Abstract
Background
Granulosa cells play an important endocrine role in folliculogenesis. They mobilize Ca2+ from intracellular stores by a coordinated action between 1,4,5 inositol trisphosphate and ryanodine receptors (IP3R and RyR). The aim of this study was to explore the isoforms of IP3Rs expressed in mouse C57BL/6 NHsd granulosa cells, characterizing their intranuclear localization and the relation with other Ca2+-handling proteins.
Methods
Ovarian tissue and granulosa cells were analyzed by multiphotonic and confocal microscopy to determine the intracellular presence of IP3R types 1, 2 and 3, RyR, thapsigargin-sensitive Ca2+-ATPase, and endomembranes. Cellular fractionation and Western blot assays were also used to further confirm the nuclear occurrence of the three IP3R isoforms. Free nuclear and cytosolic Ca2+ concentrations were measured using Fluo-4 AM by confocal microscopy.
Results
By using antibodies and specific fluorophores, was shown that granulosa cells endomembranes contain three isoforms of IP3R, the RyR, and the thapsigargin-sensitive Ca2+-ATPase (SERCA). Interestingly, all these proteins were also detected in the nuclear envelope and in well-defined intranuclear structures. Microsomal membranes depicted characteristic bands of the 3 types of IP3R, but also variants of lower molecular weight. Analysis of nuclear membranes and nucleoplasmic fraction confirmed the nuclear localization of the IP3R types 1, 2 and 3. We demonstrated ATP-induced Ca2+ transients in the nuclear and cytoplasmic compartments. Remarkably, the inhibitory effect on ATP-induced Ca2+ mobilization of brefeldin A was more accentuated in the cytoplasm than in the nucleus.
Conclusion
These findings provide evidence that granulosa cells, including nuclei, express the Ca2+-handling proteins that allow Ca2+ mobilization. All three IP3R were also detected in ovarian slices, including the nuclei of granulosa cells, suggesting that these cells use the three IP3R in situ to achieve their physiological responses.
Similar content being viewed by others
Background
Granulosa cells are derived from a keratin-positive epithelium, and function supporting the process oocyte maturation. Granulosa cells are follicular somatic cells and the main source of steroids in the ovary [1, 2]. They exert their actions by a combination of paracrine signaling and gap junction-mediated communication [3]. The physiological events characteristic of granulosa cells such as metabolic control, secretion, proliferation, differentiation, and apoptosis, are regulated by numerous factors, but one of the most prominent is the modulation of intracellular Ca2+ concentration ([Ca2+]i) [3–7].
Ca2+ is an ionic and biochemical messenger that regulates a great number of cellular functions by acting as a coordinator and effector of metabolic responses among intracellular compartments, such as cytoplasm, endoplasmic reticulum, nucleus, and mitochondria [8]. Ca2+ fulfills its physiological role when: 1) it enters the cell through plasma membrane ion- and receptor-channels, 2) it is released from intracellular stores by ion channels such IP3R and RyR, 3) it is extruded from the cell by Ca2+/Na+ exchangers and Ca2+-ATPases (PMCA) or confined within organelles by others Ca2+-ATPases (SERCA), and 4) it is mobilized from or transported into the mitochondria by proton motive force (For review see [9]). Recently, nuclear Ca2+ handling has been the focus of reports which postulate new and original roles in Ca2+ signaling for this organelle, including the presence of invaginations inside the nucleoplasm with the ability to release Ca2+[10, 11]. Albeit not much information is available regarding the physiological role played by nuclear Ca2+, it has been reported that excitation-transcription coupling in myocites is regulated in a nuclear Ca2+-dependent manner [12].
Some reports have suggested that this organelle could be acting as an independent and active Ca2+ pool [13]. Accordingly, mechanisms for Ca2+ uptake and release from the nucleus have been recognized in a variety of cells such as neurons, hepatocytes, pancreatic exocrine cells, and starfish oocytes [14]. Ca2+-handling proteins, namely IP3R, RyR, and thapsigargin-sensitive Ca2+-ATPase (SERCA), have been detected in the nuclear envelope [15, 16]. Further support for the notion that this organelle can handle Ca2+ by itself are the reports documenting the existence of a nucleoplasmic reticulum in which active IP3R, RyR, and SERCA were localized in discrete subnuclear regions [17, 18].
Previous reports have established the expression of IP3R isoforms in ovarian cells, including granulosa cells [19, 20]. Having reported for the first time the expression and subcellular localization of RyR in granulosa cells, and the coordinated activity between RyR and IP3R that make possible the ATP-induced Ca2+ mobilization [21], in the present study we further characterize the properties and the type of the Ca2+-handling proteins present in these cells. We present experimental evidence that the three isoforms of IP3Rs are expressed in the ovarian tissue of C57BL/6 NHsd mice. In addition, we demonstrate the presence of all these isoforms in the nuclei of granulosa cells. We also find specific signals in the granulosa cell nuclei using fluorescent probes that recognize RyR, SERCA, and endomembranes. Suggestive evidence of a possible independent Ca2+ handling between compartments was obtained by showing a selective inhibitory action of brefeldin A on cytosolic, but not in the nuclear ATP-induced Ca2+ transients.
Methods
Reagents
Insulin, apo-transferrin, penicillin, streptomycin, fetal bovine serum (FBS), Leibowitz medium (L-15), and α-minimal essential medium (α-MEM) were obtained from Gibco BRL (Gaithersburg, MD. USA). Ryanodine, xestospongin C, thapsigargin, and follicle-stimulant hormone (FSH) were from Calbiochem (La Jolla, CA. USA). Fluo-4 AM, BODIPY TR-X Ryanodine, BODIPY-FL thapsigargin, TO-PRO-1 Iodide, brefeldin A BODIPY 558/568 conjugate isomer 1 were obtained from Molecular Probes (Eugene, OR. USA). Sodium pyruvate, 1,4-diazabicyclo [2, 2, 2] octane (DABCO), paraformaldehyde (PFA), glutaraldehyde, ATP, brefeldin A, bovine serum bovine (BSA), dimethyl sulfoxide (DMSO), the proteinase inhibitors: phenylmethylsulfonyl fluoride (PMSF), aprotinin, leupeptin, pepstatin, and other salts were obtained from Sigma (St. Louis, MO, USA). The Complete mini, Protease inhibitor cocktail tablets was purchased from Roche, (Germany). The NE-PER Nuclear and Cytoplasmic Extraction Reagents Kit was from Pierce (Rockford, IL. USA). The Alkaline Phosphatase (AP) conjugate substrate kit was purchased from Bio-Rad (Hercules, CA. USA). Jung tissue-freezing medium was obtained from Leica (Germany).
Goat polyclonal IgG (immunoglobin G) for isoforms 1, 2, and 3 of the IP3R, rabbit anti-goat IgG-Texas Red (TR), rabbit anti-goat IgG AP, and rabbit polyclonal IgG α-actin (H-196) were obtained from Santa Cruz (Santa Cruz, CA. USA). Rabbit anti-goat IgG (H-L) FITC-Conjugate was purchased from ZYMED (San Francisco, CA. USA).
Cell culture
Cells were obtained based on a published protocol [3, 21]. Briefly, C57BL/6 NHsd female mice (all animal work was conducted using procedures reviewed and approved by our Institutional animal care, Mexican University), 40–60 days old in different stages of the estrous cycle, were killed by cervical dislocation, and the ovaries were dissected and transferred to a Petri dish with L-15 medium (supplemented with 50 μl/ml FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin). Ovaries were cleaned of neighboring tissue and opened to allow visualization of follicles. Antral follicles (usually 8–10 per ovary) were manually separated from the ovaries and transferred to α-MEM (supplemented with 100 ng/ml FSH, 1 mM sodium pyruvate, 10 μg/ml apo-transferrin, 10 μg/ml insulin, 100 U/ml penicillin, and 100 μg/ml streptomycin). Each follicle was opened using fine forceps, and the granulosa cells were carefully removed and transferred to freshly prepared α-MEM, disaggregated mechanically, and placed on glass coverslips coated with poly-D-lysine. The primary culture was maintained in an incubator at 37°C and 5% CO2 for 2–3 days.
Ovary histology
In independent experiments, female C57BL/6 NHsd mice were anesthetized and perfused by intracardiac puncture with phosphate buffer (PBS in mM: 2 KH2PO4, 3 KCl, 10 Na2HPO4, 140 NaCl, pH adjusted to 7.4 with NaOH, containing 40 μl/ml PFA) for 8–10 min. Subsequently the mice were decapitated; and the ovaries were removed and cleaned to eliminate the adjacent tissue, then incubated in PBS-40 mg/ml PFA for 4 h at room temperature. Isolated ovaries were transferred to PBS containing 300 mg/ml sucrose and incubated for 12 h at 4°C. Finally, the ovaries were put into Jung Tissue Freezing Medium and preserved at -20°C for 12 h. Cryostat sections of 8–10 μm were used. Samples were stored at -80°C until use.
Immunochemistry
For immunochemical examination, coverslips of granulosa cells were fixed in PBS containing 40 mg/ml glutaraldehyde for 15 min at 40°C and washed three times with PBS. For cryostat sections, the slices were placed for 1 h at room temperature, transferred to 40 μl/ml PFA for 10 min, and washed three times, each for 5 min, with PBS.
Immunodetection of IP3R types 1, 2, and 3 was achieved using the method reported in reference [22], with the following modifications: After fixation, the cells were exposed to 50 mg/ml fat-free milk in PBS for 1 h at room temperature (to block protein-binding sites) and washed three times with PBS, each for 5 min. Anti-IP3R types 1, 2, and 3 antibodies diluted 1:200 with 50 mg/ml fat-free milk in PBS including 1 μl/ml Triton X-100 (PBST) (to block residual protein-binding sites) were added. The cells were incubated overnight at 4°C and then washed three times with PBS. In order to detect the primary antibody, cells were incubated with rabbit anti-goat IgG-TR or anti-goat IgG (H-L) FITC-Conjugate, diluted 1:1000 in PBST for 1 h at room temperature, and washed six times in PBST. The coverslips were protected with DAPCO, an aqueous mounting medium. Visualization was performed in a confocal microscope with appropriate filters. Images were obtained using a LSM510 laser scanning microscope with a plan apochromatic 63 × oil-immersion objective (numerical aperture = 1.4), and captured at a resolution of 1024 × 1024 pixels.
Ovarian fractionation for microsomal membranes and Western blotting
Subcellular fractionation of mouse ovary cells was done using the protocol reported by [23]. Briefly, mice were killed by cervical dislocation, and the ovaries were dissected and gently homogenized with a silicon-coated glass homogenizer in SET buffer (containing in mM): 300 sucrose, 1 EDTA, 1, 2-mercaptoethanol, 50 Tris-HCl pH 8.0. The buffer was supplemented with the following peptidase inhibitors: 0.2 mM PMSF and 10 μg/ml each of aprotinin, leupeptin, and pepstatin or Protease inhibitor cocktail tablets. The homogenized tissue was centrifuged at 2,000 g for 10 min to remove residual tissue and heavy particles. The supernatant was recovered and then centrifuged at 105,000 g for 45 min. Microsomal precipitates were diluted in SET buffer. Membranes were kept at -80°C until use. Protein was determined according to the method of Lowry [24].
Membrane fractions were boiled for 10 min and loaded onto 60 mg/ml SDS-polyacrylamide gels. Ovarian proteins were transferred to nitrocellulose membranes using a Mini Trans Blot Semi dry transfer cell (BioRad, Hercules CA). The nitrocellulose membranes were blocked 2 h in PBS-0.5 μl/ml Tween-2 supplemented with 50 mg/ml fat-free milk. After 3 washes with 150 mM NaCl-0.5 μl/ml Tween-20 (NaCl-T), each for 10 min, the membranes were incubated overnight at 4°C with the primary antibody (Goat polyclonal IgG anti-IP3R types 1, 2, and 3, dilution 1:250) in PBST supplemented with 1 mg/ml BSA, washed again three times with NaCl-T for 10 min and incubated for 1 h with the secondary antibody (rabbit anti goat IgG-AP, dilution 1:500) in PBST supplemented with 1 mg/ml BSA. After 3 washes with 0.1 M Tris pH 9.5, color associated with the complexes of IP3Rs-antibodies was developed using the AP conjugate substrate kit (Bio-Rad, Hercules CA). All fractions were incubated with rabbit polyclonal IgG α-actin (H-196) (1:1000), a rabbit polyclonal antibody raised against amino acids 180–375 of α-actin of human origin. From this point the protocol mentioned in the previous section was followed.
Nuclear fractionation
Nuclear extracts were obtained according to NE-PER Nuclear and Cytoplasmic Extraction Reagents Kit (complemented with Protease inhibitor cocktail tablets) [13]. Briefly, the isolation of cytoplasmic and nuclear fractions using the NE-PER kit maintains the integrity of the two cellular compartments before extraction. This prevents cross-contamination of proteins between the two fractions. Additionally, we performed a centrifugation (16,000 × g) to obtain nuclear membranes and nucleoplasmic fraction. The protein was calculated according to the Bradford method [25], and analyzed by Western blotting (see previous section).
Fluorescent probes
Following fixation, the mouse granulosa cells were incubated with the following fluorescent probes: BODIPY TR-X Ryanodine (1 μM) to detect the ryanodine receptor [26, 27], BODIPY-Red thapsigargin (1 μM) to localize the thapsigargin-sensitive Ca2+-ATPase (SERCA) [27], TO-PRO-1 iodide (50 nM) and DAPI (1 μg/ml) to stain nuclei [28], brefeldin A BODIPY 558/568 and BODIPY FL-conjugate isomer 1 (1 μM) to localize the ER and Golgi apparatus [29]. Fluorescent probes were incubated for 60–90 min at room temperature and washed 3 times with PBS for 5 min to minimize non-specific binding. To estimate non-specific binding of the fluorescent probes, cells were pre-incubated with 100 μM ryanodine, 100 μM thapsigargin, 100 μM brefeldin A according to [21]. Treated cells were visualized by confocal microscopy (see Immunochemistry Section).
Ca2+ dynamics by confocal microscopy
Ca2+ mobilization was determined according to [21]. Briefly, mouse granulosa cells were loaded for 20–30 min at room temperature with 5 μM Fluo-4 AM in Krebs solution (KS; containing in mM): 150 NaCl, 1 KCl, 1 MgCl2, 1.8 CaCl2, 4 Glucose, 10 HEPES, pH adjusted to 7.4, with the addition of 5 mg/ml of BSA and 0.1 mg/ml of pluronic acid. The solution was filtered to eliminate particles. Cells were washed 3 times with KS to remove extracellular Fluo-4 AM and incubated for 10 min to complete de-esterification of the dye. The coverslips were mounted in a recording chamber and placed in a Nikon Eclipse E600 microscope. To apply the drugs, a home-made multichannel perfusion system was used. Perfusion of the solutions was continuous at 1 ml/min. Confocal images were obtained using a Nikon Plan-Flour × 20 multi-immersion objective (numerical aperture = 0.75) and captured at a resolution of 640 × 640 pixels in the scan mode (1 image/min). Photo-bleaching and photo-damage were minimized by reducing the laser power (95% attenuation). Fluorescent measurements were performed with an Argon laser (Fluo-4 AM was excited at 506 nm, and emission was collected at 526 nm).
Fluorescent images analyzes and data fitting
Multiphotonic and confocal images (for control and experimental conditions) were obtained and analyzed in identical situation by LSM image and SIMPLE PCI software respectively. Immunochemical analyzes involved ≈ 20 cells, in which each cell was divided in 4–5 quadrant of similar dimensions (10 μm2) in both cytoplasmic and nuclear areas (n = 5). For analyses of Ca2+ dynamics experiments, the complete nuclear and cytoplasmic areas of ≈ 200 cells were considered (n = 4).
Values are expressed as means ± S.E. Significance was tested by the Student's t-test. P < 0.05 was considered significant. Graphics were made with the Origin program (Version 5.0), and the images were processed with Photoshop program.
Results
Nuclear localization of the IP3R types 1, 2, and 3 in granulosa cells
Commercial specific antibodies, tested in numerous previous reports [23, 30], coupled to fluorescent moieties were used to determine the presence and intracellular distribution of IP3Rs in primary cultures of mouse granulosa cells by confocal microscopy (Figure 1) (analyzed cells ≈ 20; n = 5). All three types of IP3Rs were expressed at discernible levels, but in different patterns. Apparently, IP3R types 2 and 3 were the most abundant, whereas IP3R type 1 was detected in lesser amount, especially in the cytoplasm (Figure 1, images in the first column). Remarkably, the nuclear signal of all three IP3R isoforms was intense and well defined. Confirmation of the nuclear localization of the three IP3Rs isoforms was obtained by co-localization with DAPI, which is a specific nuclear marker (Nuclei and Merge columns in Figure 1). Images seen at higher magnification show an intricate cytoplasmic pattern for the three types of IP3Rs, with the signals for type 2 and 3 extended more towards the periphery of the cell (fourth column of Figure 1). In addition, magnified images clearly display nuclear signals for the three isoforms in both the nuclear envelope and the interior of the organelle. To further confirm the nuclear localization of the IP3R isoforms, the fifth column depicts a z-stack reconstruction (≈13 optical slices corresponding to 4 μm section) of the granulosa cells nuclei. It can be seen that the 3 types of IP3R show intranuclear localization, being more abundant the isoform 2 and 3. Therefore, primary cultures of mouse granulosa cells co-express all three IP3R isoforms, and unexpectedly, all of them are present within the nuclei. Low signal, barely detectable, was observed when the primary antibodies were omitted (Figure 1, insets in first column). To estimate the difference between the cytoplasmic and the nuclear IP3R distribution, a semi-quantitative analyzes was done (histograms at the bottom of Figure 1; cells analyzed ≈ 20; n = 5). IP3R type 1 and 2 showed an increased nuclear presence, whereas IP3R type 3 displayed similar signals in both compartments.
Nuclear and cytoplasmic location of IP 3 R isoforms in mouse granulosa cells. Confocal microscope images depict the subcellular location of IP3R isoforms detected by specific antibodies whereas nuclei were stained with DAPI. The first column shows the fluorescent signal elicited by the primary antibodies against IP3R types 1, 2, and 3, followed by the TX-Red-conjugated secondary antibody (Magenta color). No signal was detected when the primary antibodies were omitted (insets in first column). The second column depicts the fluorescent signal associated with the nuclear marker DAPI (Cyan color). The third column is the combination of the images in the first and second columns (merge), and the fourth column is a magnification of a selected sector of the merged image that strongly suggests the intranuclear presence of the three IP3R isoforms. The fifth column is a z-stack reconstruction of the fourth column (13 optics slices corresponding to 4 μm section). Histograms at the bottom show the quantification of the cytoplasmic (C) and nuclear (N) signals associated with each IP3R isoform. The measurements were obtained from the fluorescent signals within nuclei and cytoplasm using the LSM510 laser scanning microscope program. Scale bar corresponds to 20 μm. Results are expressed in arbitrary fluorescent units (AFU) and are the mean ± SEM of 5 independent experimental observations.
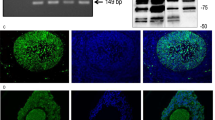
Figure 2 panel A shows biochemical detection of IP3Rs isoforms by Western blot in reductive conditions, in granulosa cells (108 cells; n = 3), ovary and cerebellum (8 mice; n = 3). IP3R type 1 was detected in granulosa cells, ovary and cerebellum with a expected high molecular weight band (≈230–250 kDa [22, 31, 32]. In cerebellum, a second band (≈200 kDa) was also observed. IP3R type 2 was also seen in granulosa cells in a similar high molecular weight band (≈230–250 kDa), but in addition, three smaller bands were also detected (≈120, 100 and 90 kDa). The high molecular weight and smaller bands were also observed in ovary and cerebellum. The low molecular weight variants of IP3R type 2 might correspond to regulated proteolytic activity or to alternative mRNA processing [31–36]. IP3R type 3 in granulosa cells was detected as a high molecular weight band (≈230–250 kDa), but also in a band with lower molecular weight (≈130 kDa). IP3R type 3 was also present in ovary and cerebellum. At the right side of Figure 2 panel A, note that the signal corresponding to α-actin was detected in ovary tissue and not in granulosa cells treated with FSH in vitro, since the cytoskeleton of these cells does not contain this protein [37]. Taken together these results, it is shown that granulosa cells express the 3 IP3R isoforms, and in our experimental conditions granulosa cells are luteinized, and then they do not express α-actin.
Biochemical detection of IP 3 R isoforms by Western blot assays. Arrows indicate the bands corresponding to the high molecular weight forms of the non-processed IP3Rs (≈230–250 kDa). Panel A. The presence of IP3R isoforms and low molecular weight variants in microsomal fractions from granulosa cells (G); tissues such as ovary (O), cerebellum (C), were obtained and analyzed by electrophoretic mobility. At the right side of Panel A the signal corresponds to α-actin (45 kDa). Panel B. The nuclear fractions (nuclear membranes (NM) and nucleoplasm (NP) from granulosa cells) were obtained and analyzed by electrophoretic mobility to detect the isoforms and low molecular weight variants of IP3Rs. Representative experiments of 3 independent observations.
Figure 2 panel B shows the presence of the three IP3R isoforms in isolated nuclei of granulosa cells (108 cells; n = 3), in two fractions: nuclear membranes (NM) and nucleoplasm (NP). For all three isoforms, a high molecular weight band (≈230–250 kDa) corresponding to the complete form of IP3R was observed in the NM fraction. However the band of IP3R type 1 was clearly fainter than the other two types. Two smaller variants of IP3R types 2 and 3 were also detected in this fraction. The NP fraction showed no signal for the complete IP3R type 1; in contrast a weak band corresponding to IP3R type 2 whereas the band for type 3 was stronger. Low molecular weight variant was clearly detected for types 2 and 3.
Cytoplasmic and nuclear Ca2+ mobilization in granulosa cells
To examine the functionality of the nuclear Ca2+-handling proteins detected in the mouse granulosa cells, ATP-induced Ca2+ mobilization was assessed by confocal microscopy (analyzed cells = 800; n = 4). Application of ATP (50 μM) to the assay promoted Ca2+ mobilization by activation of purinergic G-protein-coupled receptor P2Y2 in mouse granulosa cells [3, 21]. The Fluo-4 associated signal was higher in the nuclei than in the cytoplasm, even in basal, non-stimulated conditions (Figure 3, first block, and column α). However, this fact could be due to nuclear milieu influence over the fluorescent signal, and not necessarily represent an elevated Ca2+ level within the nucleus. Upon ATP stimulation, the Ca2+ transient elicited in the nucleus was higher than the one recorded in the cytoplasm (Figure 3, first block, and column β). After the transient, intracellular Ca2+ returned to basal levels, but the nuclear fluorescent signal remained higher than in the cytosol (Figure 3, first block, and column γ). Treatment with the IP3R non-competitive inhibitor xestospongin C (5 μM for 15 min) prevented the ATP-induced Ca2+ mobilization in both compartments, without changes in the basal Ca2+ (Figure 3, second block, columns α, β, and γ). Interestingly, the ATP-induced Ca2+ transient in the presence of the antibiotic brefeldin A (5 μg/ml for 2 h) showed a differential response comparing the cytoplasmic and nuclear compartments. In this case, Ca2+ mobilization was completely suppressed in the cytoplasm, but in the nucleus the brefeldin A effect was only partial, and a net Ca2+ mobilization could be observed (Figure 3, third block, and column β). In addition, brefeldin A treatment promoted a decrease in the basal Fluo-4 associated-signal within the nucleus (Figure 3, third block, columns α, β and γ). These data support the notion that the nuclei in the granulosa cells, as the nuclei of others cellular systems, also have the capacity to mobilize Ca2+ [14].
ATP-induced Ca2+ mobilization in the nucleus and cytoplasm of mouse granulosa cells. Cells were incubated with Fluo-4 AM (5 μM) and analyzed by confocal microscopy. Each image represents the temporal changes in [Ca2+]i of a set of nearly 200 granulosa cells. Plots in the left column show the temporal pattern of fluorescent fluctuations of each experiment. Images were acquired every second, and the signals are expressed in arbitrary fluorescent units (AFU). N = nucleus and C = cytoplasm. The period of ATP (50 μM) delivery is indicated in the black box, whereas application of xestospongin C (Xes) (5 μM for 15 min) and brefeldin A (Bf A) (5 μg/ml for 2 h) is depicted in white boxes. Greek letters represent Ca2+ mobilization before (α), during (β), and after (γ) ATP application. In the plot, light symbols indicate the temporal pattern of Ca2+ mobilization within the nucleus, whereas dark symbols correspond to the variation of cytoplasmic Ca2+. First block: Representative experiment of Ca2+ mobilization by ATP. Second and third blocks: Inhibitory action of pre-treatment with xestospongin C and brefeldin A on the nuclear and cytoplasmic ATP-induced Ca2+ mobilization. Representative of 4 independent experiments.
Ryanodine receptor, thapsigargin-sensitive Ca2+-ATPase, and endomembranes are also present in the nuclei of granulosa cells
By means of specific fluorescent probes, the subcellular localization of RyR, SERCA, and the network of endomembranes in granulosa cells were investigated (n = 5). The results are shown in Figure 4. RyR formed clusters throughout the cytosol, and its expression within the nucleus was demonstrated by the coincidence of the fluorescent ryanodine derivative with the nuclear marker DAPI. The magnified image depicts more clearly the nuclear and perinuclear localization of the RyR. The fluorescent signal associated with thapsigargin showed the presence of SERCA in the cytoplasm as thread-like structures, and as with the RyR, the nuclear localization of SERCA was also evident from the coincidence with the DAPI signal. With higher magnification, the widespread distribution of SERCA throughout the entire cytoplasm as well as its intranuclear localization can be appreciated. Additionally, mouse granulosa cells were incubated with a fluorescent derivative of brefeldin A, a fungal metabolite that binds selectively to endomembranes, namely endoplasmic reticulum and the Golgi complex [29]. As with RyR and SERCA, the endomembrane marker was present in the cytoplasm, where it formed numerous clusters, and also within the nucleus. Co-localization with DAPI further supported the intranuclear localization of fluorescent brefeldin A, suggesting the presence of a membranous system within the nucleus (possibly the nucleoplasmic reticulum) in mouse granulosa cells. These characteristics are apparent in the magnified image. Taken together, these results indicate that the nuclei of granulosa cells in culture conditions express the principal Ca2+-handling proteins, as well as membranal structures in the nucleoplasmic compartment. These findings are similar to results reported in other cellular systems [10–14].
Subcellular location of ryanodine receptor, thapsigargin-sensitive Ca2+-ATPase, and endomembranes in mouse granulosa cells. The first block shows the intracellular location of the ryanodine receptor visualized by BODIPY FL-ryanodine. The second block depicts the intracellular location of thapsigargin-sensitive Ca2+-ATPase using BODIPY-FL thapsigargin. The third block shows the distribution of endomembranes by means of the BODIPY-FL conjugate of brefeldin A. All fluorescent probes were assayed at 1 μM (Yellow color). The second column shows the signal of the nuclear marker DAPI (Cyan color), whereas the third column depicts the merging the first and second columns. The fourth column is a magnification of a selected sector of the merge image. The results strongly suggest the intranuclear presence of all the Ca2+-handling proteins and the endomembranes in the mouse granulosa cells. Scale bar corresponds to 20 μm. Representative of 5 independent experiments.
Isoforms of IP3Rs in mouse ovary
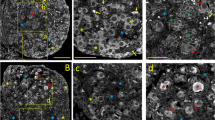
To eliminate possibly that our results reflected an artifact related to the culture conditions, we tested if the three IP3R isoforms were also observed within the granulosa cells nuclei in histological slices of mouse ovarian tissue (n = 3). Figure 5 shows the signals associated with the same isoform-specific antibodies against the IP3R types 1, 2, and 3 that were used in the assays with granulosa cells in vitro. Consistent with the previous results, the three IP3R isoforms were also detected in the granulosa cell nuclei in situ when these cells are still part of the ovary, which are located between the oocyte and the theca cells. The specificity of the experiments was confirmed by the very low unspecific signal seen when only the secondary antibodies were used (Figure 5, Control). These results confirm previous reports in which the three IP3R isoforms were detected in ovary [19, 20].
Detection of IP 3 R isoforms in mouse ovarian tisuue. Ovarian tissue was incubated with specific antibodies against IP3R types 1, 2, and 3, as well as with TO-PRO-1 iodide (50 nM) to label nuclei. Control images done in absence of primary antibody are at the top of columns 1 and 2. The first column shows entire ovarian structures, whereas the second column depicts an enhanced image delimiting granulosa (G) and theca (T) cells (Magenta color). The third column shows nuclear signal associated with TO-PRO-1 (Cyan color), and the fourth column represents the convergence of signals between IP3Rs and nuclear structures (merge). Scale bar for ovary slices corresponds to 100 μm, and for granulosa cells to 20 μm. These results provide evidence for the intranuclear presence of all the IP3R isoforms in the mouse granulosa cells in situ. Representative of 3 independent experiments.
The distribution of the three types of IP3R was similar along the slices of ovarian tissue. The nuclear expression of the three IP3R isoforms was evident from their co-localization with the nuclear marker TO-PRO 1 iodide. Analogous results were also obtained with the theca cells. These data indicate that mouse granulosa cells express, both in culture and in situ, all three types of IP3Rs with a cytoplasmic and nuclear localization.
Discussion
Expression and subcellular localization of IP3R isoforms in granulosa cells
Granulosa cells, like many other cellular types, contain at least two forms of Ca2+ release channels: RyR and IP3R [21]. Both channels are localized in the endoplasmic reticulum membranes, but also within the nuclear structure (Figures 1 and 4). Our data show that granulosa cells express the three IP3R isoforms (Figure 1). Detection of the IP3R isoforms was similar in both experimental conditions tested: in primary cultures (Figures 1 and 2), and in ovary slices (Figure 5). Certainly, we do not know if the immuno-detected IP3Rs are homo or heterotetramers. However, we have evidence that granulosa cells express low molecular weight variants of IP3R types 2 and 3 which could result from regulated proteolysis or mRNA splicing (Figure 2) [31–36]. Hence, granulosa cells have the potential to display a great variety of Ca2+ signaling responses based in the molecular diversity of IP3Rs forms.
The occurrence of at least two different isoforms of IP3R has been reported in several cellular types, such as hepatocytes (types 1 and 2) [38], lung (types 2 and 3) [32], colonic epithelium (types 2 and 3) [39], and deep cerebellar nuclei (types 1 and 3) [40]. The congregation of the three isoforms of IP3R in a unique cellular population is less frequent. Besides the finding from this study in mouse granulosa cells, the three types of IP3R have been reported to coexist in rat bile duct epithelial cells or cholangiocytes [41] and in bovine adrenal chromaffin cells [42].
When two or three IP3R isoforms are expressed in the same cell, they are usually distributed in different subcellular regions: For example, IP3R type 3 is localized in the apical section of cholangiocytes and non-pigmented epithelium cells, whereas the other isoforms are present in the rest of the cell structure, especially in the basolateral region [38, 43]. In the case of the mouse granulosa cells, we did not observe any preferential subcellular location for the IP3R isoforms: The signal from the three types of IP3Rs occurs throughout most of cytoplasm, presumably along the granulosa cells endomembranes, with a less intense signal for the type 1 (Figure 1). The identity of the organelles in which the IP3R isoforms were detected will be treated later.
In heterologous systems (Sf9 insect cells) the expression of each isoform of the IP3Rs presents the same Ca2+ gating and similar ionic conductance [44]. However, they differ in their sensitivity to IP3, intracellular Ca2+, and ATP: type 1 shows medium IP3-affinity, high ATP-affinity and low Ca2+ affinity. IP3R type 2 has a high IP3-affinity, a medium Ca2+ affinity, and is ATP independent. IP3R type 3 shows a low IP3-affinity, a low ATP-affinity, and a high Ca2+ affinity [44].
IP3R type 3 is related to the triggering of Ca2+ waves in diverse tissues, such as polarized epithelia [38], as well as to act as an apoptotic mediator in different cellular systems [45]. As to granulosa cells, it is not clear if they can be considered polarized, but some authors postulate a directionality in their function when, as a cellular population, the granulosa cells are surrounding the oocyte. For example, the handling of intracellular Ca2+ in the granulosa cells close to the theca is different from the granulosa cells close to the oocyte [46]. However, at least in the ovarian slice, there was no indication of the existence of a cellular sub-population with regard to the three IP3R isoforms. As to the role of Ca2+ release channels in promoting Ca2+ signaling, it was reported that the activity of RyR was necessary for ATP-induced Ca2+ mobilization in mouse granulosa cells [21]. More systematic studies are needed to define the precise role of the three IP3R isoforms during spontaneous and ligand-induced Ca2+ transients in granulosa cells. Although it is well documented that granulosa cells are prone to apoptosis according to hormonal and nutritional factors [47], the exact role of IP3R type 3 or the other two isoforms has not be substantiated during this process when this cell population is luteinized.
Nuclear location of IP3R isoforms in granulosa cells
IP3Rs have long been known to be present within the sarco-endoplasmic reticulum membranes of many cellular types and tissues (for review see [48]). However, growing evidence indicates the existence of the IP3Rs in other intracellular organelles such as the Golgi apparatus [49], plasma membrane [50, 51], and nucleus [8–18, 52]. Interestingly, in several cell types the IP3R has been detected in the nuclei, specifically in the nuclear envelope membranes, but also within membranous reticular structures known as the nucleoplasmic reticulum [17]. This intranuclear system is able to store and release Ca2+ in the same way as the endoplasmic reticulum does in the cytoplasm [17, 53]. To our knowledge, the results reported here are the first to show that the nuclei of granulosa cells contain all three isoforms of the IP3R. The intranuclear distribution of each isoform of the IP3Rs is variable; for example, types 1 and 3 were preferentially localized in the inner nuclear membrane of skeletal muscle myocytes [54]. In contrast, isoform 1 in the nuclei of bovine aortic endothelial cells, bovine adrenal glomerulosa cells, COS-7 cells [55], and ventricular myocytes [56] was not confined to the nuclear envelope, but distributed uniformly within the nucleus. Type 2 was detected forming part of the nucleoplasmic reticulum in rat hepatocytes [17]. The exact role of each IP3R isoform in the generation and kinetics of Ca2+ transients in the nucleus and cytoplasm of all these cells remains to be explored.
Nuclei in the granulosa cells contain the most important elements to accomplish Ca2+ release and uptake: besides IP3Rs, we detected positive and specific signals for RyR, thapsigargin-sensitive SERCA, and endomembranes (Figures 1 and 4). It has been postulated that the cellular pattern of Ca2+ dynamics depends on the isoforms of the IP3Rs, RyRs, and SERCAs present in the different organelles [11]. Indeed, the results in Figure 3 indicate that cytoplasm and nuclei differ in their capacity for ATP-induced Ca2+ mobilization due to differences in the extent to which brefeldin A alters Ca2+ dynamics in the two compartments. Nuclear Ca2+ has been shown to be specifically and autonomously mobilized in a great variety of other cellular types [53]. Questions arise regarding the potential functions of Ca2+ fluctuations within the nucleus. Several reports have shown that nuclear Ca2+ can control the transcriptional activity of certain genes [57], protein transport across the nuclear envelope [58], and translocation of protein kinases [17, 59]. More experiments are needed to determine how these ion channels and metabolic pumps are coordinated to enable nuclear Ca2+ transients in synchronization with cytosolic Ca2+ dynamics in granulosa cells.
IP3R isoforms in granulosa cells: In situ and in culture conditions
Granulosa cells are part of an ovarian complex where different follicular cell types are congregated to promote the development and maturation of the oocyte. Within this complex, granulosa cells are found in a precise location between the oocyte and the theca cells. It is a controversial issue if granulosa cells change their physiological characteristics when they are dissected and placed in culture conditions [60]. However, there are numerous reports that consider granulosa cells in vitro to be a suitable experimental system. Hence, studies of granulosa cells properties in culture conditions regarding hormonal action, signal transduction, and cell differentiation are common [61].
The findings of this study indicate that the presence of the three IP3R isoforms in the cytoplasmic and nuclear membranes is comparable in granulosa cells maintained in in vitro conditions with granulosa cells as part of the in vivo histological architecture; however, this conclusion must be confirmed. There are three potential interpretations of these observations: First, the expression of the three IP3R isoforms is not an artifact associated with the manipulation of granulosa cells when they are put in culture. Second, it is highly probable that granulosa cells express the three IP3R types in vivo, which would indicate a potential enriched repertoire in the intracellular Ca2+ dynamics of these ovarian endocrine cells. Third, granulosa cells are among the cellular types that express consistently the three isoforms of the IP3R, and the only type known so far that expresses all of them within the nucleus.
Conclusion
1) We are reporting for the first time that one type of murine granulosa cell expresses in cytoplasmic endomembranes and nucleus all three types of IP3Rs. 2) Both compartments mobilized Ca2+ in response to ATP, but the nucleus was less sensitive to the inhibitory action of brefeldin A. 3) Studies on intracellular Ca2+ mobilization in mouse granulosa cells should provide further information regarding the molecular and cellular events that are relevant for the participation of somatic cells in regulating the ovarian follicular development.
References
Liu YX: Interaction and signal transduction between oocyte and somatic cells in the ovary. Front Biosci. 2007, 12: 2782-2796.
Stocco C, Telleria C, Gibori G: The molecular control of corpus luteum formation, function, and regression. Endocr Rev. 2007, 28 (1): 117-149. 10.1210/er.2006-0022.
Arellano RO, Martinez-Torres A, Garay E: Ionic currents activated via purinergic receptors in the cumulus cell-enclosed mouse oocyte. Biol Reprod. 2002, 67 (3): 837-846. 10.1095/biolreprod.102.003889.
Flores JA, Aguirre C, Sharma OP, Veldhuis JD: Luteinizing hormone (LH) stimulates both intracellular calcium ion ([Ca2+]i) mobilization and transmembrane cation influx in single ovarian (granulosa) cells: recruitment as a cellular mechanism of LH-[Ca2+]i dose response. Endocrinology. 1998, 139 (8): 3606-3612. 10.1210/en.139.8.3606.
Park DW, Cho T, Kim MR, Kim YA, Min CK, Hwang KJ: ATP-induced apoptosis of human granulosa luteal cells cultured in vitro. Fertil Steril. 2003, 80 (4): 993-1002. 10.1016/S0015-0282(03)01118-X.
Balakier H, Dziak E, Sojecki A, Librach C, Michalak M, Opas M: Calcium-binding proteins and calcium-release channels in human maturing oocytes, pronuclear zygotes and early pre-implantation embryos. Hum Reprod. 2002, 17 (11): 2938-2947. 10.1093/humrep/17.11.2938.
Goud PT, Goud AP, Leybaert L, Van Oostveldt P, Mikoshiba K, Diamond MP, Dhont M: Inositol 1,4,5-trisphosphate receptor function in human oocytes: calcium responses and oocyte activation-related phenomena induced by photolytic release of InsP(3) are blocked by a specific antibody to the type I receptor. Mol Hum Reprod. 2002, 8 (10): 912-918. 10.1093/molehr/8.10.912.
Alonso MT, Villalobos C, Chamero P, Alvarez J, Garcia-Sancho J: Calcium microdomains in mitochondria and nucleus. Cell Calcium. 2006, 40 (5–6): 513-525. 10.1016/j.ceca.2006.08.013.
Carafoli E, Santella L, Branca D, Brini M: Generation, control, and processing of cellular calcium signals. Crit Rev Biochem Mol Biol. 2001, 36 (2): 107-260. 10.1080/20014091074183.
Gerasimenko J, Maruyama Y, Tepikin A, Petersen OH, Gerasimenko O: Calcium signalling in and around the nuclear envelope. Biochem Soc Trans. 2003, 31 (Pt 1): 76-78.
Gerasimenko O, Gerasimenko J: New aspects of nuclear calcium signalling. J Cell Sci. 2004, 117 (Pt 15): 3087-3094. 10.1242/jcs.01295.
Wu X, Zhang T, Bossuyt J, Li X, McKinsey TA, Dedman JR, Olson EN, Chen J, Brown JH, y Bers DM: Local InsP3-dependent perinuclear Ca2+ signaling in cardiac myocyte excitation-transcription coupling. J Clin Invest. 2006, 116 (3): 675-682. 10.1172/JCI27374.
Quesada I, Martin F, Roche E, Soria B: Nutrients induce different Ca(2+) signals in cytosol and nucleus in pancreatic beta-cells. Diabetes. 2004, 53 (Suppl 1): S92-95. 10.2337/diabetes.53.2007.S92.
Santella L, Carafoli E: Calcium signaling in the cell nucleus. Faseb J. 1997, 11 (13): 1091-1109.
Gerasimenko OV, Gerasimenko JV, Tepikin AV, Petersen OH: ATP-dependent accumulation and inositol trisphosphate- or cyclic ADP-ribose-mediated release of Ca2+ from the nuclear envelope. Cell. 1995, 80 (3): 439-444. 10.1016/0092-8674(95)90494-8.
Quesada I, Rovira JM, Martin F, Roche E, Nadal A, Soria B: Nuclear KATP channels trigger nuclear Ca(2+) transients that modulate nuclear function. Proc Natl Acad Sci USA. 2002, 99 (14): 9544-9549. 10.1073/pnas.142039299.
Echevarria W, Leite MF, Guerra MT, Zipfel WR, Nathanson MH: Regulation of calcium signals in the nucleus by a nucleoplasmic reticulum. Nat Cell Biol. 2003, 5 (5): 440-446. 10.1038/ncb980.
Marius P, Guerra MT, Nathanson MH, Ehrlich BE, Leite MF: Calcium release from ryanodine receptors in the nucleoplasmic reticulum. Cell Calcium. 2006, 39 (1): 65-73.
Fujino I, Yamada N, Miyawaki A, Hasegawa M, Furuichi T, Mikoshiba K: Differential expression of type 2 and type 3 inositol 1,4,5-trisphosphate receptor mRNAs in various mouse tissues: in situ hybridization study. Cell Tissue Res. 1995, 280 (2): 201-210.
Fissore RA, Longo FJ, Anderson E, Parys JB, Ducibella T: Differential distribution of inositol trisphosphate receptor isoforms in mouse oocytes. Biol Reprod. 1999, 60 (1): 49-57. 10.1095/biolreprod60.1.49.
Morales-Tlalpan V, Arellano RO, Diaz-Munoz M: Interplay between ryanodine and IP3 receptors in ATP-stimulated mouse luteinized-granulosa cells. Cell Calcium. 2005, 37 (3): 203-213. 10.1016/j.ceca.2004.10.001.
Rosado JA, Sage SO: Coupling between inositol 1,4,5-trisphosphate receptors and human transient receptor potential channel 1 when intracellular Ca2+ stores are depleted. Biochem J. 2000, 350 (Pt 3): 631-635. 10.1042/0264-6021:3500631.
He CL, Damiani P, Ducibella T, Takahashi M, Tanzawa K, Parys JB, Fissore RA: Isoforms of the inositol 1,4,5-trisphosphate receptor are expressed in bovine oocytes and ovaries: the type-1 isoform is down-regulated by fertilization and by injection of adenophostin A. Biol Reprod. 1999, 61 (4): 935-943. 10.1095/biolreprod61.4.935.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ: Protein measurement with the Folin phenol reagent. J Biol Chem. 1951, 193 (1): 265-275.
Bradford MM: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976, 72: 248-254. 10.1016/0003-2697(76)90527-3.
Cifuentes F, Gonzalez CE, Fiordelisio T, Guerrero G, Lai FA, Hernandez-Cruz A: A ryanodine fluorescent derivative reveals the presence of high-affinity ryanodine binding sites in the Golgi complex of rat sympathetic neurons, with possible functional roles in intracellular Ca(2+) signaling. Cell Signal. 2001, 13 (5): 353-362. 10.1016/S0898-6568(01)00132-2.
Vazquez-Martinez O, Canedo-Merino R, Diaz-Munoz M, Riesgo-Escovar JR: Biochemical characterization, distribution and phylogenetic analysis of Drosophila melanogaster ryanodine and IP3 receptors, and thapsigargin-sensitive Ca2+ ATPase. J Cell Sci. 2003, 116 (Pt 12): 2483-2494. 10.1242/jcs.00455.
Mangiardi DA, McLaughlin-Williamson K, May KE, Messana EP, Mountain DC, Cotanche DA: Progression of hair cell ejection and molecular markers of apoptosis in the avian cochlea following gentamicin treatment. J Comp Neurol. 2004, 475 (1): 1-18. 10.1002/cne.20129.
Deng Y, Bennink JR, Kang HC, Haugland RP, Yewdell JW: Fluorescent conjugates of brefeldin A selectively stain the endoplasmic reticulum and Golgi complex of living cells. J Histochem Cytochem. 1995, 43 (9): 907-915.
Zhang D, Boulware MJ, Pendleton MR, Nogi T, Marchant JS: The inositol 1,4,5-trisphosphate receptor (Itpr) gene family in Xenopus: identification of type 2 and type 3 inositol 1,4,5-trisphosphate receptor subtypes. Biochem J. 2007, 404 (3): 383-391. 10.1042/BJ20070101.
Patterson RL, Boehning D, Snyder SH: Inositol 1,4,5-trisphosphate receptors as signal integrators. Annu Rev Biochem. 2004, 73: 437-465. 10.1146/annurev.biochem.73.071403.161303.
Choi JY, Beaman-Hall CM, Vallano ML: Granule neurons in cerebellum express distinct splice variants of the inositol trisphosphate receptor that are modulated by calcium. Am J Physiol Cell Physiol. 2004, 287 (4): C971-C980. 10.1152/ajpcell.00571.2003.
Iwai M, Tateishi Y, Hattori M, Mizutani A, Nakamura T, Futatsugi A, Inoue T, Furuichi T, Michikawa T, Mikoshiba K: Molecular cloning of mouse type 2 and type 3 inositol 1,4,5-trisphosphate receptors and identification of a novel type 2 receptor splice variant. J Biol Chem. 2005, 280 (11): 10305-10317. 10.1074/jbc.M413824200.
Bhanumathy CD, Nakao SK, Joseph SK: Mechanism of proteasomal degradation of inositol trisphosphate receptors in CHO-K1 cells. J Biol Chem. 2006, 281 (6): 3722-3730. 10.1074/jbc.M509966200.
Boehning D, Joseph SK: Direct association of ligand-binding and pore domains in homo- and heterotetrameric inositol 1,4,5-trisphosphate receptors. EMBO Journal. 2000, 19 (20): 5450-5459. 10.1093/emboj/19.20.5450.
Sinha M, Hasan G: Sequencing and exon mapping of the inositol 1,4,5-trisphosphate receptor cDNA from Drosophila embryos suggests the presence of differentially regulated forms of RNA and protein. Gene. 1999, 233 (1–2): 271-276. 10.1016/S0378-1119(99)00158-4.
Ben-Ze'ev A, Amsterdam A: In vitro regulation of granulosa cell differentiation. Involvement of cytoskeletal protein expression. J Biol Chem. 1987, 262 (11): 5366-5376.
Hirata K, Dufour JF, Shibao K, Knickelbein R, O'Neill AF, Bode HP, Cassio D, St-Pierre MV, Larusso NF, Leite MF, Nathanson MH: Regulation of Ca(2+) signaling in rat bile duct epithelia by inositol 1,4,5-trisphosphate receptor isoforms. Hepatology. 2002, 36 (2): 284-296. 10.1053/jhep.2002.34432.
Siefjediers A, Hardt M, Prinz G, Diener M: Characterization of inositol 1,4,5-trisphosphate (IP(3)) receptor subtypes at rat colonic epithelium. Cell Calcium. 2006
Sharp AH, Nucifora FC, Blondel O, Sheppard CA, Zhang C, Snyder SH, Russell JT, Ryugo DK, Ross CA: Differential cellular expression of isoforms of inositol 1,4,5-triphosphate receptors in neurons and glia in brain. J Comp Neurol. 1999, 406 (2): 207-220. 10.1002/(SICI)1096-9861(19990405)406:2<207::AID-CNE6>3.0.CO;2-7.
Pusl T, Nathanson MH: The role of inositol 1,4,5-trisphosphate receptors in the regulation of bile secretion in health and disease. Biochem Biophys Res Commun. 2004, 322 (4): 1318-1325. 10.1016/j.bbrc.2004.08.036.
Huh YH, Chu SY, Park SY, Huh SK, Yoo SH: Role of nuclear chromogranin B in inositol 1,4,5-trisphosphate-mediated nuclear Ca2+ mobilization. Biochemistry. 2006, 45 (4): 1212-1226. 10.1021/bi051594r.
Hirata K, Nathanson MH, Burgstahler AD, Okazaki K, Mattei E, Sears ML: Relationship between inositol 1,4,5-trisphosphate receptor isoforms and subcellular Ca2+ signaling patterns in nonpigmented ciliary epithelia. Invest Ophthalmol Vis Sci. 1999, 40 (9): 2046-2053.
Tu H, Wang Z, Nosyreva E, De Smedt H, Bezprozvanny I: Functional characterization of mammalian inositol 1,4,5-trisphosphate receptor isoforms. Biophys J. 2005, 88 (2): 1046-1055. 10.1529/biophysj.104.049593.
Khan AA, Soloski MJ, Sharp AH, Schilling G, Sabatini DM, Li SH, Ross CA, Snyder SH: Lymphocyte apoptosis: mediation by increased type 3 inositol 1,4,5-trisphosphate receptor. Science. 1996, 273 (5274): 503-507. 10.1126/science.273.5274.503.
Tai CJ, Kang SK, Leung PC: Adenosine triphosphate-evoked cytosolic calcium oscillations in human granulosa-luteal cells: role of protein kinase C. J Clin Endocrinol Metab. 2001, 86 (2): 773-777. 10.1210/jc.86.2.773.
Quirk SM, Cowan RG, Harman RM: The susceptibility of granulosa cells to apoptosis is influenced by oestradiol and the cell cycle. J Endocrinol. 2006, 189 (3): 441-453. 10.1677/joe.1.06549.
Berridge MJ, Bootman MD, Roderick HL: Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003, 4 (7): 517-529. 10.1038/nrm1155.
Surroca A, Wolff D: Inositol 1,4,5-trisphosphate but not ryanodine-receptor agonists induces calcium release from rat liver Golgi apparatus membrane vesicles. J Membr Biol. 2000, 177 (3): 243-249. 10.1007/s002320010008.
Guillemette G, Balla T, Baukal AJ, Catt KJ: Characterization of inositol 1,4,5-trisphosphate receptors and calcium mobilization in a hepatic plasma membrane fraction. J Biol Chem. 1988, 263 (10): 4541-4548.
Taylor CW, Dellis O: Plasma membrane IP(3) receptors. Biochem Soc Trans. 2006, 34 (Pt 5): 910-912.
Stehno-Bittel L, Luckhoff A, Clapham DE: Calcium release from the nucleus by InsP3 receptor channels. Neuron. 1995, 14 (1): 163-167. 10.1016/0896-6273(95)90250-3.
Gomes DA, Leite MF, Bennett AM, Nathanson MH: Calcium signaling in the nucleus. Can J Physiol Pharmacol. 2006, 84 (3–4): 325-332. 10.1139/Y05-117.
Cardenas C, Liberona JL, Molgo J, Colasante C, Mignery GA, Jaimovich E: Nuclear inositol 1,4,5-trisphosphate receptors regulate local Ca2+ transients and modulate cAMP response element binding protein phosphorylation. J Cell Sci. 2005, 118 (Pt 14): 3131-3140. 10.1242/jcs.02446.
Laflamme K, Domingue O, Guillemette BI, Guillemette G: Immunohistochemical localization of type 2 inositol 1,4,5-trisphosphate receptor to the nucleus of different mammalian cells. J Cell Biochem. 2002, 85 (1): 219-228. 10.1002/jcb.10124.
Bare DJ, Kettlun CS, Liang M, Bers DM, Mignery GA: Cardiac type 2 inositol 1,4,5-trisphosphate receptor: interaction and modulation by calcium/calmodulin-dependent protein kinase II. J Biol Chem. 2005, 280 (16): 15912-15920. 10.1074/jbc.M414212200.
Thompson M, Andrade VA, Andrade SJ, Pusl T, Ortega JM, Goes AM, Leite MF: Inhibition of the TEF/TEAD transcription factor activity by nuclear calcium and distinct kinase pathways. Biochem Biophys Res Commun. 2003, 301 (2): 267-274. 10.1016/S0006-291X(02)03024-3.
Perez-Terzic C, Stehno-Bittel L, Clapham DE: Nucleoplasmic and cytoplasmic differences in the fluorescence properties of the calcium indicator Fluo-3. Cell Calcium. 1997, 21 (4): 275-282. 10.1016/S0143-4160(97)90115-9.
Wu X, Bers DM: Sarcoplasmic reticulum and nuclear envelope are one highly interconnected Ca2+ store throughout cardiac myocyte. Circ Res. 2006, 99 (3): 283-291. 10.1161/01.RES.0000233386.02708.72.
Senbon S, Hirao Y, Miyano T: Interactions between the oocyte and surrounding somatic cells in follicular development: lessons from in vitro culture. J Reprod Dev. 2003, 49 (4): 259-269. 10.1262/jrd.49.259.
Matsuda-Minehata F, Inoue N, Goto Y, Manabe N: The Regulation of Ovarian Granulosa Cell Death by Pro- and Anti-apoptotic Molecules. J Reprod Dev. 2006, 52 (6): 695-705. 10.1262/jrd.18069.
Acknowledgements
This study was supported by projects IN-201807 to MDM (PAPIIT) and PI200406 to VMT (PFAMU). We thank Dr. Dorothy Pless for editing the manuscript, as well as Biol. Olivia Vázquez-Martínez and Dr. Edith Garay for expert technical assistance. We also acknowledge M.V.Z. José Martín García Servín, I.S.C. Omar González Hernández, Lic. María del Pilar Galarza Barrios, and Ing. Elsa Nydia Hernández Rios for their kind participation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
MDM participated in designing the study, in the analysis and discussion of the results and drafted critical revision of the manuscript. PdlRS participated in the initial immunofluorescent experiments. ABJE and ROA in the experimental design of the ovary histology. VMT designed the study, performed most of the experiments, and participated in the analysis and discussion of the results and drafted the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Díaz-Muñoz, M., de la Rosa Santander, P., Juárez-Espinosa, A.B. et al. Granulosa cells express three inositol 1,4,5-trisphosphate receptor isoforms: cytoplasmic and nuclear Ca2+ mobilization. Reprod Biol Endocrinol 6, 60 (2008). https://doi.org/10.1186/1477-7827-6-60
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1477-7827-6-60