Abstract
Background
There is conflicting evidence about the association between low vitamin D levels in children and development of asthma in later life. The objective of this study was to systematically review the evidence for an epidemiological association between low serum levels of vitamin D and the diagnosis of asthma in children.
Methods
We used the Cochrane methodology for conducting systematic reviews. The search strategy included an electronic search of MEDLINE and EMBASE in February 2013. Two reviewers completed, in duplicate and independently, study selection, data abstraction, and assessment of risk of bias.
Results
Of 1081 identified citations, three cohort studies met eligibility criteria. Two studies found that low serum vitamin D level is associated with an increased risk of developing asthma late in childhood, while the third study found no association with either vitamin D2 or vitamin D3 levels. All three studies suffer from major methodological shortcomings that limit our confidence in their results.
Conclusions
Available epidemiological evidence suggests a potential association between low serum levels of vitamin D and the diagnosis of asthma in children. High quality studies are needed to reliably answer the question of interest.
Similar content being viewed by others
Background
Asthma is a highly prevalent respiratory condition in childhood [1]. Development of asthma is associated with many immunological markers [2]. Hypovitaminosis is prevalent worldwide, and an increasing body of evidence supports pleotropic effects of vitamin D on various chronic disorders including those associated with immune regulatory function [3–5]. This includes associations with a number of childhood disorders [6], such as type I diabetes mellitus [7–11], celiac disease, and asthma [12, 13].
The hypothesis is that vitamin D has immunoregulatory properties [14, 15] that protect from asthma [16, 17]. Vitamin D has been shown to play a role in both the innate and adaptive immune responses by promoting phagocytosis and modulating the effect of Th1, Th2 and regulatory T cells [18]. Vitamin D has also been shown to inhibit the production of TH17 cytokines, which are associated with the severity of asthma and low steroid responsiveness [19]. A number of studies have suggested that low vitamin D levels are associated with increased risk of developing asthma [20–22] but other studies failed to confirm these findings [23, 24]. One cohort study has shown that vitamin D supplementation in childhood might be associated with an increased risk of developing asthma [25].
Given these conflicting results and the high prevalence of both asthma and vitamin D deficiency in many countries [26, 27], we aimed to systematically review the evidence for the epidemiological association between low levels of serum vitamin D and asthma diagnosis in children.
Methods
Protocol and registration
Prior to starting the review process, we registered the systematic review protocol with PROSPERO (CRD42013004204) [28].
Selection criteria
We included studies meeting the following eligibility criteria:
-
Types of studies: cohort studies. We excluded case–control studies and cross sectional studies.
-
Types of participants: children less than 18 years old and free of asthma at the time of inclusion in the cohort. We did not consider other kinds of allergic conditions.
-
Types of exposure: serum vitamin D levels in the child. We excluded studies of vitamin D levels in the pregnant mother or in the cord blood at the time of delivery and studies of vitamin D intake or supplementation.
-
Types of outcome measures: asthma diagnosed based on doctor’s diagnosis, questionnaires, or spirometry measures.
We did not exclude studies based on language or date of publication, but excluded meeting abstracts.
Search strategy
The OVID interface was used to electronically search MEDLINE and EMBASE (from date of inception to February 2013). The search strategy was designed with the help of a medical librarian. The search combined terms for asthma, vitamin D, and pediatric age group. It used both free text words and medical subject heading. We did not use any search filter for study design. Additional file 1 provides the full details of the search strategy.
In addition, we searched the grey literature (theses and dissertations) and the abstracts and proceedings from the following scientific meetings: American Thoracic Society (ATS), American College of Chest Physicians (ACCP), Pediatric Academic Societies, European Respiratory Society, and European Society for Pediatric Research. We also reviewed the references lists of included studies and publications available in the authors’ libraries. We searched forward for papers citing our included papers (ISI Web of Science). Finally, we contacted the authors of included studies inquiring about potentially eligible studies that we might have missed.
Selection of studies
Two reviewers (LA, MF) screened the titles and abstracts of identified citations for potential eligibility in duplicate and independently. We obtained the full text for citations judged as potentially eligible by at least one of the 2 reviewers. The two reviewers (LA, MF) then screened the full texts for eligibility, in duplicate, and independently.
Data collection
Two reviewers abstracted data from included studies in duplicate and independently. A senior team member (EAA) provided oversight. For each included study, the following information was abstracted: type, funding, population characteristics, exposure, outcomes assessed and the statistical data.
For both study selection and data collection steps, the reviewers used a pilot tested and standardized screening form and detailed instructions and resolved disagreement by discussion. A senior team member (EAA) provided oversight.
Assessment of risk of bias in included studies
The two reviewers assessed the risk of bias in each included study in duplicate and independently. They resolved disagreements by discussion or with the help of a third reviewer (EAA) who provided oversight. Risk of bias was assessed using the following criteria: [29].
-
Failure to develop and apply appropriate eligibility criteria (e.g., selection of exposed and unexposed in cohort studies from different populations).
-
Flawed measurement of exposure (i.e., serum vitamin D levels).
-
Flawed measurement of outcome (i.e., asthma diagnosis).
-
Failure to adequately control confounding variables (e.g., failure of accurate measurement of all known prognostic factors, failure to match for prognostic factors and/or adjustment in statistical analysis).
-
Incomplete follow-up.
We graded each potential source of bias as high, low or unclear.
Data analysis and synthesis
We used the kappa statistic to calculate the agreement between the two reviewers for the assessment of trial eligibility. We were not able to meta-analyze the results of the included studies, as two analyzed vitamin D as a continuous variable [13, 24], while the third study analyzed it categorized into tertiles [12]. We report both unadjusted and adjusted odds ratios (ORs) where available.
Results
Description of study selection
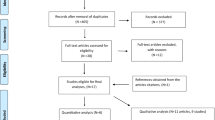
Figure 1 shows the study flow. Out of 39 potentially eligible studies, three met our eligibility criteria [12, 13, 24]. Additional file 2 lists the 36 excluded studies along with the reasons for exclusion.
Study characteristics
Table 1 lists the characteristics of the included studies. All three studies used data collected in the context of larger cohort studies conducted in the late 1980’s and 1990’s. The first study was conducted in Australia and included a population of 989 six-year-old subjects followed up until 14 years of age. The second one was conducted in the Netherlands and included a population of 372 four-year-old subjects followed up until 8 years of age. The last study was conducted in England and included a population of 3,323 children with a mean age of 9.8 years and followed up until a mean age of 15.5 years.
Risk of bias
Table 2 describes the assessment of the risk of bias in those studies. In our judgment, the risk of bias associated with subject selection in the study by Van Oeffelen et al. was high as only a small percentage of the inception cohort was assessed in this study. The risk of bias associated with measurement of the exposure (Vitamin D levels) in the Van Oeffelen study was high due to the use of tests for which no validation is described, and due to the low reliability of a single measurement. The risk of bias associated with the outcome measurement (asthma) was low for both the Hollams et al. and Van Oeffelen et al. studies and uncertain for the Tolppanen et al. study in which non-validated questionnaires were used to diagnose asthma. We judged the risk of bias associated with confounding as high in one study due to lack of adjustment (Hollmans et al.). The risk of bias associated with missing data was judged as high in two studies due to high rates of missing data (30% for Hollams et al. and 42% for Tolppanen et al.).
Statistical results
Asthma
Hollams et al. [13] found an unadjusted OR for the association between increasing vitamin D levels and risk of asthma of 0.11 (95% CI 0.02–0.84). Van Oeffelen et al. found an adjusted OR of 0.39 (95% CI 0.27–0.53) when comparing vitamin D tertile 2 to tertile 1 (the lowest tertile) and an adjusted OR of 0.45 (95% CI 0.32–0.57) when comparing tertile 3 to tertile 1 (see Table 2 for variables used for adjustment) [12]. Tolppanen et al. found no association between vitamin D3 and asthma with an adjusted OR of 1.02 (95% CI 0.93–1.12) and an unadjusted OR of 0.98 (95% CI 0.92–1.05) [24]. Similarly, they found no association between vitamin D2 and asthma with an adjusted OR of 0.89 (95% CI 0.78–1.02) and an unadjusted OR of 0.98 (95% CI 0.89–1.08) (see Table 2 for variables used for adjustment). Hollams et al. also assessed the association between increasing vitamin D levels and severe asthma and found an OR of 0.28 (95% CI 0.06–1.37).
Spirometric outcomes
Both Hollams et al. and van Oeffelen et al. assessed bronchial hyperresponsiveness using the methacholine challenge test. Hollams et al. [13] found an unadjusted OR for the association between increasing vitamin D levels and bronchial hyperresponsiveness of 0.28 (95% CI 0.06–1.37). Van Oeffelen [12] found an adjusted OR of 0.72 (95% CI 0.39–1.35) when comparing vitamin D tertile 2 to tertile 1, and an adjusted OR of 0.66 (95% CI 0.35–1.25) when comparing tertile 3 to tertile 1 (see Table 2 for variables used for adjustment).
Tolppanen et al. assessed lung function through the bronchodilator response to 400 ug dose of salbutamol. They reported the results using “SD change in outcome per doubling of exposure” [24]. This refers to reporting a standardized measure of the lung function outcome (typically equivalent to the mean divided by standard deviation) for every “doubling of exposure”. The authors calculated the doubling of exposure after scaling the two forms of 25(OH) D by multiplying the beta coefficients from the regression models by loge. The highest SD change reached among all analytical models was 0.06, which is typically considered a small effect size.
Discussion
Summary of main results
In summary, our systematic review identified three cohort studies assessing the association between low vitamin D levels in children and the incidence of asthma. Two of the included studies reported results suggesting that lower levels of vitamin D during childhood are associated with later development of asthma [12, 13]. The third one found no association with either vitamin D2 or D3 levels [24]. Besides the inconsistent results, all three included studies suffered from major methodological limitations that limit our confidence in their findings. They all suffered to different degrees from selection bias (e.g., unclear selection criteria), inadequate measurement of the outcome (e.g., non-validated questionnaires), confounding (e.g., inadequate adjustment for potential confounders such as maternal atopy), and incompleteness of outcome data 12%-50% of subjects in the three studies had missing data). The risk of bias associated with exposure measurement was common to the three studies. Indeed, and as detailed in Table 2, vitamin D was measured once, between 5–8 years depending on the study, before the assessment of the outcomes, thus limiting the reliability of the value obtained as a reflection of integrated vitamin D nutritional status over the follow-up time [30]. In addition, the methods of handling the samples (e.g., thawing procedure) may have affected the accuracy of the results. Our confidence in the findings is further decreased by the imprecision of the results as indicated by the wide confidence intervals that include the value of 1 for most odds ratios.
Overall completeness and applicability of evidence
Autier et al. recently published a systematic review of “vitamin D status and ill health” [31]. Although the reviewers included prospective cohort studies, they did not capture any of the studies we included in our review. This makes the possibility that we missed eligible studies unlikely. We have excluded studies of cord blood vitamin D levels because cord blood in part reflects maternal exposure, and evidence of their correlation with actual vitamin D levels during childhood is lacking. Also, we only included cohort studies to minimize the risk of bias in the results of this review. However, we observed that studies we excluded for their study design, and described as “case–control”, were actually cross-sectional in nature. Indeed, these studies assessed both the exposure (vitamin D level) and the outcome (asthma) simultaneously at the time of inclusion into the study.
Agreements and disagreements with other reviews
Recently, Theodoratou et al. published an umbrella review that included systematic reviews and meta-analyses of observational studies of vitamin D and multiple health outcomes [32]. They identified no published systematic review or meta-analysis assessing the asthma outcome. We identified two systematic reviews [33, 34] and two narrative reviews related to our study [35, 36]. Neither of the two systematic reviews identified the studies we included in our review. The systematic review by Nurmatov et al. included studies related to maternal levels of vitamin D as opposed to children’s levels’. Most of the studies systematic review by Zhang et al. were either studies of chronic obstructive pulmonary disease or non-cohort studies [34]. The one cohort study of asthma that they included measured cord blood levels [37]. The narrative review by Gupta et al. addressed a range of questions around vitamin D and asthma in children [35]. That review did not include any of the three studies we included. The narrative review by Hollmas [36] identified only one of the three included studies [13].
Implications for practice
The finding of a possible association between lower vitamin D levels and incidence of asthma may imply that vitamin D supplementation may be effective in preventing asthma. However, the considerable uncertainty regarding the validity of this association precludes at this point any translation of the findings into clinical practice. Indeed, we have identified 12 ongoing trials posted on clinicaltrials.gov that are assessing the effects of vitamin D supplementation in children with asthma symptoms.
Implications for research
Future studies should address the methodological limitations of the available evidence. This includes proper assessment of the exposure, by assessing vitamin D status by multiple measurements throughout the study period, and accounting for seasonal variation and other environmental factors. It also requires setting appropriate eligibility criteria, valid measurement of outcomes, controlling for all confounders, and minimizing missing data.
Abbreviations
- BHR:
-
Bronchial hyper responsiveness
- 25(OH) Vitamin D:
-
25-Hydroxy Vitamin D.
References
Page TF, Beck-Sague CM, Pinzon-Iregui MC, Cuddihy A, Tyler T, Forno E, Dean AG, Siven J, Pottinger S, Gasana J: Asthma in underserved schoolchildren in Miami, Florida: results of a school- and community-based needs assessment. J Asthma: official j Assoc Care of Asthma. 2013, 50: 480-487. 10.3109/02770903.2013.790416.
Chien JW, Lin CY, Yang KD, Lin CH, Kao JK, Tsai YG: Increased IL-17A secreting CD4(+) T cells, serum IL-17 levels and exhaled nitric oxide are correlated with childhood asthma severity. Clin Exp Allergy: J Br Soc Allergy and Clinical Immunol. 2013, 43: 1018-1026. 10.1111/cea.12119.
Holick MF: Vitamin D Deficiency. N Engl J Med. 2007, 357: 266-281. 10.1056/NEJMra070553.
Holick MF: The vitamin D deficiency pandemic and consequences for nonskeletal health: mechanisms of action. Mol Asp Med. 2008, 29: 361-368. 10.1016/j.mam.2008.08.008.
Makariou S, Liberopoulos EN, Elisaf M, Challa A: Novel roles of vitamin D in disease: what is new in 2011?. Eur J Intern Med. 2011, 22: 355-362. 10.1016/j.ejim.2011.04.012.
Moreno LA, Valtuena J, Perez-Lopez F, Gonzalez-Gross M: Health effects related to low vitamin D concentrations: beyond bone metabolism. Ann Nutr Metab. 2011, 59: 22-27. 10.1159/000332070.
Hypponen E, Laara E, Reunanen A, Jarvelin MR, Virtanen SM: Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001, 358: 1500-1503. 10.1016/S0140-6736(01)06580-1.
The EURODIAB Substudy 2 Study Group: Vitamin D supplement in early childhood and risk for Type I (insulin-dependent) diabetes mellitus. Diabetologia. 1999, 42: 51-54. 10.1007/s001250051112.
Bener A, Alsaied A, Al-Ali M, Al-Kubaisi A, Basha B, Abraham A, Guiter G, Mian M: High prevalence of vitamin D deficiency in type 1 diabetes mellitus and healthy children. Acta Diabetol. 2009, 46: 183-189. 10.1007/s00592-008-0071-6.
Bin-Abbas BS, Jabari MA, Issa SD, Al-Fares AH, Al-Muhsen S: Vitamin D levels in Saudi children with type 1 diabetes. Saudi Med J. 2011, 32: 589-592.
Lerner A, Shapira Y, Agmon-Levin N, Pacht A, Ben-Ami Shor D, Lopez HM, Sanchez-Castanon M, Shoenfeld Y: The clinical significance of 25OH-Vitamin D status in celiac disease. Clin Rev Allergy Immunol. 2012, 42: 322-330. 10.1007/s12016-010-8237-8.
van Oeffelen AA, Bekkers MB, Smit HA, Kerkhof M, Koppelman GH, Haveman-Nies A, Van Der AD, Jansen EH, Wijga AH: Serum micronutrient concentrations and childhood asthma: the PIAMA birth cohort study. Pediatr Allergy Immunol. 2011, 22: 784-793. 10.1111/j.1399-3038.2011.01190.x.
Hollams EM, Hart PH, Holt BJ, Serralha M, Parsons F, de Klerk NH, Zhang G, Sly PD, Holt PG: Vitamin D and atopy and asthma phenotypes in children: a longitudinal cohort study. Eur Respir J. 2011, 38: 1320-1327. 10.1183/09031936.00029011.
Cantorna MT, Mahon BD: D-hormone and the immune system. J Rheumatol Suppl. 2005, 76: 11-20.
Hewison M: Vitamin D and immune function: autocrine, paracrine or endocrine?. Scand J Clin Lab Invest Suppl. 2012, 243: 92-102.
Gorman S, Weeden CE, Tan DH, Scott NM, Hart J, Foong RE, Mok D, Stephens N, Zosky G, Hart PH: Reversible control by vitamin D of granulocytes and bacteria in the lungs of mice: an ovalbumin-induced model of allergic airway disease. PLoS One. 2013, 8: e67823-10.1371/journal.pone.0067823.
Majak P, Jerzynska J, Smejda K, Stelmach I, Timler D, Stelmach W: Correlation of vitamin D with Foxp3 induction and steroid-sparing effect of immunotherapy in asthmatic children. Ann Allergy Asthma Immunol. 2012, 109: 329-335. 10.1016/j.anai.2012.08.002.
Matheu V, Bäck O, Mondoc E, Issazadeh-Navikas S: Dual effects of vitamin D-induced alteration of TH1/TH2 cytokine expression: enhancing IgE production and decreasing airway eosinophilia in murine allergic airway disease. J Allergy Clin Immunol. 2003, 112: 585-592. 10.1016/S0091-6749(03)01855-4.
Nanzer AM, Chambers ES, Ryanna K, Richards DF, Black C, Timms PM, Martineau AR CJG, Corrigan CJ, Hawrylowicz CM: Enhanced production of IL-17A in patients with severe asthma is inhibited by 1α,25-dihydroxyvitamin D3 in a glucocorticoid-independent fashion. J Allergy Clin Immunol. 2013, 132: 297-304. 10.1016/j.jaci.2013.03.037. e293
Carraro S, Giordano G, Reniero F, Carpi D, Stocchero M, Sterk PJ, Baraldi E: Asthma severity in childhood and metabolomic profiling of breath condensate. Allergy: Eur J Allergy Clinical Immunol. 2013, 68: 110-117. 10.1111/all.12063.
Robinson CL, Baumann LM, Gilman RH, Romero K, Combe JM, Cabrera L, Hansel NN, Barnes K, Gonzalvez G, Wise RA, Breysse PN, Checkley W: The Peru urban versus rural asthma (PURA) study: Methods and baseline quality control data from a cross-sectional investigation into the prevalence, severity, genetics, immunology and environmental factors affecting asthma in adolescence in Peru. BMJ Open. 2012, 2: e000421-doi: 10.1136/bmjopen-2011-000421. Print 2012
Bener A, Ehlayel MS, Tulic MK, Hamid Q: Vitamin D deficiency as a strong predictor of asthma in children. Int Arch Allergy Immunol. 2012, 157: 168-175. 10.1159/000323941.
Gergen PJ, Teach SJ, Mitchell HE, Freishtat RF, Calatroni A, Matsui E, Kattan M, Bloomberg GR, Liu AH, Kercsmar C, O'Connor G, Pongracic J, Rivera-Sanchez Y, Morgan WJ, Sorkness CA, Binkley N, Busse W: Lack of a relation between serum 25-hydroxyvitamin D concentrations and asthma in adolescents. Am J Clin Nutr. 2013, 97: 1228-1234. 10.3945/ajcn.112.046961.
Tolppanen AM, Sayers A, Granell R, Fraser WD, Henderson J, Lawlor DA: Prospective association of 25-hydroxyvitamin d3 and d2 with childhood lung function, asthma, wheezing, and flexural dermatitis. Epidemiology. 2013, 24: 310-319. 10.1097/EDE.obo13e318280dd5e.
Hypponen E, Sovio U, Wjst M, Patel S, Pekkanen J, Hartikainen AL, Jarvelin MR: Infant vitamin D supplementation and allergic conditions in adulthood: Northern Finland birth cohort 1966. Ann New York Acad Sci. 2004, 1037: 84-95. 10.1196/annals.1337.013.
El-Hajj Fuleihan G: Vitamin D Deficiency in the Middle East and its Health Consequences. Clin Rev Bone Miner Metab. 2009, 7: 77-93. 10.1007/s12018-009-9027-9.
Braegger C, Campoy C, Colomb V, Decsi T, Domellof M, Fewtrell M, Hojsak I, Mihatsch W, Molgaard C, Shamir R, Turck D, van Goudoever J, ESPGHANCommittee on Nutrition: Vitamin D in the Healthy Paediatric Population: A Position Paper by the ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2013, 56 (6): 692-701. 10.1097/MPG.0b013e31828f3c05.
Mroueh S, Alkhaled L, Fares M, Akl E: Vitamin D for asthma in children. PROSPERO. 2013, CRD42013004204 Available from http://www.crd.york.ac.uk/PROSPERO_REBRANDING/display_record.asp?ID=CRD42013004204
Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso-Coello P, Montori V, Akl EA, Djulbegovic B, Falck-Ytter Y, Norris SL, Williams JW, Atkins D, Meerpohl J, Schünemann HJ: GRADE guidelines: 4. Rating the quality of evidence--study limitations (risk of bias). J Clin Epidemiol. 2011, 64: 407-415. 10.1016/j.jclinepi.2010.07.017.
Major JM, Graubard BI, Dodd KW, Iwan A, Alexander BH, Linet MS, Freedman DM: Variability and reproducibility of circulating vitamin D in a nationwide U.S. population. J Clin Endocrinol Metab. 2013, 98: 97-104. 10.1210/jc.2012-2643.
Autier P, Boniol M, Pizot C, Mullie P: Vitamin D status and ill health: a systematic review. The Lancet Diab Endocrinol. 2014, 2: 76-89.
Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JPA: Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ. 2014, 348: g2035-10.1136/bmj.g2035.
Nurmatov U, Devereux G, Sheikh A: Nutrients and foods for the primary prevention of asthma and allergy: systematic review and meta-analysis. J Allergy Clin Immunol. 2011, 127: 724-733. 10.1016/j.jaci.2010.11.001. e721-730
Zhang LL, Gong J, Liu CT: Vitamin D with asthma and COPD: not a false hope? A systematic review and meta-analysis. Genet Mol Res: GMR. 2014, 13: 13-(AOP). [Epub ahead of print]
Gupta A, Bush A, Hawrylowicz C, Saglani S: Vitamin D and Asthma in Children. Paediatr Respir Rev. 2012, 13: 236-243. 10.1016/j.prrv.2011.07.003.
Hollams EM: Vitamin D and atopy and asthma phenotypes in children. Curr Opin Allergy Clin Immunol. 2012, 12: 228-234. 10.1097/ACI.0b013e3283534a32.
Rothers J, Wright AL, Stern DA, Halonen M, Camargo CA: Cord blood 25-hydroxyvitamin D levels are associated with aeroallergen sensitization in children from Tucson, Arizona. J Allergy Clin Immunol. 2011, 128: 1093-1099. 10.1016/j.jaci.2011.07.015. e1091-1095
Acknowledgements
We would like to thank Miss Aida Farha for her valuable help in designing the search strategy.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
SM, EAA and LA conceived of the study and participated in the design of the study. LA and MF participated in study selection process. MR and TL participated in the data collection process. EA, GEHF and SM participated in data analysis and interpretation of the study. MR and EA helped in drafting the manuscript. All authors reviewed, edited, and approved the final manuscript.
Electronic supplementary material
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Rajabbik, M.H., Lotfi, T., Alkhaled, L. et al. Association between low vitamin D levels and the diagnosis of asthma in children: a systematic review of cohort studies. All Asth Clin Immun 10, 31 (2014). https://doi.org/10.1186/1710-1492-10-31
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1710-1492-10-31