Abstract
Background
Acute myeloid leukemia (AML) is an immunophenotypically heterogenous malignant disease, in which CD34 positivity is associated with poor prognosis. CD34+ AML cells are 10-15-fold more resistant to daunorubicin (DNR) than CD34- AML cells. Curcumin is a major component of turmeric that has shown cytotoxic activity in multiple cancers; however, its anti-cancer activity has not been well studied in DNR-insensitive CD34+ AML cells. The aim of this study was to therefore to explore curcumin-induced cytotoxicity in DNR-insensitive CD34+ AML cell lines (KG1a, Kasumi-1), DNR-sensitive U937 AML cells, and primary CD34+ AML bone-marrow-derived cells.
Methods
Primary human CD34+ cells were isolated from peripheral blood mononuclear cells or bone marrow mononuclear cells using a CD34 MicroBead kit. The growth inhibitory effects of curcumin were evaluated by MTT and colony-formation assays. Cell cycle distribution was examined by propidium iodide (PI) assay. Apoptosis was analyzed by Wright-Giemsa, Hoechst 33342 and Annexin-V/PI staining assays. The change in mitochondrial membrane potential (MMP) was examined by JC-1 staining and flow cytometry. Expression of apoptosis-related proteins was determined by reverse transcription-polymerase chain reaction and Western blotting. Short interfering RNA (siRNA) against Bcl-2 was used in CD34+ KG1a and Kasumi-1 cells incubated with/without DNR.
Results
Curcumin inhibited proliferation and induced apoptosis and G1/S arrest in both DNR-insensitive KG1a, Kasumi-1 and DNR-sensitive U937 cells. Curcumin-induced apoptosis was associated with reduced expression of both Bcl-2 mRNA and protein, subsequent loss of MMP, and activation of caspase-3 followed by PARP degradation. Curcumin synergistically enhanced the cytotoxic effect of DNR in DNR-insensitive KG1a and Kasumi-1 cells, consistent with decreased Bcl-2 expression. Accordingly, siRNA against Bcl-2 increased the susceptibility of KG1a and Kasumi-1 cells to DNR-induced apoptosis. More importantly, curcumin suppressed Bcl-2 expression, selectively inhibited proliferation and synergistically enhanced the cytotoxicity of DNR in primary CD34+ AML cells, while showing limited lethality in normal CD34+ hematopoietic progenitors.
Conclusion
Curcumin down-regulates Bcl-2 and induces apoptosis in DNR-insensitive CD34+ AML cell lines and primary CD34+ AML cells.
Similar content being viewed by others
Background
Acute myeloid leukemia (AML) is an immunophenotypically heterogenous malignant disease, in which CD34 positivity has been significantly correlated with a lower complete response (CR) rate, drug resistance and poor outcome [1–3]. Treatment of AML has generally consisted of a combination of cytarabine and an anthracycline such as daunorubicin (DNR), or the anthracenedione mitoxantrone [4]. Although conventional chemotherapy regimens induce CR in 65-80% of newly diagnosed AML patients, most patients who achieve a CR relapse within 2 years from diagnosis [5]. At relapse, blast cells usually display a more immature phenotype, with one of the most common antigenic changes being a gain in expression of the stem cell antigen CD34 [6, 7]. This is reflected in the resistance of these immature phenotype CD34+ AML progenitors to current chemotherapies.
CD34+ AML cells are 10-15-fold more resistant to DNR than CD34- AML cells [8]. CD34+ KG1a and TF-1 AML cell lines are 30-40 fold more resistant to mitoxantrone than more mature HL-60 and U937 cells, and this resistance appears to be associated with the lack of apoptosis [9]. Increasing evidence indicates that CD34+ AML cells are less sensitive to spontaneous apoptosis and have higher levels of Bcl-2 and Bcl-xl gene and protein expression than the CD34- subpopulation [6, 10–12]. CD34 positivity has been reported to be another indicator of poor prognosis in AML [3, 12], and use of more effective drugs to eliminate this early immature CD34+ AML cell subpopulation might therefore improve the outcome of AML.
DNR is one of the most commonly used anti-leukemia agents. Bcl-2 overexpression can block DNR-induced apoptosis in more mature U937 AML cells [13]. The anti-apoptotic proteins Bcl-2 and Bcl-xl also contribute to the survival and chemoresistance of quiescent leukemia CD34+ cells [14]. These findings suggest that Bcl-2 plays a critical role in CD34+ AML cell survival and that agents aimed at down-regulating Bcl-2 protein might be effective for the treatment of DNR-insensitive CD34+ AML.
Curcumin, a major yellow pigment in turmeric, has been proven to be a powerful therapeutic drug [15, 16]. Curcumin induces apoptosis in a variety of tumor cells, including more mature HL-60 and U937 cell lines, through activation of caspase-3, cytochrome c release, and down-regulation of Bcl-2 [17–20]. Curcumin inhibits proliferation in a variety of cancer cells through targeting multiple cellular signaling pathways [21], including the mitogen-activated protein kinase [22], nuclear factor kappaB [23], phosphoinositide-3 kinase/Akt/mammalian target of rapamycin [24, 25], Wnt [26], and Notch-mediated signaling pathways [27]. Curcumin has also been found to be a powerful chemosensitizing agent in tumor cells. It demonstrated no major toxicities in phase I and II clinical studies at doses of up to 8 g/day [28, 29]. However, the cytotoxic effects of curcumin in DNR-insensitive CD34+ immature AML cells remain unclear.
In this study, we examined the cytotoxic efficiency and molecular mechanisms underlying the anticancer activity of curcumin in both DNR-insensitive CD34+ immature AML cell lines and in primary CD34+AML cells.
Methods
Materials
Curcumin (Sigma, St. Louis, MO) was dissolved in dimethyl sulfoxide (DMSO) to prepare a 100-mM stock solution that was stored at -20°C. DNR was purchased from Pharmacia & Upjohn SpA (Milan, Italy). Annexin-V assay kit was purchased from Molecular Probes (Eugene, OR, USA). Anti-cleaved PARP, cleaved caspase-3, and Bcl-2 antibodies were purchased from Cell Signaling Technologies (Beverly, MA, USA). Anti-GAPDH antibody and goat anti-rabbit/mouse-horseradish peroxidase (HRP)-conjugated secondary antibody were purchased from Protein Tech Group (Chicago, IL, USA). JC-1 kit was purchased from Beyotime (China). CD34-PE and IgG1-PE monoclonal antibodies were purchased from BD Biosciences (San Jose, CA, USA). CD34 MicroBead kit was purchased from Miltenyi biotec (Auburn, CA, USA).
Cell lines, primary samples, and cell culture
KG1a and Kasumi-1 cell lines were obtained from Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (DSMZ) (Braunschweig, Germany) and grown in RPMI 1640 medium (Gibco; Invitrogen, Carlsbad, CA, USA) supplemented with 20% (v/v) fetal bovine serum (FBS; Hyclone, Logan, UT). According to immunological studies by DSMZ and others [30, 31], KG1a and Kasumi-1 cells are characterized by high expression of CD34 surface antigen. U937 cells were obtained from the American Type Culture Collection (ATCC) and grown in RPMI 1640 medium supplemented with 10% FBS. Cells were cultured at 37°C in a humidified atmosphere containing 5% CO2. Control cultures received an equivalent amount of DMSO only. Bone marrow mononuclear cells (BMMCs) or mobilized peripheral blood mononuclear cells (PBMCs) were obtained from 9 newly diagnosed AML patients and 8 healthy donors. All donors provided written informed consent, and the study had the approval of the Institute Research Ethics Committee at Sun Yan-sen University, in accordance with the Declaration of Helsinki. Patient characteristics are shown in Table 1. PBMCs and BMMCs were enriched by Ficoll-Hypaque density gradient centrifugation and isolated using a CD34 MicroBead kit. BMMCs and PBMCs were stained with PE-conjugated anti-CD34 to determine the purity of CD34+ cells.
MTT assay
Viability was assessed by MTT assay. Briefly, 1.0×104 cells were incubated in triplicate in a 96-well plate in the presence or absence of the indicated test samples in a final volume of 0.2 ml for various lengths of time at 37°C. Thereafter, 20 μl MTT solution (5 mg/ml in PBS) was then added to each well. After 4-h incubation at 37°C, 150 μl DMSO was added. Finally the plates were shaken and the optical density at 490 nm was measured using a multiwell plate reader (Microplate Reader; Bio-Rad, Hercules, CA). Percent cell viability was calculated as cell viability of the experimental samples/cell viability of the control samples × 100. At least three independent experiments were performed.
Colony-forming assay
Treated and untreated cells were cultured in RPMI 1640 medium supplemented with 0.9% methylcellulose and 20% FBS at 37°C in 5% CO2. The colonies (containing 50 or more cells) were counted by light microscopy after 14 days. All semi-solid cultures were performed in triplicate. Three independent experiments were performed.
Wright-Giemsa staining
Morphological signs of apoptosis were detected by Wright-Giemsa staining. Cells were treated with 0-80 μM curcumin for 24 h. Smears of control and treated cells were stained with Wright-Giemsa solution for 25 min, rinsed with distilled water and air dried. Cell morphology was studied by light microscopy.
Hoechst 33342 staining
Nuclear fragmentation was examined by Hoechst 33342 (Sigma). Cells treated with 0-80 μM curcumin for 24 h were washed and stained with Hoechst 33342 (10 μg/ml) for 15 min at 37°C. Slides were viewed using a fluorescence microscope.
Measurement of apoptosis by Annexin V analysis
An Annexin V-binding assay was used according to the manufacturer's instructions. Briefly, approximately 5×105/ml cells in 6-well plates were treated with various concentrations of the indicated test samples. The cells were harvested and used for Annexin V-Alexa Fluor-488/PI staining. The stained cells were analyzed by flow cytometry to determine the percentages of AnnexinV+/PI- (early apoptosis) and AnnexinV+/PI+ (late apoptosis) cells.
Cell cycle analysis
Cell cycle was analyzed by flow cytometry. Approximately 5 × 105/ml cells in 6-well plates were treated with various concentrations of curcumin for 24 h. Cell cycle analysis was performed using the CycleTEST™ PLUS DNA kit (BD Biosciences).
Detection of mitochondrial membrane potential (MMP, Δψm) using JC-1
MMP was estimated by flow cytometry after staining with JC-1 fluorescent dye. When the cell is in a normal state, MMP is high and JC-1 predominantly appears as red fluorescence. When the cell is in an apoptotic or necrotic state, the MMP is reduced and JC-1 appears as a monomer indicated by green fluorescence. A change in the florescence from red to green indicates a decrease in the MMP. Approximately 5×105/ml cells in 6-well plates were treated with various concentrations of curcumin for 24 h. The cells were then washed with PBS and incubated with JC-1 working solution for 20 min at 37°C in the dark. Cells were washed with PBS and resuspended in 500 μl PBS. The stained cells were analyzed by flow cytometry to determine the change in the florescence from red to green.
RNA isolation and semiquantitative reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted using Trizol isolation reagent (Invitrogen, USA). Reverse transcription was performed using a reverse transcriptase first strand cDNA synthesis kit (Takara, Japan). The sequences of the sense and antisense primers were: 5'-CTGGTGGACAACATCGC-3' (sense) and 5'-GGAGAAATCAAACAGAGGC-3' (anti-sense) for Bcl-2, 5'-TGACTTTTCCTGTGAACTCT-3' (sense) and 5'-GCCTTTCATTCGTATCAAGA-3' (anti-sense) for c-IAP-1, 5'-GCAGGGTTTCT TTATACTG-3' (sense) and 5'-TGTCCCTTCTGTTCTAACAG-3' (anti-sense) for XIAP [32], 5'-GTGGACATCCGCAAAGAC-3' (sense) and 5'-GAAAGGGTGTAA CGCAACT-3' (anti-sense) for β-actin. The PCR conditions were as follows: for c-IAP-1 and XIAP, 94°C for 1 min, 62°C for 1 min, and 72°C for 1 min; for Bcl-2, 94°C for 30 s, 62°C for 30 s, 72°C for 10 s; and for β-actin: 94°C for 30 s, 55°C for 30 s, 72°C for 1 min. Thirty cycles of amplification were used. PCR (10 μl) products were analyzed by electrophoresis on 2% (w/v) agarose gel.
Western blot analysis
Total cellular proteins were isolated with lysis buffer (20 mM Tris, pH 7.5; 150 mM NaCl; 0.25% NP40; 2.5 mM sodium pyprophosphate; 1 mM EGTA, 1 mM EDTA; 1 mM β-glycerophosphate; 1 mM Na3VO4; 1 mM PMSF; 1 μg/ml leupeptin). Equal amounts of protein were subjected to 10% or 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. The membranes were treated with primary antibodies overnight at 4°C and incubated with a HRP-conjugated anti-mouse or anti-rabbit secondary antibody at room temperature for 1 h. The protein bands were visualized using an enhanced chemiluminescence reagent (Pierce Biotechnology, USA), according to the manufacturer's instructions.
Short interfering RNA (siRNA) transfection
KG1a and Kasumi-1 cells were seeded onto 6-well plates for 24 h before transfection. Control scrambled siRNA was synthesized and purchased from GenePharma (Shanghai Co. Ltd., China). SiRNA Bcl-2 (50 nM): 5'-GGGAGAUAGUGAUGAAG UAUU-3' [33] or control scramble sequences were transfected using Lipofectamine 2000 reagent (Invitrogen), according to the manufacturer's protocol. Briefly, for each well, 5 μl Lipofectamine 2000 was diluted in 250 μl Opti-MEM medium (Invitrogen). The mixture was gently added to a solution containing siRNA in 250 μl Opti-MEMI medium and incubated for 20 min. The mixture was then added to the plates. After transfection with siRNA for 24 h, cells were harvested for further assay.
Statistical analysis
Data were presented as mean ± SD. One-way ANOVA followed by Bonferroni multiple comparison was performed to assess the differences between two groups under multiple conditions. If the data failed the normality test, the Kruskal-Wallis one-way ANOVA on ranks was used. A value of p < 0.05 was considered statistically significant. Both Calcusyn software (Biosoft, Ferguson, MO, USA) [34, 35] and Jin's formula [36] were used to evaluate the synergistic effects of drug combinations. Jin's formula is given as: Q = Ea + b/(Ea + Eb-Ea × Eb). Ea+b represents the cell proliferation inhibition rate of the combined drugs, while Ea and Eb represent the rates for each drug respectively. A value of Q = 0.85-1.15 indicates a simple additive effect, while Q > 1.15 indicates synergism. Combination index (CI) plots were generated using CalcuSyn software. A value of CI < 1 indicates synergism.
Results
CD34+ KG1a and Kasumi-1cells were insensitive to DNR
KG1a, Kasumi-1 and U937 AML cells were stained with PE-conjugated CD34 antibody and subjected to flow cytometry to determine the purity of CD34+ cells. The percentages of CD34+ cells were 99.43 ± 0.60% in KG1a cells, 96.67 ± 0.11% in Kasumi-1 cells, but CD34+ was absent in U937 cells (Figure 1A). After treatment of these three cell lines with different concentrations of DNR for 48 h, MTT and apoptosis analyses showed that DNR inhibited proliferation and induced apoptosis in more mature U937 cells, but not in immature CD34+ KG1a or Kasumi-1 cell lines (Figure 1B, C). This was in accord with previous studies indicating that CD34+ AML cells were insensitive to DNR. The concentration of DNR used in this study was clinically achievable in patients [37, 38].
CD34+ KG1a and Kasumi-1cells were insensitive to DNR. (A) KG1a, Kasumi-1 and U937 cells were stained with PE-conjugated CD34 antibody and subjected to flow cytometry to determine the purity of CD34+ cells. (B, C) These three cell lines were treated with different concentrations of DNR for 48 h. MTT assay (B) was performed as described in "Methods" and apoptosis (C) was assessed by Annexin V/PI assays. Cells in the lower right quadrant represent early apoptosis and those in the upper right quadrant represent late apoptosis. The graph displays the means ± SD of three independent experiments. * p < 0.05, ** p < 0.01, *** p < 0.001 (compared with control).
Curcumin suppressed cell growth and induced G1/S cell cycle arrest in both DNR-insensitive and -sensitive AML cell lines
KG1a, Kasumi-1 and U937 cell lines were exposed to curcumin (0-100 μM) for 24, 48, 72 and 96 h. The cytotoxic effects of curcumin were determined by MTT assay. Curcumin had a significant cytotoxic effect in all tested cell lines in both dose- and time-dependent manners (Figure 2B, C, D). The IC50 values at 24, 48, 72, and 96 h were 230.5, 86.9, 60.0, and 35.7 μM for KG1a, 68.5, 46.6, 28.8, and 23.5 μM for Kasumi-1, and 58.3, 26.0, 10.6, and 4.4 μM for U937 cells, respectively. The antiproliferative effects of curcumin in these cell lines were further determined using clonogenic assays. Curcumin inhibited clonogenic growth in a dose-dependent manner, and completely inhibited colony formation at a dose as low as 20 μM (Figure S1A, B, Additional file 1).
Curcumin suppressed cell growth and induced G1/S arrest. (A) Structure of curcumin. (B, C, D) KG1a, Kasumi-1 and U937 cell lines were treated with different concentrations of curcumin for 24, 48, 72, and 96 h. MTT assay was performed. (E) These three cell lines were treated with different concentrations of curcumin for 24 h and analyzed for DNA content by flow cytometry, as described in "Methods." The bar represents means ± SD of three independent experiments.
Cell cycle distributions in KG1a, Kasumi-1, and U937 cells were examined after treatment with curcumin for 24 h. As shown in Figure 2E, treatment of KG1a cells with 80 μM curcumin resulted in a significant increase in the percentage of cells in the G1 phase, from 46-62%, and a decrease in the percentage of cells in the S phase, from 39-23%. Similar results were found for Kasumi-1 and U937 cells. These results demonstrated that curcumin induced G1/S arrest in both DNR-insensitive and -sensitive AML cell lines.
Curcumin induced apoptosis through activation of caspase-3 followed by PARP degradation in both DNR-insensitive and -sensitive AML cell lines
To determine if growth inhibition induced by curcumin was a result of apoptosis, the pro-apoptotic effect was examined using Wright-Giemsa, Hoechst 33342 and Annexin-V/PI staining. Both Wright-Giemsa and Hoechst 33342 staining showed that curcumin induced morphological changes such as cell shrinkage and nuclear condensation, which are typical characteristics of apoptosis (Figure 3A; Figure S2A, Additional file 2). These morphological changes were confirmed by flow cytometry. Treatment with curcumin at 40 μM for 24 h resulted in apoptosis rates of 23.5 ± 8.8%, 36.1 ± 5.3%, and 40.1 ± 17.8% in KG1a, Kasumi-1 and U937 cells, respectively (Figure 3B). Western blotting analysis further showed that curcumin induced caspase-3 activation and PARP cleavage, two hallmarks of apoptosis (Figure 3C). Both Annexin-V/PI and Western blotting showed that curcumin induced apoptosis in a dose-dependent manner. U937cells were the most sensitive to curcumin-induced apoptosis, followed by Kasumi-1, then KG1a cells.
Curcumin induced apoptosis through activation of caspase-3 followed by PARP degradation. KG1a, Kasumi-1 and U937 cells were incubated with indicated concentrations of curcumin for 24 h. (A) Cells were stained with Wright-Giemsa and then examined under a light microscope. Arrows indicate apoptotic cells (magnification ×400). (B) Cells were stained with Annexin V/PI to analyze apoptotic cell populations. The graph displays the means ± SD of four independent experiments. (C) Western blotting analysis showed cleaved caspase-3 (17, 19 kDa) and cleaved PARP (89 kDa) fragment. Three independent experiments were performed with similar results, and representative data are shown.
Curcumin decreased Bcl-2 mRNA and protein levels and reduced MMP in both DNR-insensitive and -sensitive AML cell lines
The mechanisms underlying curcumin-induced apoptosis were investigated. The IAP and Bcl-2 family play an important role in the regulation of cell apoptosis, and the effects of curcumin on mRNA levels of c-IAP-1, XIAP and Bcl-2 were therefore assessed by RT-PCR. As shown in Figure 4A, Bcl-2 mRNA levels were significantly down-regulated in both DNR-insensitive AML cell lines (KG1a and Kasumi-1) and in DNR-sensitive U937 cells, while the levels of c-IAP-1 and XIAP were unchanged. Western blotting also demonstrated that curcumin significantly down-regulated Bcl-2 protein levels in a dose-dependent manner (Figure 4B). These results suggest that down-regulation of Bcl-2 could contribute to curcumin-induced apoptosis.
Curcumin decreased Bcl-2 mRNA and protein levels and caused the loss of MMP. KG1a, Kasumi-1 and U937 cells were exposed to different concentrations of curcumin for 24 h. (A) The effects on Bcl-2, c-IAP-1, and XIAP mRNA levels were determined by RT-PCR. (B) The effect on Bcl-2 protein levels was determined by Western blotting assay. Three independent experiments were performed with similar results, and representative data are shown. (C) MMP was estimated by flow cytometry showing decrease in the red to green fluorescence ratio. The results shown are representative of three independent experiments. The bar represents mean ± SD of three independent experiments.
Disruption of the function of Bcl-2 protein leads to permeabilization of the mitochondrial membrane [39]. We therefore investigated the effects of curcumin on MMP using JC-1 fluorescent dye and flow cytometry. Exposure of the three cell lines to increasing doses of curcumin for 24 h led to a significant reduction in the MMP (Figure 4C). These results suggest that curcumin-induced apoptosis is mitochondria-dependent.
Curcumin synergistically enhanced the cytotoxic effect of DNR in DNR-insensitive KG1a and Kasumi-1 cells, associated with down-regulation of Bcl-2
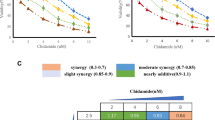
To determine if curcumin could enhance the cytotoxic activity of DNR, DNR-insensitive KG1a and Kasumi-1 cells were cultured with combinations of these two drugs at different doses but in a constant ratio (curcumin to DNR: 20 μM to 0.1 μg/ml, 40 μM to 0.2 μg/ml, and 80 μM to 0.4 μg/ml, respectively) for 48 h, as shown in Figure 5A, B and Table S1 (Additional file 3). Both CalcuSyn software and Jin's formula were used to determine synergy, and the results were consistent. With the exception of co-treatment of KG1a cells with 20 μM curcumin and 0.1 μg/ml DNR, which showed an additive effect (CI = 1.03, Q = 0.99), co-treatment with other doses in KG1a cells and with all doses in Kasumi-1 cells exhibited synergistic effects. For example, the combination of 40 μM curcumin with 0.2 μg/ml DNR in KG1a cells caused growth inhibition of 45.12%, compared to curcumin (26.31%) or DNR (5.47%) alone, indicating synergism (CI = 0.654, Q = 1.49). Notably, co-treatment with 40 μM curcumin and 0.2 μg/ml DNR caused more attenuation of Bcl-2 protein levels than treatment with either agent alone (Figure 5C).
Curcumin synergistically enhanced the cytotoxic effects of DNR associated with down-regulation of Bcl-2. KG1a and Kausumi-1cells were exposed to different concentrations of curcumin, DNR, or their combination as indicated, for 48 h. (A, B) CI-effect plots and median-effect plots were generated using CalcuSyn software. The points a, b, and c represent CI values for the combinations 20, 40, and 80 μM curcumin with 0.1, 0.2, and 0.4 μg/ml DNR in a constant ratio, respectively. The CI values at ED50, ED75, ED90 were 0.667, 0.490, and 0.364 for KG1a cells and 0.529, 0.456, and 0.394 for Kasumi-1 cells, respectively. (C) Bcl-2 protein levels were determined by Western blotting assay. Three independent experiments were performed with similar results, and representative data are shown.
Suppression of Bcl-2 with siRNA induced apoptosis and increased the susceptibility of KG1a and Kasumi-1 cells to DNR-induced apoptosis
To clarify if down-regulation of Bcl-2 by curcumin plays an important role in this synergistic effect, Bcl-2 expression was suppressed by siRNA and the effect on apoptosis and DNR sensitivity was examined by flow cytometry. Bcl-2 siRNA-induced apoptosis in 24 h (28.58% in KG1a cells, 37.12% in Kasumi-1 cells) was similar to that in curcumin-treated KG1a (31.71%, 60 μM, Figure 3B) and Kasumi-1 cells (36.10%, 40 μM, Figure 3B), respectively (Figure 6A, B). As shown in Figure 6C, suppression of Bcl-2 by siRNA increased the susceptibility of these cell lines to DNR-induced apoptosis (40.15% in KG1a cells and 86.23% in Kasumi-1 cells), compared to DNR only (3.17% in KG1a cells, 5.94% in Kasumi-1 cells). These results suggest that suppression of Bcl-2 could contribute to curcumin-induced apoptosis and the synergistic effect of curcumin and DNR.
Suppression of Bcl-2 with siRNA induced apoptosis and increased susceptibility to DNR. KG1a and Kasumi-1cells were transfected with siRNA Bcl-2 or siRNA control for 24 h. (A) Bcl-2 protein levels were determined by Western blotting assay. (B) Cells were stained with Annexin V/PI to analyze apoptotic cell populations. The graph displays the means ± SD of three independent experiments. (C) KG1a and Kasumi-1 cells were transfected with siRNA Bcl-2 or siRNA control for 24 h, and then treated with DNR (0.2 μg/ml) for 48 h. Cells were stained with Annexin V/PI to analyze apoptotic cell populations. The bar represents mean ± SD of three independent experiments. ** p < 0.01, *** p < 0.001 (compared with control).
Curcumin was effective against primary CD34+ AML cells
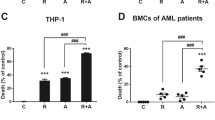
The cytotoxic effects of either curcumin and/or DNR on primary CD34+AML cells were also examined. CD34+ cells were sorted from BMMCs or PBMCs from 9 AML patients and 8 healthy donors. The sorted samples yielded more than 95% CD34+ cells with greater than 90% viability, determined by trypan blue exclusion (Figure 7A). The antiproliferative effects of curcumin on CD34+ cells from 3 AML patients (patients 1, 2, 3) and 3 healthy donors (donors 1, 2, 3) were determined by MTT assay, and compared with the results of DNR treatment. CD34+ cells were treated with curcumin (0, 10, 20, 40, 80 μM) or DNR (0, 0.4, 0.8, 1.6 μg/ml) for 24 h. Curcumin significantly inhibited proliferation of CD34+ AML cells, but only exhibited modest lethality in normal CD34+ hematopoietic progenitors (Figure 7B). However, CD34+ cells derived from the 3 AML patients were insensitive to DNR (Figure 7B). Synergy between curcumin and DNR was examined in another set of 3 AML patients (patients 7, 8, 9) and 3 healthy donors (donors 6, 7, 8). CD34+ cells were treated with curcumin (0, 10, 20, 40, 80 μM) and/or DNR (0.2 μg/ml) for 48 h. Curcumin at 20, 40, or 80 μM synergistically enhanced the cytotoxic effect of DNR (0.2 μg/ml) in CD34+ AML cells, with Q values of 1.60, 1.35 and 1.33, respectively. Normal CD34+ progenitors were less susceptible to the combined toxic effects (Figure 7C). 4 AML patients (patients 4, 5, 6, 7) and 3 donors (donors 4, 5, 6) yielded sufficient numbers of cells for apoptosis assay by flow cytometry. As shown in Figure 7D, E, curcumin induced significant apoptosis in CD34+ AML cells, but minimal apoptosis in normal CD34+ hematopoietic progenitors. 3 AML samples (patients 5, 7, 8) with sufficient cell numbers were further analyzed for Bcl-2 protein expression by Western blotting assay. A dose of 80 μM curcumin was used in primary CD34+ AML cells, because curcumin significantly down-regulated the Bcl-2 protein levels in CD34+ AML cell lines at 80 μM. The results showed that treatment with 80 μM curcumin significantly down-regulated Bcl-2 protein levels (Figure 7F).
Curcumin was effective against primary CD34+ AML cells. (A) Primary CD34+ cells isolated from BMMCs or PBMCs of 9 AML patients and 8 healthy donors were isolated and subjected to flow cytometry to determine the purity of CD34+ cells. (B) 3 CD34+ AML and 3 CD34+ normal samples were treated with different concentrations of curcumin (0, 10, 20, 40, and 80 μM) for 24 h. The same CD34+ AML samples were also exposed to DNR (0, 0.4, 0.8, and 1.6 μg/ml) for 24 h. MTT assay was performed. The bar represents mean ± SD of three independent experiments. (C) Another set of 3 CD34+ AML samples and 3 CD34+ normal samples were exposed to different concentrations of curcumin, DNR, and their combination as indicated, for 48 h. MTT assay was performed. * P < 0.05, ** P < 0.01 (compared with either curcumin or DNR alone). (D, E) 4 CD34+ AML samples and 3 CD34+ normal samples were treated with 0 and 40 μM curcumin for 24 h. Apoptosis was assessed by AnnexinV/PI assay. The graph displays the mean ± SD of four independent experiments (D). A representative figure is shown (E). (F) 3 CD34+ AML samples were treated with 0 and 80 μM curcumin for 24 h. Bcl-2 protein levels were determined by Western blotting assay.
Discussion
CD34 positivity has been reported to be an indicator of poor prognosis in AML [3]. In the present study, we evaluated the cytotoxicity of curcumin in DNR-insensitive CD34+ AML cell lines (KG1a and Kasumi-1) and in CD34+ primary AML samples. We showed that curcumin selectively induced apoptosis in KG1a and Kasumi-1 cell lines, as well as in primary CD34+ AML cells, in association with down-regulation of Bcl-2 expression. Importantly, co-treatment with curcumin and DNR synergistically inhibited proliferation, consistent with decreased Bcl-2 expression. Accordingly, suppression of Bcl-2 with siRNA increased the susceptibility of KG1a and Kasumi-1 cells to DNR-induced apoptosis. These results provide the first evidence for the ability of curcumin to overcome insensitivity to DNR by down-regulation of Bcl-2 in CD34+ AML progenitors.
Insensitivity to chemotherapy is a major obstacle to cancer treatment. CD34+ cell lines display natural resistance to mitoxantrone associated with an absence of apoptosis [9], giving these immature myeloid leukemia cells a survival advantage over the more mature leukemia hematopoietic compartment. Curcumin induced apoptosis in more mature HL-60 AML cells by releasing cytochrome c and activating caspase-3 [18]. The results of the present study demonstrated that curcumin induced apoptosis in both DNR-sensitive U937 cells and DNR-insensitive KG1a and Kasumi-1 cells via the intrinsic apoptosis pathway involving down-regulation of Bcl-2 protein, loss of MMP and activation of caspase-3, followed by PARP degradation. Furthermore, suppression of Bcl-2 with siRNA caused significant apoptosis, similar to that seen in curcumin-treated cells, suggesting an important role for Bcl-2 in curcumin-induced apoptosis in these CD34+AML cell lines.
Accumulating evidence has shown that curcumin potentiates the effects of chemotherapeutic drugs such as bortezomib, cisplatin, and 5-fluorouracil plus oxaliplatin (FOLFOX) in vitro and vivo[40–42]. Notably, Yu et al. revealed that curcumin, either alone or together with FOLFOX, could effectively eliminate FOLFOX-resistant colon cancer stem cells (CSCs) [42]. CSCs have been proposed to be responsible for disease progression or relapse following conventional therapy [43], and the results of the current study suggest that curcumin could act as a potentially powerful chemosensitizing agent in tumor cells, including CSCs. A recent study indicated that the combination of curcumin with carnosic acid also produced a synergistic antiproliferative effect on KG1a cells; however, this synergism was not associated with alterations in Bcl-2 levels [44]. In contrast, our study demonstrated that curcumin synergistically enhanced the cytotoxic effects of DNR in association with decreased Bcl-2 expression in KG1a and Kasumi-1 cells. Accordingly, siRNA against Bcl-2 increased the susceptibility of these CD34+ cell lines to DNR-induced apoptosis, indicating that Bcl-2 down-regulation played an important role in this curcumin-induced synergistic effect.
Anti-apoptotic Bcl-2 contributes to the survival and chemoresistance of quiescent leukemia CD34+ cells [14]. CD34+ AML cells have higher levels of Bcl-2 gene and protein than CD34- AML cells [6]. DNR-induced apoptosis can be blocked by Bcl-2 overexpression in DNR-sensitive CD34- U937 cells [13]. Conversely, suppression of Bcl-2 expression with siRNA enhanced DNR-induced apoptosis in DNR-insensitive CD34+ KG1a and Kasumi-1 cells. These results suggest that high levels of Bcl-2 expression could contribute to DNR-insensitivity, and that down-regulation of Bcl-2 by curcumin could be a molecular mechanism whereby curcumin can overcome the insensitivity of CD34+ AML cells to DNR.
We further demonstrated that primary CD34+ AML cells also underwent proliferation inhibition and apoptosis with curcumin exposure. This effect was replicated in 9 individual patient samples representative of different French-American-British (FAB) classifications. Furthermore, curcumin also suppressed Bcl-2 expression and synergistically enhanced DNR cytotoxicity in primary CD34+ AML cells. These primary cells with different FAB classifications represented a broad cross-section of common AML types, suggesting that down-regulation of Bcl-2 and induction of apoptosis by curcumin could be a common death mechanism in CD34+ AML cells.
Several phase I and phase II clinical trials have indicated the potential therapeutic efficacy and lack of toxic side effects associated with curcumin [28, 29]. However, its poor bioavailability has limited its use for the treatment of cancers outside the gastrointestinal tract [45]. Modern techniques such as the use of synthetic analogs, derivatives, different formulations and heat-solubilized curcumin have been explored with the aim of improving its bioavailability [46–48]; e.g., the water solubility of curcumin could be increased 12-fold by heating, without destroying its biological activity [47, 48].
Conclusion
In summary, this study demonstrated a potential new mechanism whereby curcumin could overcome DNR insensitivity by down-regulating Bcl-2 in both CD34+ AML cell lines and in primary CD34+ AML cells. Curcumin, either alone or in combination with DNR, could thus be a potential anti-leukemic agent for the treatment of DNR-insensitive CD34+ AML cells.
Abbreviations
- MAPK:
-
mitogen-activated protein kinase
- NF-κB:
-
nuclear factor kappa B
- mTOR:
-
mammalian target of rapamycin
- PI3K:
-
phosphoinositide 3-kinase
- Bcl-2:
-
B cell lymphoma 2
- IAP:
-
inhibitor of apoptosis protein.
References
Geller RB, Zahurak M, Hurwitz CA, Burke PJ, Karp JE, Piantadosi S, Civin CI: Prognostic importance of immunophenotyping in adults with acute myelocytic leukaemia: the significance of the stem-cell glycoprotein CD34 (My10). Br J Haematol. 1990, 76: 340-347. 10.1111/j.1365-2141.1990.tb06365.x.
Myint H, Lucie NP: The prognostic significance of the CD34 antigen in acute myeloid leukaemia. Leuk Lymphoma. 1992, 7: 425-429. 10.3109/10428199209049798.
Repp R, Schaekel U, Helm G, Thiede C, Soucek S, Pascheberg U, Wandt H, Aulitzky W, Bodenstein H, Sonnen R, Link H, Ehninger G, Gramatzki M, AML-SHG Study Group: Immunophenotyping is an independent factor for risk stratification in AML. Cytometry B Clin Cytom. 2003, 53: 11-19.
Tallman MS, Gilliland DG, Rowe JM: Drug therapy for acute myeloid leukemia. Blood. 2005, 106: 1154-1163. 10.1182/blood-2005-01-0178.
Estey E, Dohner H: Acute myeloid leukaemia. Lancet. 2006, 368: 1894-1907. 10.1016/S0140-6736(06)69780-8.
Shman TV, Fedasenka UU, Savitski VP, Aleinikova OV: CD34+ leukemic subpopulation predominantly displays lower spontaneous apoptosis and has higher expression levels of Bcl-2 and MDR1 genes than CD34- cells in childhood AML. Ann Hematol. 2008, 87: 353-360. 10.1007/s00277-008-0439-2.
Baer MR, Stewart CC, Dodge RK, Leget G, Sule N, Mrozek K, Schiffer CA, Powell BL, Kolitz JE, Moore JO, Stone RM, Davey FR, Carroll AJ, Larson RA, Bloomfield CD: High frequency of immunophenotype changes in acute myeloid leukemia at relapse: implications for residual disease detection (Cancer and Leukemia Group B Study 8361). Blood. 2001, 97: 3574-3580. 10.1182/blood.V97.11.3574.
Bailly JD, Muller C, Jaffrezou JP, Demur C, Gassar G, Bordier C, Laurent G: Lack of correlation between expression and function of P-glycoprotein in acute myeloid leukemia cell lines. Leukemia. 1995, 9: 799-807.
Bailly JD, Skladanowski A, Bettaieb A, Mansat V, Larsen AK, Laurent G: Natural resistance of acute myeloid leukemia cell lines to mitoxantrone is associated with lack of apoptosis. Leukemia. 1997, 11: 1523-1532. 10.1038/sj.leu.2400762.
van-Stijn A, van-der-Pol MA, Kok A, Bontje PM, Roemen GM, Beelen RH, Ossenkoppele GJ, Schuurhuis GJ: Differences between the CD34+ and CD34- blast compartments in apoptosis resistance in acute myeloid leukemia. Haematologica. 2003, 88: 497-508.
Suarez L, Vidriales MB, Moreno MJ, Lopez A, Garcia-Larana J, Perez-Lopez C, Tormo M, Lavilla E, Lopez-Berges MC, de Santiago M, San Miguel JF, Orfao A, PETHEMA Cooperative Group: Differences in anti-apoptotic and multidrug resistance phenotypes in elderly and young acute myeloid leukemia patients are related to the maturation of blast cells. Haematologica. 2005, 90: 54-59.
Chang H, Salma F, Yi QL, Patterson B, Brien B, Minden MD: Prognostic relevance of immunophenotyping in 379 patients with acute myeloid leukemia. Leuk Res. 2004, 28: 43-48. 10.1016/S0145-2126(03)00180-2.
Kim YH, Park JW, Lee JY, Surh YJ, Kwon TK: Bcl-2 overexpression prevents daunorubicin-induced apoptosis through inhibition of XIAP and Akt degradation. Bioche Pharmacol. 2003, 66: 1779-1786. 10.1016/S0006-2952(03)00545-8.
Konopleva M, Zhao S, Hu W, Jiang S, Snell V, Weidner D, Jackson CE, Zhang X, Champlin R, Estey E, Reed JC, Andreeff M: The anti-apoptotic genes Bcl-xl and Bcl-2 are over-expressed and contribute to chemoresistance of non-proliferating leukaemic CD34+ cells. Brit J Haematol. 2002, 18: 521-534.
Anand P, Sundaram C, Jhurani S, Kunnumakkara AB, Aggarwal BB: Curcumin and cancer: an "old-age" disease with an "age-old" solution. Cancer Lett. 2008, 267: 133-164. 10.1016/j.canlet.2008.03.025.
Aggarwal BB, Sung B: Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends Pharmacol Sci. 2009, 30: 85-94. 10.1016/j.tips.2008.11.002.
Bae JH, Park JW, Kwon TK: Ruthenium red, inhibitor of mitochondrial Ca2+ uniporter, inhibits curcumin-induced apoptosis via the prevention of intracellular Ca2+ depletion and cytochrome c release. Biochem Biophys Res Commun. 2003, 303: 1073-1079. 10.1016/S0006-291X(03)00479-0.
Mukherjee S, Ghosh U, Bhattacharyya NP, Bhattacharya RK, Dey S, Roy M: Curcumin-induced apoptosis in human leukemia cell HL-60 is associated with inhibition of telomerase activity. Mol Cell Biochem. 2007, 297: 31-39. 10.1007/s11010-006-9319-z.
Hussain AR, Al-Rasheed M, Manogaran PS, Al-Hussein KA, Platanias LC, Al-Kuraya K, Uddin S: Curcumin induces apoptosis via inhibition of PI3'-kinase/AKT pathway in acute T cell leukemias. Apoptosis. 2006, 11: 245-254. 10.1007/s10495-006-3392-3.
Tomita M, Kawakami H, Uchihara JN, Okudaira T, Masuda M, Takasu N, Matsuda T, Ohta T, Tanaka Y, Ohshiro K, Mori N: Curcumin (diferuloylmethane) inhibits constitutive active NF-kappaB, leading to suppression of cell growth of human T-cell leukemia virus type I-infected T-cell lines and primary adult T-cell leukemia cells. Int J Cancer. 2006, 118: 765-772. 10.1002/ijc.21389.
Kunnumakkara AB, Anand P, Aggarwal BB: Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 2008, 269: 199-225. 10.1016/j.canlet.2008.03.009.
Lev-Ari S, Starr A, Vexler A, Karaush V, Loew V, Greif J, Fenig E, Aderka D, Ben-Yosef R: Inhibition of pancreatic and lung adenocarcinoma cell survival by curcumin is associated with increased apoptosis, down-regulation of COX-2 and EGFR and inhibition of Erk1/2 activity. Anticancer Res. 2006, 26: 4423-4430.
Shishodia S, Amin HM, Lai R, Aggarwal BB: Curcumin (diferuloylmethane) inhibits constitutive NF-kappaB activation, induces G1/S arrest, suppresses proliferation, and induces apoptosis in mantle cell lymphoma. Biochem Pharmacol. 2005, 70: 700-713. 10.1016/j.bcp.2005.04.043.
Yu S, Shen G, Khor TO, Kim JH, Kong AN: Curcumin inhibits Akt/mammalian target of rapamycin signaling through protein phosphatase-dependent mechanism. Mol Cancer Ther. 2008, 7: 2609-2620. 10.1158/1535-7163.MCT-07-2400.
Choi BH, Kim CG, Lim Y, Shin SY, Lee YH: Curcumin down-regulates the multidrug-resistance mdr1b gene by inhibiting the PI3K/Akt/NF kappa B pathway. Cancer Lett. 2008, 259: 111-118. 10.1016/j.canlet.2007.10.003.
Prasad CP, Rath G, Mathur S, Bhatnagar D, Ralhan R: Potent growth suppressive activity of curcumin in human breast cancer cells: Modulation of Wnt/beta-catenin signaling. Chem Biol Interact. 2009, 181: 263-271. 10.1016/j.cbi.2009.06.012.
Wang Z, Zhang Y, Banerjee S, Li Y, Sarkar FH: Notch-1 down-regulation by curcumin is associated with the inhibition of cell growth and the induction of apoptosis in pancreatic cancer cells. Cancer. 2006, 106: 2503-2513. 10.1002/cncr.21904.
Lao CD, Ruffin MT, Normolle D, Heath DD, Murray SI, Bailey JM, Boggs ME, Crowell J, Rock CL, Brenner DE: Dose escalation of a curcuminoid formulation. BMC Complement Altern Med. 2006, 6: 10-13. 10.1186/1472-6882-6-10.
Dhillon N, Aggarwal BB, Newman RA, Wolff RA, Kunnumakkara AB, Abbruzzese JL, Ng CS, Badmaev V, Kurzrock R: Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. 2008, 14: 4491-4499. 10.1158/1078-0432.CCR-08-0024.
Koeffler HP, Billing R, Lusis AJ, Sparkes R, Golde DW: An undifferentiated variant derived from the human acute myelogenous leukemia cell line (KG-1). Blood. 1980, 56: 265-273.
Asou H, Tashiro S, Hamamoto K, Otsuji A, Kita K, Kamada N: Establishment of a human acute myeloid leukemia cell line (Kasumi-1) with 8;21 chromosome translocation. Blood. 1991, 77: 2031-2036.
Notarbartolo M, Poma P, Perri D, Dusonchet L, Cervello M, D'Alessandro N: Antitumor effects of curcumin, alone or in combination with cisplatin or doxorubicin, on human hepatic cancer cells. Analysis of their possible relationship to changes in NF-kB activation levels and in IAP gene expression. Cancer Lett. 2005, 16: 53-65.
Anderson EM, Miller P, Ilsley D, Marshall W, Khvorova A, Stein CA, Benimetskaya L: Gene profiling study of G3139- and Bcl-2-targeting siRNAs identifies a unique G3139 molecular signature. Cancer Gene Ther. 2006, 13: 406-414. 10.1038/sj.cgt.7700901.
Chou TC: Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006, 58: 621-681. 10.1124/pr.58.3.10.
Chou TC: Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70: 440-446. 10.1158/0008-5472.CAN-09-1947.
Jin ZJ: [Addition in drug combination (author's transl)]. Zhongguo Yao Li Xue Bao. 1980, 1: 70-76.
Greene RF, Collins JM, Jenkins JF, Speyer JL, Myers CE: Plasma pharmacokinetics of adriamycin and adriamycinol: implications for the design of in vitro experiments and treatment protocols. Cancer Res. 1983, 43: 3417-3421.
Lagadinou ED, Ziros PG, Tsopra OA, Dimas K, Kokkinou D, Thanopoulou E, Karakantza M, Pantazis P, Spyridonidis A, Zoumbos NC: c-Jun N-terminal kinase activation failure is a new mechanism of anthracycline resistance in acute myeloid leukemia. Leukemia. 2008, 22: 1899-1908. 10.1038/leu.2008.192.
Henry-Mowatt J, Dive C, Martinou JC, James D: Role of mitochondrial membrane permeabilization in apoptosis and cancer. Oncogene. 2004, 23: 2850-2860. 10.1038/sj.onc.1207534.
Park J, Ayyappan V, Bae EK, Lee C, Kim BS, Kim BK, Lee YY, Ahn KS, Yoon SS: Curcumin in combination with bortezomib synergistically induced apoptosis in human multiple myeloma U266 cells. Mol Oncol. 2008, 2: 317-326. 10.1016/j.molonc.2008.09.006.
Duarte VM, Han E, Veena MS, Salvado A, Suh JD, Liang LJ, Faull KF, Srivatsan ES, Wang MB: Curcumin enhances the effect of cisplatin in suppression of head and neck squamous cell carcinoma via inhibition of IKKβ protein of the NFκB pathway. Mol Cancer Ther. 2010, 9: 2665-2675. 10.1158/1535-7163.MCT-10-0064.
Yu Y, Kanwar SS, Patel BB, Nautiyal J, Sarkar FH, Majumdar AP: Elimination of Colon Cancer Stem-Like Cells by the Combination of Curcumin and FOLFOX. Transl Oncol. 2009, 2: 321-328.
Dean M, Fojo T, Bates S: Tumour stem cells and drug resistance. Nat Rev Cancer. 2005, 5: 275-284. 10.1038/nrc1590.
Pesakhov S, Khanin M, Studzinski GP, Danilenko M: Distinct combinatorial effects of the plant polyphenols curcumin, carnosic acid, and silibinin on proliferation and apoptosis in acute myeloid leukemia cells. Nutr Cancer. 2010, 62: 811-824. 10.1080/01635581003693082.
Garcea G, Berry DP, Jones DJ, Singh R, Dennison AR, Farmer PB, Sharma RA, Steward WP, Gescher AJ: Consumption of the putative chemopreventive agent curcumin by cancer patients: assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiol Biomarkers Prev. 2005, 14: 120-125.
Anand P, Thomas SG, Kunnumakkara AB, Sundaram C, Harikumar KB, Sung B, Tharakan ST, Misra K, Priyadarsini IK, Rajasekharan KN, Aggarwal BB: Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochem Pharmacol. 2008, 76: 1590-1611. 10.1016/j.bcp.2008.08.008.
Kurien BT, Scofield RH: Oral administration of heat-solubilized curcumin for potentially increasing curcumin bioavailability in experimental animals. Int J Cancer. 2009, 125: 1992-1993. 10.1002/ijc.24547.
Kurien BT, Singh A, Matsumoto H, Scofield RH: Improving the solubility and pharmacological efficacy of curcumin by heat treatment. Assay Drug Dev Technol. 2007, 5: 567-576. 10.1089/adt.2007.064.
Acknowledgements
We thank Fucheng Zhang and Huiqiong Lu (Central Lab, Third Affiliated Hospital, Sun Yat-sen University) for their technical supports. We thank Min Yan, Jie Xu, Yan Zhao and other members of Liu Lab for technical assistance. This work was supported by a grant from National Nature Science Fund for Distinguished Young Scholars (30888003 to Q.L.) and National Nature Science Foundation of China (30873084 to Q.L. and 81000217 to Zi-Jie Long).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
JR carried out protein studies, apoptosis analysis, statistical analysis, and drafted the manuscript. FZ and ZL performed the protein studies, apoptosis analysis. SH, XW and WZ participated in the statistical analysis. QL and RH conceived of the study, and participated in its design and coordination. DX contributed effort in designing, performing, and analyzing the results and conclusion. All authors read and approved the final manuscript.
Electronic supplementary material
12967_2010_740_MOESM1_ESM.PDF
Additional file 1: Figure S1 Curcumin inhibited clonogenic growth. (A) The colonies (containing ≥50) were counted after 14 days by light microscopy (magnification ×40). (B) Results show numbers of colonies in the curcumin-treated group expressed as a percentage of number of colonies in the DMSO-treated group. The graph displays the means ± SD of three independent experiments. (PDF 169 KB)
12967_2010_740_MOESM2_ESM.PDF
Additional file 2: Figure S2 Morphological changes in nuclei in curcumin-treated cells. (A) KG1a, Kasumi-1 and U937 cells were incubated with the indicated concentrations of 0, 40, 60, and 80 μM curcumin for 24 h. Cells were stained with Hoechst 33342 and then examined under a light microscope. (PDF 130 KB)
12967_2010_740_MOESM3_ESM.PDF
Additional file 3: Table S1 Q value. Q values are shown. Q = 0.85-1.15 indicates simple addition; Q > 1.15 indicates synergism. (PDF 14 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Rao, J., Xu, DR., Zheng, FM. et al. Curcumin reduces expression of Bcl-2, leading to apoptosis in daunorubicin-insensitive CD34+ acute myeloid leukemia cell lines and primary sorted CD34+ acute myeloid leukemia cells. J Transl Med 9, 71 (2011). https://doi.org/10.1186/1479-5876-9-71
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1479-5876-9-71