significant. The mechanism responsible for complement activation on normal dopamine neurons, as with injured dopamine neurons, is unknown. Oxidative stress, which can activate complement [38], may be involved. The basal level of oxidative stress in the human substantia nigra is higher than in other brain regions [39], probably due to the production of H2O2 as a byproduct of dopamine metabolism [40]. Early complement activation on normal dopamine neurons could play a protective role, as discussed earlier, whereas MAC deposition on these neurons, if it occurs, is likely to be sublytic. There is a substantial literature on the cellular effects of sublytic levels of the MAC, including cell cycle activation, cell proliferation, enhancement of cell survival, and cytokine synthesis [41–44], but its influence on neurons has apparently not been examined. In addition to the concentrations of complement proteins deposited on melanized neurons, neuronal expression of complement inhibitory molecules [45] and complement receptors [46] in normal and diseased substantia nigra is also likely to be important in determining the influence of complement activation on these neurons.
Conclusion
This study confirms the occurrence of complement activation on Lewy bodies in melanized neurons in the PD substantia nigra, and indicates that this process also occurs on some non-Lewy body-bearing melanized neurons and on melanin fragments in this region. Complement activation on melanized neurons tends to increase in the PD substantia nigra, but is also present in normal individuals and in subjects with other neurodegenerative disorders. Complement activation on melanized neurons may decrease during normal aging. Further studies are indicated to clarify the mechanism (or mechanisms) responsible for complement activation on normal and injured dopamine neurons, and the significance of this process.
References
Beal MF: Mitochondria, oxidative damage, and inflammation in Parkinson's disease. Ann NY Acad Sci. 2003, 991: 120-31.
McGeer PL, Itagaki S, Boyes BE, McGeer EG: Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology. 1988, 38: 1285-91.
Teismann P, Schulz JB: Cellular pathology of Parkinson's disease: astrocytes, microglia and inflammation. Cell Tissue Res. 2004, 318: 149-61. 10.1007/s00441-004-0944-0.
Imamura K, Hishikawa N, Sawada M, Nagatsu T, Yoshida M, Hashizume Y: Distribution of major histocompatibility class II-positive microglia and cytokine profile of Parkinson's disease brains. Acta Neuropathol (Berl). 2003, 106: 518-26. 10.1007/s00401-003-0766-2.
McGeer PL, Itagaki S, Akiyama H, McGeer EG: Rate of cell death in parkinsonism indicates active neuropathological process. Ann Neurol. 1988, 24: 574-6. 10.1002/ana.410240415.
Mogi M, Harada M, Kondo T, Riederer P, Inagaki H, Minami M, Nagatsu T: Interleukin-1 beta, interleukin-6, epidermal growth factor and transforming growth factor-alpha are elevated in the brain from parkinsonian patients. Neurosci Lett. 1994, 180: 147-50. 10.1016/0304-3940(94)90508-8.
Nagatsu T, Mogi M, Ichinose H, Togari A: Changes in cytokines and neurotrophins in Parkinson's disease. J Neural Transm Suppl. 2000, 60: 277-90.
Kurkowska-Jastrzebska I, Wronska A, Kohutnicka M, Czlonkowski A, Czlonkowska A: The inflammatory reaction following 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine intoxication in mouse. Exp Neurol. 1999, 156: 50-61. 10.1006/exnr.1998.6993.
Depino AM, Earl C, Kaczmarczyk E, Ferrari C, Besedovsky H, del Rey A, Pitossi FJ, Oertel WH: Microglial activation with atypical proinflammatory cytokine expression in a rat model of Parkinson's disease. Eur J Neurosci. 2003, 18: 2731-42. 10.1111/j.1460-9568.2003.03014.x.
Chen H, Zhang SM, Hernan MA, Schwarzschild MA, Willett WC, Colditz GA, Speizer FE, Ascherio A: Non-steroidal anti-inflammatory drugs and the risk of Parkinson's disease. Arch Neurol. 2003, 60: 1059-64. 10.1001/archneur.60.8.1059.
Chen H, Jacobs E, Schwarzschild MA, McGullough ML, Calle EE, Thun MJ, Ascherio A: Nonsteroidal antiinflammatory drug use and the risk for Parkinson's disease. Ann Neurol. 2005, 58: 963-7. 10.1002/ana.20682.
Ton TG, Heckbert SR, Longstreth WT, Rossing MA, Kukull WA, Franklin GM, Swanson PD, Smith-Weller T, Checkoway H: Nonsteroidal anti-inflammatory drugs and risk of Parkinson's disease. Mov Disord. 2006, 21: 964-9. 10.1002/mds.20856.
Yao J, Harvath L, Gilbert DL, Colton CA: Chemotaxis by a CNS macrophage, the microglia. J Neurosci Res. 1990, 27: 36-42. 10.1002/jnr.490270106.
Nolte C, Moller T, Walter T, Kettenmann H: Complement 5a controls motility of murine microglial cells in vitro via activation of an inhibitory G-protein and the rearrangement of the actin cytoskeleton. Neuroscience. 1996, 73: 1091-107. 10.1016/0306-4522(96)00106-6.
Grondahl G, Hohannisson A, Jensen-Waern M, Nilsson Ekdahl K: Opsonization of yeast cells with equine iC3b, C3b, and IgG. Vet Immunol Immunopathol. 2001, 80: 209-23. 10.1016/S0165-2427(01)00262-8.
Cunnion KM, Zhang HM, Frank MM: Availability of complement bound to Staphylococcus aureus to interact with membrane complement receptors influences efficiency of phagocytosis. Infect Immun. 2003, 71: 656-62. 10.1128/IAI.71.2.656-662.2003.
Erdei A, Andrasfalvy M, Peterfy H, Toth G, Pecht I: Regulation of mast cell activation by complement-derived peptides. Immunol Lett. 2004, 92: 39-42. 10.1016/j.imlet.2003.11.019.
Shen Y, Halperin JA, Lee CM: Complement-mediated neurotoxicity is regulated by homologous restriction. Brain Res. 1995, 671: 282-292. 10.1016/0006-8993(94)01264-I.
McGeer PL, McGeer EG: The possible role of complement activation in Alzheimer disease. Trends Mol Med. 2002, 9: 519-23. 10.1016/S1471-4914(02)02422-X.
Shen Y, Meri S: Yin and Yang: complement activation and regulation in Alzheimer's disease. Prog Neurobiol. 2003, 70: 463-72.
Yamada T, McGeer PL, McGeer EG: Lewy bodies in Parkinson's disease are recognized by antibodies to complement proteins. Acta Neuropathol (Berl). 1992, 84: 100-4. 10.1007/BF00427222.
Iseki E, Marui W, Akiyama H, Ueda K, Kosaka K: Degeneration process of Lewy bodies in the brains of patients with dementia with Lewy bodies using alpha-synuclein-immunohistochemistry. Neurosci Lett. 2000, 286: 69-73. 10.1016/S0304-3940(00)01090-9.
Rozemuller AJ, Eikelenboom P, Theeuwes JW, Jansen Steur EN, de Vos RA: Activated microglial cells and complement factors are unrelated to cortical Lewy bodies. Acta Neuropathol (Berl). 2000, 100: 701-8. 10.1007/s004010000225.
Fasano M, Bergamasco B, Lopiano L: Modifications of the iron-neuromelanin system in Parkinson's disease. J Neurochem. 2006, 96: 909-16. 10.1111/j.1471-4159.2005.03638.x.
Kingsbury AE, Marsden CD, Foster OJ: The vulnerability of nigral neurons to Parkinson's disease is unrelated to their intrinsic capacity for dopamine synthesis: an in situ hybridization study. Mov Disord. 1999, 14: 206-18. 10.1002/1531-8257(199903)14:2<206::AID-MDS1002>3.0.CO;2-I.
Wright SP: Adjusted P-values for simultaneous inference. Biometrics. 1992, 48: 1005-1013. 10.2307/2532694.
Humbles AA, Lu B, Nilsson CA, Lilly C, Israel E, Fujiwara Y, Gerard NP, Gerard C: A role for the C3a anaphylatoxin receptor in the effector phase of asthma. Nature. 2000, 406: 998-1001. 10.1038/35023175.
Basta M, Van Goor F, Luccioli S, Billings EM, Vortmeyer AO, Baranyi L, Szebeni J, Alving CR, Carroll MC, Berkower I, Stojilkovic SS, Metcalfe DD: F(ab)'2-mediated neutralization of C3a and C5a anaphylatoxins: a novel effector function of immunoglobulins. Nat Med. 2003, 9: 431-8. 10.1038/nm836.
Soto E, Romero R, Richani K, Espinoza J, Nien JK, Chaiworapongsa T, Santolava-Forgas J, Edwin SS, Mazor M: Anaphylatoxins in preterm and term labor. J Perinat Med. 2005, 33: 306-13. 10.1515/JPM.2005.051.
Gasque P, Dean YD, McGreal EP, VanBeek J, Morgan BP: Complement components of the innate immune system in health and disease in the CNS. Immunopharmacology. 2000, 49: 171-86. 10.1016/S0162-3109(00)80302-1.
van Beek J, Nicole O, Ali C, Ischenko A, MacKenzie ET, Buisson A, Fontaine M: Complement anaphylatoxin C3a is selectively protective against NMDA-induced neuronal cell death. Neuroreport. 2001, 12: 289-93. 10.1097/00001756-200102120-00022.
Heese K, Hock C, Otten U: Inflammatory signals induce neurotrophin expression in human microglial cells. J Neurochem. 1998, 70: 699-707.
Webster S, Lue LF, Brachova L, Tenner AJ, McGeer PL, Terai K, Walker DG, Bradt B, Cooper NR, Rogers J: Molecular and cellular characterization of the membrane attack complex, C5b-9, in Alzheimer's disease. Neurobiol Aging. 1997, 18: 415-21. 10.1016/S0197-4580(97)00042-0.
Robert-Offerman SR, Leers MP, van Suylen RJ, Nap M, Daemen MJ, Theunissen PH: Evaluation of the membrane attack complex of complement for the detection of a recent myocardial infarction in man. J Pathol. 2000, 191: 48-53. 10.1002/(SICI)1096-9896(200005)191:1<48::AID-PATH583>3.0.CO;2-9.
Xi G, Hua Y, Keep RF, Younger JG, Hoff JT: Brain edema after intracerebral hemorrhage: the effects of systemic complement depletion. Acta Neurochir Suppl. 2002, 81: 253-6.
Schultz SJ, Aly H, Hasanen BM, Khashaba MT, Lear SC, Bendon RW, Gordon LE, Feldhoff PW, Lassiter HA: Complement component 9 activation, consumption, and neuronal deposition in the post-hypoxic-ischemic central nervous system of human newborn infants. Neurosci Lett. 2005, 378: 1-6. 10.1016/j.neulet.2004.12.008.
Orr CF, Rowe DB, Mizuno Y, Mori H, Halliday GM: A possible role for humoral immunity in the pathogenesis of Parkinson's disease. Brain. 2005, 128: 2665-74. 10.1093/brain/awh625.
Collard CD, Montalto MC, Reenstra WR, Buras JA, Shahl GL: Endothelial oxidative stress activates the lectin complement pathway: role of cytokeratin 1. Am J Pathol. 2001, 159: 1045-54.
Floor E, Wetzel MG: Increased protein oxidation in human substantia nigra pars compacta in comparison with basal ganglia and prefrontal cortex measured with an improved dinitrophenylhydrazine assay. J Neurochem. 1998, 70: 268-75.
Cohen G: Oxy-radical toxicity in catecholamine neurons. Neurotoxicology. 1984, 5: 77-82.
Hila S, Soane L, Koski CL: Sublytic C5b-9-stimulated Schwann cell survival through PI 3-kinase-mediated phosphorylation of BAD. Glia. 2001, 36: 58-67. 10.1002/glia.1095.
Badea TD, Park JH, Soane L, Niculescu T, Nuculescu F, Rus H, Shin ML: Sublytic terminal complement attack induces c-fos transcriptional activation in myotubes. J Neuroimmunol. 2003, 142: 58-66. 10.1016/S0165-5728(03)00261-3.
Rus H, Cudrici C, Niculescu F: C5b-9 complement complex in autoimmune demyelination and multiple sclerosis: dual role in neuroinflammation and neuroprotection. Ann Med. 2005, 37: 97-104. 10.1080/07853890510007278.
Cudrici C, Niculescu F, Jensen T, Zafranskaia E, Fosbrink M, Rus V, Shin ML, Rus H: C5b-9 terminal complex protects oligodendrocytes from apoptotic cell death by inhibiting caspase-8 processing and up-regulating FLIP. J Immunol. 2006, 176: 3173-80.
van Beek J, van Meurs M, 't hart BA, Brok HP, Neal JW, Chatagner A, Harris CL, Omidvar N, Morgan BP, Laman JD, Gasque P: Decay-accelerating factor (CD55) is expressed by neurons in response to chronic but not acute autoimmune central nervous system inflammation associated with complement activation. J Immunol. 2005, 174: 2353-65.
Boos L, Campbell IL, Ames R, Wetsel RA, Barnum SR: Deletion of the complement anaphylatoxin C3a receptor attenuates, whereas ectopic expression of C3a in the brain exacerbates, experimental autoimmune encephalomyelitis. J Immunol. 2004, 173: 4708-14.
Acknowledgements
Brain tissues used in this project were provided by the Institute for Brain Aging and Dementia Brain Tissue Resource and University of California Irvine Alzheimer's Disease Research Center (supported by NIA grant P50 AG16573), the Harvard Brain Tissue Resource Center (McLean Hospital, Belmont, MA; supported by PHS #R24 MH 068855), the Massachusetts General Hospital Alzheimer Disease Research Center (Charlestown, MA), and the University of California Davis Department of Pathology and Laboratory Medicine (supported by NIA #AD12435 and IVD #AG10129). Thanks are expressed to Donna Selenich and Paul Juneau for technical assistance. This study was supported by a grant to D.A.L. from the Michael J. Fox Foundation for Parkinson's Research, and by a donation from Marcia and Howard Parven.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
DAL performed the immunocytochemical staining and cell counts and wrote the manuscript. DMC generated the figures, performed the statistical analyses, and assisted with the writing of the manuscript. SBC performed preliminary experiments to develop the staining methods and reviewed the manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Loeffler, D.A., Camp, D.M. & Conant, S.B. Complement activation in the Parkinson's disease substantia nigra: an immunocytochemical study. J Neuroinflammation 3, 29 (2006). https://doi.org/10.1186/1742-2094-3-29
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1742-2094-3-29