Abstract
Na+/K+-ATPase alpha 2 (Atp1a2) is an integral plasma membrane protein belonging to the P-type ATPase family that is responsible for maintaining the sodium (Na+) and potassium (K+) gradients across cellular membranes with hydrolysis of ATP. Atp1a2 contains two subunits, alpha and beta, with each having various isoforms and differential tissue distribution. In humans, mutations in ATP1A2 are associated with a rare form of hereditary migraines with aura known as familial hemiplegic migraine type II. Genetic studies in mice have revealed other neurological effects of Atp1a2 in mice including anxiety, fear, and learning and motor function disorders. This paper reviews the recent findings in the literature concerning Atp1a2.
Similar content being viewed by others
Introduction
The integral plasma membrane protein Na+/K+-ATPase is a member of the P-type ATPase family. P-type ATPases maintain the essential plasma membrane potential in all eukaryotic cells and are found in all cell type membranes. The plasma membrane potential is upheld by adjusting ion concentrations on the intracellular and extracellular sides of the membrane. This electrochemical gradient fuels central cellular processes, such as the secondary transport of metabolites, and it also provides the basis for electrical excitation in neurons. The mechanism of action of Na+/K+-ATPase involves a conformation change driven by ATP hydrolysis (Figure 1). While bound to ATP, the pump binds three intracellular sodium ions (Figure 1, step 1). ATP is then hydrolyzed to ADP, and the pump is phosphorylated at a highly conserved aspartate residue (Figure 1, step 2). This phosphorylation causes a conformation change, resulting in the release of the sodium ions into the extracellular space and the binding of two potassium ions (Figure 1, step 3). This binding of potassium ions causes the pump to dephosphorylate (Figure 1, step 4), which returns the pump to its previous conformational state (Figure 1, step 5), and transports the potassium ions into the cell (Figure 1, step 6). The process then repeats itself when ATP again binds to the pump.
The Na+/K+-ATPases consist of an α subunit and a β subunit and, only in the kidney, a γ subunit [1, 4]. The α and β subunits are synthesized separately, assembled in the endoplasmic reticulum, and are delivered to the plasma membrane [1, 5]. The α subunit is the catalytic region of the enzyme and contains the binding sites for the sodium and potassium ions, ATP, and cardiac glycosides such as ouabain, while the glycosylated β subunit is needed for proper folding and function of the catalytic subunit. The X-ray crystal structure of Na+/K+-ATPase was examined from pig kidney, which showed the binding sites in the α subunit and the interactions between the α and β subunits [6]. Different genes encode each of the multiple α and β isoforms and the γ isoform; four α isoforms (ATP1A1, ATP1A2, ATP1A3, and ATP1A4), four β isoforms (ATP1B1, ATP1B2, ATP1B3 and ATP1B4), and one γ isoform (FXYD2) have been identified (Table 1, [7, 8]).
The α isoforms differ in tissue distribution and are regulated developmentally [9]. The α1 subunit is expressed ubiquitously, the α2 isoform is expressed in the brain, heart, and skeletal muscle, the α3 subunit is expressed in the brain and heart, and the α4 isoform is expressed in sperm and its precursor cells [10, 11]. The four isoforms have a high degree of amino acid identity but have differences in kinetic properties and substrate affinity [12, 13]. Homology between human and mouse protein is between 83% and 99%, and within subunits and species, it is approximately between 80% and 90% (Table 1) [14]. In the adult brain, the α1 isoform is found in multiple central nervous system (CNS) cell types, the α2 isoform is primarily expressed in astrocytes and pyramidal cells in the hippocampus, and α3 is expressed only in neurons [15–17].
ATP1A2, as well as the other isoforms, is responsible for maintaining the resting membrane potential and for driving nutrient and neurotransmitter uptakes. Na+/K+-ATPases are important in clearing extracellular potassium during neuronal activity and are essential in the clearance of released glutamate in the synaptic cleft because reuptake in astrocytes and neurons is driven by the sodium and potassium gradients [18]. ATP1A2 is co-localized with other ion transporters, such as the sodium/calcium (Na+/Ca2+) exchanger and the glutamate transporter, which are important in the clearance of glutamate and potassium from the extracellular space in the CNS [19–21].
The role of ATP1A2in FHM2
Familial hemiplegic migraine (FHM) is a rare autosomal dominant form of migraine with aura. FHM attacks are generally longer than the common migraine with aura; however, they share similar symptoms (Table 2, [22]) including visual, sensory, motor, and aphasia [23]. Aura symptoms are different for each person but are generally described as disturbances in light perception, such as spots or lines in vision, and changes in sensory perception, such as heightened sensitivity to smells and sounds [24]. There are three types of FHM: type 1 is associated with mutations in the neuronal calcium channel gene and is the most prevalent form of FHM, type 2 is caused by mutations present in ATP1A2, and type 3 is a rare form of FHM related to mutations in the sodium channel gene [24].
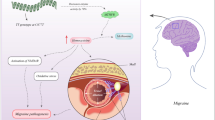
FHM2 patients suffer from migraines with hemiplegia and partial paralysis during the aura phase and, in some cases, accompanied by seizures or cognitive dysfunction. Neuroimaging studies have shown that migraine aura is caused by cortical spreading depression (CSD). CSD is a wave of continual strong neuronal depolarization that slowly progresses across the cortex, generating a brief intense spike of activity that is followed by long-lasting neural suppression [25]. CSD has been shown to activate the trigeminovascular system (TGVS), which is responsible for the headache associated with migraines [24]. Inhibition of ATP1A2 leads to high levels of extracellular potassium, causing neurons to become depolarized which can cause CSD [26].
ATP1A2 was identified as a gene associated with FHM2 in 2003 in two Italian families [27]. The mutations and deficiencies in ATP1A2 that cause FHM2 are responsible for approximately 20% of FHM in families [2]. There are over 50 mutations in ATP1A2 that have been identified in association with FHM2. Almost all FHM2 mutations are non-synonymous SNPs, but there are also small deletions [28] and a mutation affecting the stop codon, causing an extension of the ATP1A2 protein by 27 amino acid residues [29]. Most of the mutations are associated with pure FHM without additional clinical symptoms [27–31]. Recently, a number of ATP1A2 mutations were reported to be associated with FHM and cerebellar problems, specifically motor problems [32], childhood convulsions [33], epilepsy [29, 34], and mental retardation [29, 35]. Some ATP1A2 mutations have been shown to be associated with non-hemiplegic migraine phenotypes, such as basilar migraine [36] and the common migraine [37].
One of the most important experimental drugs to study ATP1A2, as well as ATP1A1 and ATP1A3, is ouabain which is a plant-derived steroid that binds to the E2P form of Na+/K+-ATPases, as seen in Figure 1. Ouabain acts as a non-selective antagonist and inhibits the enzyme transport activity [38]. The three isoforms in humans show similar affinity for ouabain; however, ouabain binding is altered due to changes in extracellular potassium levels, resulting in varying sensitivities [39]. In rats, Atp1a3 is very sensitive to ouabain, Atp1a2 is less sensitive, and Atp1a1 is insensitive [40].
Cell-based studies have shown that multiple individual mutations in ATP1A2 result in dysfunctional ion pump activity. The functional effects of mutations in ATP1A2 have been studied in HeLa cells and Xenopus oocytes (Table 3). Recombinant ATP1A2 subunits containing mutations that cause the pump to be insensitive to ouabain were expressed in HeLa cells; the ouabain-insensitive mutants were used to distinguish between endogenous ATP1A2 activity and the effects of other mutations engineered into the recombinant ATP1A2 [27, 33, 35, 41, 42]. Three mutations in HeLa cells and two mutations in Xenopus oocytes produced severe or complete loss of function of pump activity, which led to cell death [3, 27, 35, 41]. Five other FHM2 mutants were analyzed, and pump activity was reduced but sufficient to allow survival of the HeLa cells [41–43].
ATP1A2 is expressed primarily in astrocytes in the adult, where it appears functionally coupled to various transporters (glutamate transporter and Na+/Ca2+ exchanger), and is essential in the clearance of released glutamate and potassium from the extracellular space during neuronal activity. FHM2 mutations that cause loss of function of ATP1A2 may lead to decreased glutamate clearance and an increase of potassium in the synaptic cleft during neuronal activity, which could lead to prolonged recovery time after neuronal excitation, and may render the brain to be more susceptible to CSD [23, 26].
There are two hypothesized mechanisms for the effects of ATP1A2 mutations. First, the mutations cause an increase in extracellular potassium, which can result in the impaired clearance of potassium ions and therefore induce CSD [43]. Second, since the distribution of ATP1A2 is co-localized with the Na+/Ca2+ exchanger, the mutations to ATP1A2 would cause intracellular sodium to increase, which increases intracellular calcium levels through the Na+/Ca2+ exchanger, similar to FHM1, resulting in glutamate release and a decrease in glutamate clearance which can also lead to CSD [23, 44]. Both hypotheses result in making the brain more susceptible to CSD and therefore migraines with aura.
Currently, the medications used to treat FHM2 are standard migraine prophylactic drugs, such as antidepressants, beta blockers, and calcium channel blockers [45], which treat the symptoms, not the cause. Further investigation of ATP1A2 in humans and animal models are needed to better determine treatment options.
Atp1a2 studies in animal models
Over the last 20 years, animal studies using either Atp1a2 knock-out or knock-in mutations have increased our understanding of its effect on behavior. Atp1a2 heterozygous mice have been studied by two separate groups, one at the University of Cincinnati led by Dr. Jerry Lingrel and the second at Jichi Medical School in Japan overseen by Dr. Kiyoshi Kawakami. Both groups have shown that modulation of Atp1a2 activity affects neural activity and whole animal behavior. More recently, a group led by Giorgio Casari at the Vita-Salute San Raffaele University and Center for Translational Genomics and Bioinformatics in Italy generated the first FHM2 knock-in mouse. The constructs for the gene alterations are shown in Figure 2.
Three separate groups have mutated the Atp1a2 gene in mice. Two groups created knock-outs through disruption of the gene at two different locations and the other has created a knock-in mutation to mimic one of the FHM2 mutations in exon 19. Atp1a2 is located in the antisense strand of chromosome 1 [18, 19, 26].
Lingrel's lab has been investigating the functional roles of the various Na+/K+-ATPase isoforms for over 20 years. This lab developed heterozygous mice for Atp1a2 as well as for Atp1a1 and Atp1a3[19]. Atp1a2 heterozygous (Atp1a2±) mice were tested for differences in behavior as well as to investigate the role of Atp1a2 in the heart. Kawakami's group also created an Atp1a2± mouse by deleting a portion of the gene, resulting in only one functioning copy [18]. They examined the role of Atp1a2 in neural activity by measuring fear and anxiety in heterozygous mice. The Atp1a2 heterozygous mice also showed hyperphagia during the light period and suffered from late onset obesity [46]. The homozygous null Atp1a2 mice are neonatally lethal due to lack of synchronized neuronal firing in the breathing center of the brain [16, 47, 48], and the Atp1a2± mice have approximately half the protein compared to with wild type [19].
The heterozygous mice from both groups consistently show an anxiety-related phenotype (Table 4). The Atp1a2± mice were hypoactive compared with the wild type based on measurements of total distance traveled and time spent in the corners of the open-field testing box [17]. They also spent less time in and had fewer entries into the open arm of the plus maze [11, 17, 41]. In the light/dark test, the knock-outs spent less time in the light compartment and had fewer transitions between the two compartments [18]. Taken together, these results show that the Atp1a2± mice are more anxious than the wild type.
Learning and memory behavioral tests were interpreted differently between the two groups. The Lingrel lab examined spatial learning and memory using the Morris water maze and found that the knock-out mouse had a longer latency to find the hidden platform, suggesting that they have impaired learning. The Kawakami lab studied the knock-out mouse in the conditioned fear test, which showed that the knock-out mouse had increased freezing time which is typically interpreted as improved learning and memory [18]. However, the authors concluded that the knock-out mouse had enhanced fear; other effects such as increased learning and memory and altered sensory perception could explain the results. The authors did not examine or control these other possibilities.
The role of Atp1a2 has also been examined in the hearts of the heterozygous mice. While the hearts were histologically the same in Atp1a2± and wild-type mice, the Atp1a2± mice had hypercontractile hearts [19, 41, 42]. As noted above, the Atp1a2± mice have 50% less protein than the wild type, and reduction of Atp1a2 alters calcium levels in cardiomyocytes, suggesting that the intracellular concentration of sodium ions would increase. This increased concentration results in an increase in intracellular calcium levels because the sodium-calcium exchanger is inhibited by the high intracellular sodium levels. The excess intracellular calcium causes the contractions in the cardiomyocytes to increase in strength [19, 41]. These results suggest that Atp1a2 regulates calcium levels and, therefore, contractibility in the heart.
The group at the Vita-Salute San Raffaele University and Center for Translational Genomics and Bioinformatics in Italy has generated the first FHM2 knock-in mouse. This mouse model was created by inserting the T2763C mutation, in exon 19 of the mouse, which is one of the first mutations that was associated with FHM2 [26, 41, 42]. The T2763C mutation causes the amino acid substitution W887R, which affects the β subunit binding site in Atp1a2, resulting in a misfolding of the protein and, in cell-based studies, a complete loss of pump function. The homozygous knock-in mice are neonatally lethal, similar to the knock-out mice described previously; therefore, heterozygous knock-in mice were used for experiments. The authors suggested that because the W887R mutant protein does not translocate efficiently to the plasma membrane, it is degraded via the proteasome, which results in reduced overall protein in the brain. The knock-in mice were tested for CSD by electrical stimulation, and results showed that they were more susceptible to CSD: they had a higher threshold and velocity but equal duration of CSD than the wild type. These results may be due to the impaired clearance of extracellular potassium and glutamate by astrocytes, which is comparable to the effects of FHM2 mutations in humans [23, 26]. Further testing on this mouse model will be beneficial to understanding the mechanism of at least this mutation in FHM and possible treatment avenues.
Conclusions
Mutations and deficiencies in Atp1a2 are associated with FHM2 in patients suffering from migraines with hemiplegia and partial paralysis during the aura phase. FHM2 patients may also have increased susceptibility to CSD, which may be due to the increase in extracellular potassium and/or a decrease in glutamate clearance. CSD has been shown to cause the aura coupled with migraines and may activate the TGVS, resulting in the headache portion of a migraine.
Studies in Atp1a2-deficient mice suggest an association of the protein with anxiety, learning disorders, and fear. Multiple groups have created knock-out and knock-in heterozygous Atp1a2 mice and have tested them for fear and anxiety, locomotor activity, learning ability, and susceptibility to CSD. The results of these animal studies show significant increased anxiety and fear, reduced locomotor activity, and increased susceptibility to CSD in heterozygous Atp1a2 mice compared with the wild type. Learning and memory results are not consistent between the two heterozygous mouse studies. The Lingrel lab results showed poor learning and memory in the Morris water maze; however, the Kawakami lab found that the heterozygous mice had increased freezing during the conditioned fear stimuli which could be due to increased fear and anxiety, increased sensory perception, or improved learning and memory. Additional animal studies with the knock-in mutations associated with FHM2 and the Atp1a2 knock-outs are needed to better understand FHM2, treatment options, and its overall effect on behavior.
Abbreviations
- Atp1a2:
-
Na+/K+-ATPase α2
- Atp1a2±:
-
Atp1a2 heterozygous
- Ca2+:
-
calcium
- CNS:
-
central nervous system
- CSD:
-
cortical spreading depression
- FHM:
-
familial hemiplegic migraine
- FHM2:
-
familial hemiplegic migraine type II
- K+:
-
potassium
- Na+:
-
sodium
- TGVS:
-
trigeminovascular system.
References
Kaplan JH: Biochemistry of Na, K-ATPase. Annu Rev Biochem. 2002, 71: 511-535. 10.1146/annurev.biochem.71.102201.141218.
Vanmolkot KR, Kors EE, Turk U, Turkdogan D, Keyser A, Broos LA, Kia SK, van den Heuvel JJ, Black DF, Haan J, Frants RR, Barone V, Ferrari MD, Casari G, Koenderink JB, van den Maagdenberg AM: Two de novo mutations in the Na, K-ATPase gene ATP1A2 associated with pure familial hemiplegic migraine. Eur J Hum Genet. 2006, 14 (5): 555-560. 10.1038/sj.ejhg.5201607.
Bassi MT, Bresolin N, Tonelli A, Nazos K, Crippa F, Baschirotto C, Zucca C, Bersano A, Dolcetta D, Boneschi FM, Barone V, Casari G: A novel mutation in the ATP1A2 gene causes alternating hemiplegia of childhood. J Med Genet. 2004, 41 (8): 621-628. 10.1136/jmg.2003.017863.
Therien AG, Karlish SJ, Blostein R: Expression and functional role of the gamma subunit of the Na, K-ATPase in mammalian cells. J Biol Chem. 1999, 274 (18): 12252-12256. 10.1074/jbc.274.18.12252.
Geering K, Beggah A, Good P, Girardet S, Roy S, Schaer D, Jaunin P: Oligomerization and maturation of Na, K-ATPase: functional interaction of the cytoplasmic NH2 terminus of the beta subunit with the alpha subunit. J Cell Biol. 1996, 133 (6): 1193-1204. 10.1083/jcb.133.6.1193.
Morth JP, Pedersen BP, Toustrup-Jensen MS, Sorensen TL, Petersen J, Andersen JP, Vilsen B, Nissen P: Crystal structure of the sodium-potassium pump. Nature. 2007, 450 (7172): 1043-1049. 10.1038/nature06419.
NCBI: Genes. [http://www.ncbi.nlm.nih.gov/gene]
NCBI: Proteins. [http://www.ncbi.nlm.nih.gov/protein]
Orlowski J, Lingrel JB: Tissue-specific and developmental regulation of rat Na, K-ATPase catalytic alpha isoform and beta subunit mRNAs. J Biol Chem. 1988, 263 (21): 10436-10442.
Woo AL, James PF, Lingrel JB: Characterization of the fourth alpha isoform of the Na, K-ATPase. J Membr Biol. 1999, 169 (1): 39-44. 10.1007/PL00005899.
Lingrel JB, Williams MT, Vorhees CV, Moseley AE: Na, K-ATPase and the role of alpha isoforms in behavior. J Bioenerg Biomembr. 2007, 39 (5–6): 385-389.
Jewell EA, Lingrel JB: Comparison of the substrate dependence properties of the rat Na, K-ATPase alpha 1, alpha 2, and alpha 3 isoforms expressed in HeLa cells. J Biol Chem. 1991, 266 (25): 16925-16930.
Segall L, Daly SE, Blostein R: Mechanistic basis for kinetic differences between the rat alpha 1, alpha 2, and alpha 3 isoforms of the Na, K-ATPase. J Biol Chem. 2001, 276 (34): 31535-31541. 10.1074/jbc.M103720200.
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ: Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997, 25 (17): 3389-3402. 10.1093/nar/25.17.3389.
McGrail KM, Phillips JM, Sweadner KJ: Immunofluorescent localization of three Na, K-ATPase isozymes in the rat central nervous system: both neurons and glia can express more than one Na, K-ATPase. J Neurosci. 1991, 11 (2): 381-391.
Moseley AE, Lieske SP, Wetzel RK, James PF, He S, Shelly DA, Paul RJ, Boivin GP, Witte DP, Ramirez JM, Sweadner KJ, Lingrel JB: The Na, K-ATPase alpha 2 isoform is expressed in neurons, and its absence disrupts neuronal activity in newborn mice. J Biol Chem. 2003, 278 (7): 5317-5324. 10.1074/jbc.M211315200.
Moseley AE, Williams MT, Schaefer TL, Bohanan CS, Neumann JC, Behbehani MM, Vorhees CV, Lingrel JB: Deficiency in Na, K-ATPase alpha isoform genes alters spatial learning, motor activity, and anxiety in mice. J Neurosci. 2007, 27 (3): 616-626. 10.1523/JNEUROSCI.4464-06.2007.
Ikeda K, Onaka T, Yamakado M, Nakai J, Ishikawa TO, Taketo MM, Kawakami K: Degeneration of the amygdala/piriform cortex and enhanced fear/anxiety behaviors in sodium pump alpha2 subunit (Atp1a2)-deficient mice. J Neurosci. 2003, 23 (11): 4667-4676.
James PF, Grupp IL, Grupp G, Woo AL, Askew GR, Croyle ML, Walsh RA, Lingrel JB: Identification of a specific role for the Na, K-ATPase alpha 2 isoform as a regulator of calcium in the heart. Mol Cell. 1999, 3 (5): 555-563. 10.1016/S1097-2765(00)80349-4.
Rose EM, Koo JC, Antflick JE, Ahmed SM, Angers S, Hampson DR: Glutamate transporter coupling to Na, K-ATPase. J Neurosci. 2009, 29 (25): 8143-8155. 10.1523/JNEUROSCI.1081-09.2009.
Cholet N, Pellerin L, Magistretti PJ, Hamel E: Similar perisynaptic glial localization for the Na+, K+-ATPase alpha 2 subunit and the glutamate transporters GLAST and GLT-1 in the rat somatosensory cortex. Cereb Cortex. 2002, 12 (5): 515-525. 10.1093/cercor/12.5.515.
Headache Classification Subcommittee of the International Headache Society: The International Classification of Headache Disorders. 2003, Oxford: Blackwell, 2
Pietrobon D: Familial hemiplegic migraine. Neurotherapeutics. 2007, 4 (2): 274-284. 10.1016/j.nurt.2007.01.008.
de Vries B, Frants RR, Ferrari MD, van den Maagdenberg AM: Molecular genetics of migraine. Hum Genet. 2009, 126 (1): 115-132. 10.1007/s00439-009-0684-z.
Pietrobon D, Striessnig J: Neurobiology of migraine. Nat Rev Neurosci. 2003, 4 (5): 386-398. 10.1038/nrn1102.
Leo L, Gherardini L, Barone V, De Fusco M, Pietrobon D, Pizzorusso T, Casari G: Increased susceptibility to cortical spreading depression in the mouse model of familial hemiplegic migraine type 2. PLoS Genet. 2011, 7 (6): e1002129-10.1371/journal.pgen.1002129.
De Fusco M, Marconi R, Silvestri L, Atorino L, Rampoldi L, Morgante L, Ballabio A, Aridon P, Casari G: Haploinsufficiency of ATP1A2 encoding the Na+/K+ pump alpha2 subunit associated with familial hemiplegic migraine type 2. Nat Genet. 2003, 33 (2): 192-196. 10.1038/ng1081.
Riant F, De Fusco M, Aridon P, Ducros A, Ploton C, Marchelli F, Maciazek J, Bousser MG, Casari G, Tournier-Lasserve E: ATP1A2 mutations in 11 families with familial hemiplegic migraine. Hum Mutat. 2005, 26 (3): 281-
Jurkat-Rott K, Freilinger T, Dreier JP, Herzog J, Gobel H, Petzold GC, Montagna P, Gasser T, Lehmann-Horn F, Dichgans M: Variability of familial hemiplegic migraine with novel A1A2 Na+/K+-ATPase variants. Neurology. 2004, 62 (10): 1857-1861. 10.1212/01.WNL.0000127310.11526.FD.
Kaunisto MA, Harno H, Vanmolkot KR, Gargus JJ, Sun G, Hamalainen E, Liukkonen E, Kallela M, van den Maagdenberg AM, Frants RR, Farkkila M, Palotie A, Wessman M: A novel missense ATP1A2 mutation in a Finnish family with familial hemiplegic migraine type 2. Neurogenetics. 2004, 5 (2): 141-146. 10.1007/s10048-004-0178-z.
Pierelli F, Grieco GS, Pauri F, Pirro C, Fiermonte G, Ambrosini A, Costa A, Buzzi MG, Valoppi M, Caltagirone C, Nappi G, Santorelli FM: A novel ATP1A2 mutation in a family with FHM type II. Cephalalgia. 2006, 26 (3): 324-328. 10.1111/j.1468-2982.2006.01002.x.
Spadaro M, Ursu S, Lehmann-Horn F, Veneziano L, Antonini G, Giunti P, Frontali M, Jurkat-Rott K: A G301R Na+/K+-ATPase mutation causes familial hemiplegic migraine type 2 with cerebellar signs. Neurogenetics. 2004, 5 (3): 177-185. 10.1007/s10048-004-0183-2.
Vanmolkot KR, Kors EE, Hottenga JJ, Terwindt GM, Haan J, Hoefnagels WA, Black DF, Sandkuijl LA, Frants RR, Ferrari MD, van den Maagdenberg AM: Novel mutations in the Na+, K+-ATPase pump gene ATP1A2 associated with familial hemiplegic migraine and benign familial infantile convulsions. Ann Neurol. 2003, 54 (3): 360-366. 10.1002/ana.10674.
Deprez L, Weckhuysen S, Peeters K, Deconinck T, Claeys KG, Claes LR, Suls A, Van Dyck T, Palmini A, Matthijs G, Van Paesschen W, De Jonghe P: Epilepsy as part of the phenotype associated with ATP1A2 mutations. Epilepsia. 2008, 49 (3): 500-508. 10.1111/j.1528-1167.2007.01415.x.
Vanmolkot KR, Stroink H, Koenderink JB, Kors EE, van den Heuvel JJ, van den Boogerd EH, Stam AH, Haan J, De Vries BB, Terwindt GM, Frants RR, Ferrari MD, van den Maagdenberg AM: Severe episodic neurological deficits and permanent mental retardation in a child with a novel FHM2 ATP1A2 mutation. Ann Neurol. 2006, 59 (2): 310-314. 10.1002/ana.20760.
Ambrosini A, D'Onofrio M, Grieco GS, Di Mambro A, Montagna G, Fortini D, Nicoletti F, Nappi G, Sances G, Schoenen J, Buzzi MG, Santorelli FM, Pierelli F: Familial basilar migraine associated with a new mutation in the ATP1A2 gene. Neurology. 2005, 65 (11): 1826-1828. 10.1212/01.wnl.0000187072.71931.c0.
Todt U, Dichgans M, Jurkat-Rott K, Heinze A, Zifarelli G, Koenderink JB, Goebel I, Zumbroich V, Stiller A, Ramirez A, Friedrich T, Gobel H, Kubisch C: Rare missense variants in ATP1A2 in families with clustering of common forms of migraine. Hum Mutat. 2005, 26 (4): 315-321. 10.1002/humu.20229.
Crambert G, Schaer D, Roy S, Geering K: New molecular determinants controlling the accessibility of ouabain to its binding site in human Na, K-ATPase alpha isoforms. Mol Pharmacol. 2004, 65 (2): 335-341. 10.1124/mol.65.2.335.
Crambert G, Hasler U, Beggah AT, Yu C, Modyanov NN, Horisberger JD, Lelievre L, Geering K: Transport and pharmacological properties of nine different human Na, K-ATPase isozymes. J Biol Chem. 2000, 275 (3): 1976-1986. 10.1074/jbc.275.3.1976.
Munzer JS, Daly SE, Jewell-Motz EA, Lingrel JB, Blostein R: Tissue- and isoform-specific kinetic behavior of the Na, K-ATPase. J Biol Chem. 1994, 269 (24): 16668-16676.
Segall L, Scanzano R, Kaunisto MA, Wessman M, Palotie A, Gargus JJ, Blostein R: Kinetic alterations due to a missense mutation in the Na, K-ATPase alpha2 subunit cause familial hemiplegic migraine type 2. J Biol Chem. 2004, 279 (42): 43692-43696. 10.1074/jbc.M407471200.
Segall L, Mezzetti A, Scanzano R, Gargus JJ, Purisima E, Blostein R: Alterations in the alpha2 isoform of Na, K-ATPase associated with familial hemiplegic migraine type 2. Proc Natl Acad Sci U S A. 2005, 102 (31): 11106-11111. 10.1073/pnas.0504323102.
Koenderink JB, Zifarelli G, Qiu LY, Schwarz W, De Pont JJ, Bamberg E, Friedrich T: Na, K-ATPase mutations in familial hemiplegic migraine lead to functional inactivation. Biochim Biophys Acta. 2005, 1669 (1): 61-68. 10.1016/j.bbamem.2005.01.003.
Montagna P: The physiopathology of migraine: the contribution of genetics. Neurol Sci. 2004, 25 (Suppl 3): S93-96.
Jen JC: Familial hemiplegic migraine. GeneReviewsTM. Edited by: Pagon RA, Bird TD, Dolan CR, Stephens K, Adam MP. 2001, Seattle (WA): University of Washington, Seattle, PMID 20301562
Kawakami K, Onaka T, Iwase M, Homma I, Ikeda K: Hyperphagia and obesity in Na, K-ATPase alpha2 subunit-defective mice. Obes Res. 2005, 13 (10): 1661-1671. 10.1038/oby.2005.204.
Ikeda K, Onimaru H, Yamada J, Inoue K, Ueno S, Onaka T, Toyoda H, Arata A, Ishikawa TO, Taketo MM, Fukuda A, Kawakami K: Malfunction of respiratory-related neuronal activity in Na+, K+-ATPase alpha2 subunit-deficient mice is attributable to abnormal Cl- homeostasis in brainstem neurons. J Neurosci. 2004, 24 (47): 10693-10701. 10.1523/JNEUROSCI.2909-04.2004.
Onimaru H, Ikeda K, Kawakami K: Defective interaction between dual oscillators for respiratory rhythm generation in Na+, K+-ATPase {alpha}2 subunit-deficient mice. J Physiol. 2007, 584 (Pt 1): 271-284.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SMG drafted the manuscript. RAR helped draft the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Gritz, S.M., Radcliffe, R.A. Genetic effects of ATP1A2 in familial hemiplegic migraine type II and animal models. Hum Genomics 7, 8 (2013). https://doi.org/10.1186/1479-7364-7-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1479-7364-7-8