Abstract
The ability to produce transgenic animals through the introduction of exogenous DNA has existed for many years. However, past methods available to generate transgenic animals, such as pronuclear microinjection or the use of embryonic stem cells, have either been inefficient or not available in all animals, bovine included. More recently somatic cell nuclear transfer has provided a method to create transgenic animals that overcomes many deficiencies present in other methods. This review summarizes the benefits of using somatic cell nuclear transfer to create bovine transgenics as well as the possible opportunities this method creates for the future.
Similar content being viewed by others
Introduction
The bovine species has long been a focus of research due to its importance in agriculture. Advancements in bovine reproductive technologies such as artificial insemination, multiple ovulation and embryo transfer, as well as oocyte and embryo culture have improved the ability to increase superior genotypes in the dairy and beef industries [1]. In combination with these reproductive technologies, the ability to insert DNA into livestock [2, 3] has provided limitless possibilities for agriculture (e.g., inserting genes affecting milk and beef production) as well as biomedicine (e.g., producing important proteins in milk). Although there have been major advances in the field of bovine transgenics since its inception, the full potential of transgenic cattle has not been realized in part due to the limitations of commonly used transgenic technologies. More recently, the success of somatic cell nuclear transfer has provided a new and faster way to produce transgenic cattle while circumventing many of the limitations of other transgenic techniques. This review will focus on summarizing the recent past and present state of bovine transgenics and how somatic cell nuclear transfer will affect the future potential of producing transgenic cattle.
Producing Transgenic Cattle
There are multiple ways to produce a transgenic animal. Briefly discussed below are the strengths and weaknesses of the most common ways currently available to make transgenic animals. The success, or lack thereof, of each method in creating transgenic cattle is also summarized. Although there has been limited success using retroviral vectors [4] and sperm mediated transgenesis [5, 6] in cattle, these techniques will not be discussed in this review due to space limitations.
Pronuclear microinjection
One of the first techniques created to generate a transgenic animal was pronuclear microinjection or the insertion of DNA into the pronucleus of a fertilized oocyte. This technology was first used in the mouse [7] and then transferred to other animals including livestock [2, 3]. The success of pronuclear injection with respect to transgene integration ranges from around 3% for mice, rats and rabbits to only 1% for cattle, pigs and sheep [8]. In addition to the poor efficiency of pronuclear microinjection, this method usually results in a high percentage of mosaics in which not all cells of the animal contain the transgene. The time and cost of screening for germline transmission in mosaic animals like cattle can be prohibitive to generating more transgenic animals through breeding. Using pronuclear injection to create transgenic animals can also lead to high variability in transgene expression between animals due to mosaicism as well as chromosomal position effects that occur during the random integration of the transgene [9]. Testing multiple lines of animals for proper transgene expression is necessary in a pronuclear microinjection approach to creating transgenic animals. Microinjection is also limited in that it only allows for the random addition of exogenous DNA rather than targeting to specific sites. DNA targeting is necessary in generating gene knockouts to model human diseases. The success of pronuclear microinjection is evident in the generation of many transgenic cattle but its limitations have hindered the progress of bovine transgenics.
Stem cells
Embryonic stem (ES) cells are used extensively as way to create transgenic mice. These cells are isolated from the inner cell mass of a blastocyst and, kept in the right culture conditions, have the potential to divide endlessly [10]. This immortal-like characteristic allows for easy propagation and eventual DNA manipulation through the insertion of transgenes. When transgenic ES cells are isolated and inserted into a growing mouse embryo, they multiply and contribute to the resulting fetus giving rise to almost any tissue type. These chimeric animals are then tested for germline transmission and used to create fully transgenic animals through breeding strategies. One big advantage of using ES cells over microinjection is the ability to select for transgene integration through the use of selectable markers. This ability ensures the creation of transgenic offspring even if they are chimeras. ES cells also allow for the targeted alteration of DNA by homologous recombination leading to the creation of gene knockouts [11]. The ability to easily create gene knockouts has lead to the mouse being considered one of the best models for genetic studies.
Due to the success of mouse ES cells, many attempts have been made to isolate similar cells in other animals with limited success [12–14]. Success was achieved in cattle when ES-like cells were isolated from early embryos, transfected with exogenous DNA, reintroduced into preimplantation embryos and shown to contribute to tissues of the resulting calves [15]. However, genetic modification of cattle ES-like cells is not easy due to the culture system required to maintain them as undifferentiated ES cells and their inability to expand clonally in vitro [15]. Unfortunately, even if cattle ES cells were comparable to mouse ES cells the generation time and maintenance cost of multiple mosaic animals would be prohibitive to testing for germline transmission.
Somatic Cell Nuclear Transfer
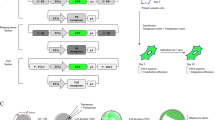
Due to absence of proven ES cells and the recent advances in nuclear transfer (NT), current emphasis for creating bovine transgenics has been placed on somatic cell nuclear transfer (SCNT). Nuclear transfer is a technique that can be used to create a genetically identical copy, or a clone, of an animal. Nuclear transfer commonly involves the transfer or placement of a donor nucleus into the cytoplasm of an enucleated MII oocyte (figure 1). Donor cells can originate from a wide variety of cell types ranging from embryonic blastomeres all the way up to adult cells. Although initial work in NT focused on using embryonic blastomeres as a donor source [16], the process was hampered by the limited number of cells available in an early embryo. More recently success in cattle cloning has been achieved using fetal [17] and adult [18] cells. The ability to use cells that can be cultured increased the number of cells available to clone with as well as facilitated the ability to make transgenic cattle. Transgenes can be introduced into cultured cells that can be used as donor cells for SCNT (figure 1).
Somatic Cell Nuclear Transfer with Transgenic Cells. (A) A mature oocyte is enucleated by a glass pipet and a transgenic somatic cell (gray striped cell) is transferred under the zona pelucida of the oocyte. An electrical pulse is then given to fuse the two cell membranes transferring the transgenic donor nucleus into the oocyte. (B) Somatic cells are transfected with a transgene and appropriate selection is used to isolate a clonal population of transgenic somatic cells.
Advantages in using SCNT for bovine transgenics
Somatic cell nuclear transfer has facilitated the ability to make transgenic animals by circumventing most of the shortcomings of other transgenic techniques [17]. One of the first advantages over microinjection is that the sex of the animal can be predetermined by choosing the donor material (ie., male or female tissue). For example, the ability to select the sex of the animal would increase efficiency and facilitate the manipulation of milk production through transgenesis [19]. Second, the use of cell culture to propagate donor cells can lead to almost limitless number of transgenic cells that can be frozen and stored for long periods of time. In conjunction with SCNT these transgenic donor cells can eventually give rise to numerous cloned transgenic animals. Transgene structure and expression can be tested by molecular techniques, such as PCR, Southern blot analysis, Fluorescence in situ Hybridization and Western blot analysis, before initiating NT and transferring the embryo to a recipient cow with a lengthy gestation time of 9 months. The proper use of SCNT also ensures that 100% of animals produced are transgenic and that every cell of an animal will have the transgene, thereby saving time and cost associated with recipient animals. The ability to use a clonal population of transgenic cells guarantees the same transgene insertion site for each clone thus decreasing animal to animal variation in transgene expression levels. Further, transgenes can be added to a specific genetic background. For example, a female that is above average on milk protein production may be used as the genetic background (donor somatic cells) in which the transgene is placed. Lastly, SCNT allows for not only the addition of DNA at random sites but also targeted insertion of DNA by homologous recombination which is vital in modulating specific gene expression as well creating gene knockouts. Gene targeted pigs [20, 21] and sheep [22, 23] have been produced using SCNT techniques.
SCNT can be improved
Although somatic cell nuclear transfer has lead to various accomplishments and offers many advantages over current transgenic techniques available, improvements can be made to increase future success. First and foremost is the need to increase overall efficiency. The success rate for somatic cell nuclear transfer averages between 1–3% in most animals including cattle [24]. The majority of embryos are lost during pregnancy with a 60% higher fetal loss between gestational days 35–60 when compared to embryos created through in vitro fertilization (IVF) [1]. In cloned cattle, there is higher perinatal loss than observed in the general population. These losses are not due to any one anomaly but rather complications that can range from increased birth weight, pulmonary abnormalities, respiratory problems, to metabolic deficiencies [reviewed in [25]]. It should be noted that these complications were first observed in blastomere derived NT offspring and reported by Willadsen and coworkers in 1991[26]. Placental abnormalities are also common in bovine NT offspring clones as first observed in bovine pluripotent cell NT pregnancies [27]. Term NT placentae often have large but few placentomes, edema and hydroallantois [28]. Most SCNT calves survive early postnatal development and seem to be quite normal and fertile [29]. Many studies have tried to increase NT efficiency and have seen small differences due to genotype [30], type of donor cell [31] and stage of donor cell [32] but no one has had extreme success.
Nuclear Reprogramming
Current research to decrease the number of pregnancy losses and thus increase the efficiency of NT has recently focused on understanding nuclear reprogramming. Nuclear reprogramming can be loosely defined as a set of epigenetic changes (those not involving a change in DNA sequence) required for a nucleus to change developmental fates.
During the NT process the oocyte changes the fate of the donor nucleus from its original status (e.g., skin, granulosa etc.) to that of a zygotic nucleus. Improper nuclear reprogramming of the donor nucleus in the oocyte is thought to be the major reason of failure in the cloning process. In cattle, studies have shown that methylation [33–35] as well as gene expression [36–38] are abnormal when compared to in vivo and in vitro matured embryos. Recently, Enright and coworkers showed that histone acetylation levels in cells changed with respect to the stage of cell, cell type and numbers of cell passages suggesting that histone acetylation could be a factor in improper nuclear reprogramming in NT [39]. Epigenetic abnormalities caused by the NT process however are not passed on to the offspring of cloned animals as shown in mice [40].
A recent idea that may increase efficiencies in nuclear reprogramming during the NT process is the exposure of donor cells to remodeling factors through in vitro systems before NT is initiated [41]. The addition of Xenopus laevis egg extracts was shown to successfully inhibit transcription, which has been hypothesized to facilitate nuclear reprogramming [41]. The addition of roscovitine, which inhibits cyclin dependent kinase 2, to donor cells successfully synchronized donor cell cycle and increased survivability of cloned calves and thus may increase the nuclear reprogramming capacity of the donor cells [42]. The addition of trichostatin A, a histone deacetylase inhibitor, to donor cells significantly increased blastocyst development rate compared to non-treated control cells when both were used for SCNT [43]. Studies such as these give evidence that increasing NT efficiency is possible and will only improve in the future.
Cell lifespan
Another difficulty in using SCNT to create transgenic animals is that unlike ES cells somatic cells have a definitive lifespan. Bovine fetal fibroblast cells, which are commonly used to make transgenic cattle, have 30–50 population doublings (PDs) before senescence [44]. Although Cibelli and coworkers were able to create transgenic calves from a clonally derived transgenic cell line with a capacity for 30 PDs [17], Clarke and coworkers have estimated that gene targeting requires around 45 PDs [45]. Recent evidence has shown that the doubling capacity can vary widely between cell lines and that genetics may play a major role in determining this capacity illustrating the importance of picking the right cell line to work with [46]. Even though there has been some success using late passage cells for NT [47, 48] the extended cell culture necessary for clonal propagation of a transgenic cell most likely leads to senescence. For example, out of the 25 gene targeted colonies identified by Denning and coworkers 23 senesced before they could be expanded for NT [49].
One approach recently suggested to overcome the problem of senescence of somatic cells is the introduction of TERT [50]. TERT is the catalytic component of telomerase and has been shown to immortalize cell lines when it is expressed [51]. Even cell lines with active TERT that have been passaged numerous times show no sign of phenotypic or chromosomal abnormalities that are hallmark of a transformed cell line [52, 53]. A reversible system has been reported, making it possible to express TERT until genetic modifications are completed and then silencing it before NT [54]. The future use of such a system would facilitate transgenic work in primary cell cultures and improve the chances of deriving SCNT offspring from these modified donor cells.
Combining transgenics and SCNT
Although SCNT efficiencies can be improved, it is currently the best method to produce transgenic livestock [55]. In this study sheep transgenic for human clotting factor IX were produced by transfecting fetal fibroblast cells with a transgene containing human clotting factor IX linked to the β-lactoglobulin gene promoter. The authors successfully produced transgenic sheep when these cells were used as donor cells for SCNT. The study also demonstrated that using SCNT improved overall efficiency 2.5 times over pronuclear microinjection mainly due to the fewer animals required as recipients. Today, most commercial companies producing transgenic livestock use SCNT extensively to produce their transgenic founders.
The success of SCNT in creating transgenic livestock has provided a means for generating gene-targeted animals. McCreath and coworkers, produced the first gene-targeted mammal other than mouse when they inserted a transgene into the ovine α1 procollagen locus [22]. Gene targeting success has also been achieved in pigs with the galactosyltransferase gene in the hopes of providing organs for organ transfers [20, 21, 49]. The number of gene targeting studies will multiply as the amount of genetic sequence information increases.
Transgenic Cattle produced through SCNT
To date, the focus of studies producing transgenic cattle through SCNT has ranged from proof of principle [17] to altering milk protein concentration [19]. The first transgenic cattle produced through SCNT were transgenic for a fusion β-galactosidase-neomycin gene and demonstrated that cattle could be produced through using transgenic culture cells for SCNT [17]. Multiple studies have used the green fluorescent protein (GFP) gene transfected donor cells and subsequently used for NT to create bovine embryos and calves. Green fluorescent protein was used in these studies as a marker for screening preimplantation embryos, proving germ line transmission and trying to increase the overall efficiency of the process [48, 56–59]. One recent study used SCNT to create calves transgenic for two casein genes involved in milk protein production [19]. When the resulting calves were induced to lactate, the levels of β- and κ-casein protein in milk were altered suggesting that the transgene did influence milk content.
The ability to generate bovine embryos using transgenic cells has differed between studies. Some studies have shown a significant decline in blastocyst development rate when using transgenic cells compared to nontransgenic cells for SCNT [56, 60]. Other studies however have not reported a difference in SCNT blastocyst development rate when using transgenic cells [19, 48]. Extended culture needed for transfection and selection may induce changes to the donor cells that decrease SCNT efficiency [60]. In addition, variation in SCNT procedures among labs and the inherent biological variability within the NT system (oocyte maturation, cell culture and donor cell lines) can add another level of variability [61]. A recent study suggested that the coordination of donor cell type and cell cycle stage is necessary to maximize NT efficiency [62]. For transfected cells, the G1 stage provided the best blastocyst development rate while for nontransfected cells the G0 stage provided the best blastocyst development rate when these cells were used for NT. Further study of factors such as the specific transgene used, integration site and methodological differences in the NT procedure should also provide insight into optimizing SCNT with transgenic cells.
Bovine models for disease
The success of SCNT has provided a means by which the generation of mammalian animal models other than mice is now available. The mouse is the usual animal model of choice due to factors like short generation time, low maintenance and cost and ease of availability. Although livestock, like pigs and cattle, do not have these attributes that one might consider as a prerequisite for an animal model, they do have similarities to humans that may make them great animal models for human disease. For instance, factors such as lifespan, size, and possibly genomic organization [63, 64] are all more similar between cattle and pigs and humans than compared to mice. Although no one would dispute the impact that the mouse has had as a model on understanding human disease, there are diseases like cystic fibrosis in which the mouse does not display all of the human phenotypes of the disease [65]. Due to similarities between the lung in sheep and humans, sheep have been suggested as a possible model for cystic fibrosis [65]. Similarities between human and livestock may help advance our understanding of certain diseases and thus successfully alleviate them.
An example of a human disease in which understanding may be advanced through the creation of a porcine or bovine model, and is currently underway in our laboratory, is ataxi telangiectasia (A-T). A-T is a genetic disease with symptoms ranging from neurodegeneration of the cerebellum to an increased risk of cancer [66, 67]. The disease originates from mutations in the ATM (ataxia telangiectasia mutated) gene that is thought to play a role in cell cycle control as well as mismatch repair [68–70]. Although mouse models for this disease have displayed early onset cancer [70–72] they have exhibited only minor abnormalities in movement [70] and subtle signs of cerebellar degeneration [73]. The severe neurodegenerative phenotype may be absent in mice due to disimilarities in lifespan, development, and genomic organization when compared to the human. The longer lifespan, similar gestational length (9 months), and analogous genomic organization in cattle and humans could provide a better model for the AT disease. Hopefully the creation of a bovine or porcine model for A-T will provide insights not currently available through the mouse and help our understanding of the disease.
Other possible uses of transgenic cattle generated through SCNT
The combination of transgenic technology and SCNT has made it far easier to make transgenic cattle than in the past. Building on the initial success of this merger of technology we hope to see more progress and past ideas realized. The host of possibilities include progress in areas like agriculture in which it now seems possible to change the protein content in milk [19] as well as target genes important in increasing beef production [74]. In biomedicine, possibilities include the production of important proteins in milk such as α-1 antitrypsin for cystic fibrosis, blood clotting factors like antithrombin III, factor IX and fibrinogen for bleeding disorders, and human serum albumin which could be useful for treatment of burns [75]. Although such possibilities originated when transgenic work was started using pronuclear microinjection, hopefully these ideas will now be realized through SCNT.
Mentioned briefly throughout this review is the fact that SCNT provides a way to make targeted modifications to the genome. This advantage offers even more future possibilities for transgenic cattle than before. For example, the inactivation of the bovine PRP gene might provide bovine spongioform encepholopathy (BSE) resistant cattle. When the PRP gene is knocked out in mice, they are resistant to scrapie without adverse effects [76]. The increase in genetic sequence information in cattle will also provide similar studies such as this one.
Conclusion
Somatic cell nuclear transfer has provided a new way to create transgenic cattle without the drawbacks of past transgenic techniques. Although the combination of transgenic technology and SCNT in cattle is still in its infancy, the possibilities it provides are almost limitless. Further research focused on improving our understanding of nuclear reprogramming will ultimately increase the efficiency of NT and facilitate the creation of transgenic SCNT cattle. Improvements in DNA transfection, colony selection and extending cell lifespan will also increase success in creating transgenic, and especially gene targeted, SCNT bovine. The future possibilities of using artificial chromosomes as vectors in donor cells [77] and increasing the number of oocytes available for NT through ES cell differentiation [78] could also boost bovine transgenics. All of these improvements will facilitate the realization of goals for transgenic cattle in agriculture and biomedicine.
References
Galli C, Duchi R, Crotti G, Turini P, Ponderato N, Colleoni S, Lagutina I, Lazzari G: Bovine embryo technologies. Theriogenology. 2003, 59: 599-616. 10.1016/S0093-691X(02)01243-8.
Hammer RE, Pursel VG, Rexroad CE, Wall RJ, Bolt DJ, Ebert KM, Palmiter RD, Brinster RL: Production of transgenic rabbits, sheep and pigs by microinjection. Nature. 1985, 315: 680-3.
Krimpenfort P, Rademakers A, Eyestone W, van der Schans A, van den Broek S, Kooiman P, Kootwijk E, Platenburg G, Pieper F, Strijker R, et al: Generation of transgenic dairy cattle using 'in vitro' embryo production. Biotechnology (N Y). 1991, 9: 844-7.
Chan AW, Homan EJ, Ballou LU, Burns JC, Bremel RD: Transgenic cattle produced by reverse-transcribed gene transfer in oocytes. Proc Natl Acad Sci U S A. 1998, 95: 14028-33. 10.1073/pnas.95.24.14028.
Rieth A, Pothier F, Sirard MA: Electroporation of bovine spermatozoa to carry DNA containing highly repetitive sequences into oocytes and detection of homologous recombination events. Mol Reprod Dev. 2000, 57: 338-45. 10.1002/1098-2795(200012)57:4<338::AID-MRD5>3.0.CO;2-K.
Shemesh M, Gurevich M, Harel-Markowitz E, Benvenisti L, Shore LS, Stram Y: Gene integration into bovine sperm genome and its expression in transgenic offspring. Mol Reprod Dev. 2000, 56: 306-308. 10.1002/(SICI)1098-2795(200006)56:2+<306::AID-MRD21>3.3.CO;2-V.
Gordon JW, Scangos GA, Plotkin DJ, Barbosa JA, Ruddle FH: Genetic transformation of mouse embryos by microinjection of purified DNA. Proc Natl Acad Sci U S A. 1980, 77: 7380-4.
Wall RJ: Transgenic Livestock: Progress and Prospects for the Future. Theriogenology. 1996, 45: 57-68. 10.1016/0093-691X(95)00355-C.
Clark AJ, Bissinger P, Bullock DW, Damak S, Wallace R, Whitelaw CB, Yull F: Chromosomal position effects and the modulation of transgene expression. Reprod Fertil Dev. 1994, 6: 589-98.
Evans MJ, Kaufman MH: Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981, 292: 154-6.
Capecchi MR: Altering the genome by homologous recombination. Science. 1989, 244: 1288-92.
Iannaccone PM, Taborn GU, Garton RL, Caplice MD, Brenin DR: Pluripotent embryonic stem cells from the rat are capable of producing chimeras. Dev Biol. 1994, 163: 288-92. 10.1006/dbio.1994.1146.
Notarianni E, Galli C, Laurie S, Moor RM, Evans MJ: Derivation of pluripotent, embryonic cell lines from the pig and sheep. J Reprod Fertil Suppl. 1991, 43: 255-60.
Thomson JA, Kalishman J, Golos TG, Durning M, Harris CP, Becker RA, Hearn JP: Isolation of a primate embryonic stem cell line. Proc Natl Acad Sci U S A. 1995, 92: 7844-8.
Cibelli JB, Stice SL, Golueke PJ, Kane JJ, Jerry J, Blackwell C, Ponce de Leon FA, Robl JM: Transgenic bovine chimeric offspring produced from somatic cell-derived stem-like cells. Nat Biotechnol. 1998, 16: 642-6.
Prather RS, Barnes FL, Sims MM, Robl JM, Eyestone WH, First NL: Nuclear transplantation in the bovine embryo: assessment of donor nuclei and recipient oocyte. Biol Reprod. 1987, 37: 859-66.
Cibelli JB, Stice SL, Golueke PJ, Kane JJ, Jerry J, Blackwell C, Ponce de Leon FA, Robl JM: Cloned transgenic calves produced from nonquiescent fetal fibroblasts. Science. 1998, 280: 1256-8. 10.1126/science.280.5367.1256.
Kato Y, Tani T, Sotomaru Y, Kurokawa K, Kato J, Doguchi H, Yasue H, Tsunoda Y: Eight calves cloned from somatic cells of a single adult. Science. 1998, 282: 2095-8. 10.1126/science.282.5396.2095.
Brophy B, Smolenski G, Wheeler T, Wells D, L'Huillier P, Laible G: Cloned transgenic cattle produce milk with higher levels of beta-casein and kappa-casein. Nat Biotechnol. 2003, 21: 157-62. 10.1038/nbt783.
Lai L, Kolber-Simonds D, Park KW, Cheong HT, Greenstein JL, Im GS, Samuel M, Bonk A, Rieke A, Day BN, et al: Production of alpha-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science. 2002, 295: 1089-92. 10.1126/science.1068228.
Dai Y, Vaught TD, Boone J, Chen SH, Phelps CJ, Ball S, Monahan JA, Jobst PM, McCreath KJ, Lamborn AE, et al: Targeted disruption of the alpha1,3-galactosyltransferase gene in cloned pigs. Nat Biotechnol. 2002, 20: 251-5. 10.1038/nbt0302-251.
McCreath KJ, Howcroft J, Campbell KH, Colman A, Schnieke AE, Kind AJ: Production of gene-targeted sheep by nuclear transfer from cultured somatic cells. Nature. 2000, 405: 1066-9. 10.1038/35016604.
Denning C, Dickinson P, Burl S, Wylie D, Fletcher J, Clark AJ: Gene targeting in primary fetal fibroblasts from sheep and pig. Cloning Stem Cells. 2001, 3: 221-31. 10.1089/15362300152725945.
Solter D: Mammalian cloning: advances and limitations. Nat Rev Genet. 2000, 1: 199-207. 10.1038/35042066.
First N, Beyhan Z, Ambroggio J: Cloning of Cattle. In: Principles of Cloning. Edited by: Cibelli J, Lanza R, Campbell K, West M. 2002, San Diego: Academic Press, 375-390.
Willadsen S, Janzen R, McAlister R, Shea B, Hamilton G, McDermand D: The Viability of Late Morulae and Blastocysts Produced by Nuclear Transplantation in Cattle. Theriogenology. 1991, 35: 161-170. 10.1016/0093-691X(91)90155-7.
Stice SL, Strelchenko NS, Keefer CL, Matthews L: Pluripotent bovine embryonic cell lines direct embryonic development following nuclear transfer. Biol Reprod. 1996, 54: 100-10.
Hill JR, Roussel AJ, Cibelli JB, Edwards JF, Hooper NL, Miller MW, Thompson JA, Looney CR, Westhusin ME, Robl JM, et al: Clinical and pathologic features of cloned transgenic calves and fetuses (13 case studies). Theriogenology. 1999, 51: 1451-65. 10.1016/S0093-691X(99)00089-8.
Enright BP, Taneja M, Schreiber D, Riesen J, Tian XC, Fortune JE, Yang X: Reproductive characteristics of cloned heifers derived from adult somatic cells. Biol Reprod. 2002, 66: 291-6.
Heyman Y, Chavatte-Palmer P, LeBourhis D, Camous S, Vignon X, Renard JP: Frequency and occurrence of late-gestation losses from cattle cloned embryos. Biol Reprod. 2002, 66: 6-13.
Kato Y, Tani T, Tsunoda Y: Cloning of calves from various somatic cell types of male and female adult, newborn and fetal cows. J Reprod Fertil. 2000, 120: 231-7. 10.1530/reprod/120.2.231.
Renard JP, Zhou Q, LeBourhis D, Chavatte-Palmer P, Hue I, Heyman Y, Vignon X: Nuclear transfer technologies: between successes and doubts. Theriogenology. 2002, 57: 203-22. 10.1016/S0093-691X(01)00667-7.
Bourc'his D, Le Bourhis D, Patin D, Niveleau A, Comizzoli P, Renard JP, Viegas-Pequignot E: Delayed and incomplete reprogramming of chromosome methylation patterns in bovine cloned embryos. Curr Biol. 2001, 11: 1542-6. 10.1016/S0960-9822(01)00480-8.
Dean W, Santos F, Stojkovic M, Zakhartchenko V, Walter J, Wolf E, Reik W: Conservation of methylation reprogramming in mammalian development: aberrant reprogramming in cloned embryos. Proc Natl Acad Sci U S A. 2001, 98: 13734-8. 10.1073/pnas.241522698.
Santos F, Zackhartchenko V, Stojkovic M, Peters A, Jenuwein T, Wolf E, Riek W, Dean W: Epigenetic Marking Correlates with Developmental Potential in Cloned Bovine Preimplantaion Embryos. Cur Bio. 2003, 13: 1116-1121. 10.1016/S0960-9822(03)00419-6.
Daniels R, Hall V, Trounson AO: Analysis of gene transcription in bovine nuclear transfer embryos reconstructed with granulosa cell nuclei. Biol Reprod. 2000, 63: 1034-40.
Daniels R, Hall VJ, French AJ, Korfiatis NA, Trounson AO: Comparison of gene transcription in cloned bovine embryos produced by different nuclear transfer techniques. Mol Reprod Dev. 2001, 60: 281-8. 10.1002/mrd.1089.
Wrenzycki C, Wells D, Herrmann D, Miller A, Oliver J, Tervit R, Niemann H: Nuclear transfer protocol affects messenger RNA expression patterns in cloned bovine blastocysts. Biol Reprod. 2001, 65: 309-17.
Enright B, Jeong B, Yang X, Tian X: Epigenetic Characteristics of Bovine Donor Cells for Nuclear Transfer: Levels of Histone Acetylation. Biol Reprod. 2003, prepublished online 11 June, doi: 10.1095/bor103.019950.
Tamashiro KL, Wakayama T, Akutsu H, Yamazaki Y, Lachey JL, Wortman MD, Seeley RJ, D'Alessio DA, Woods SC, Yanagimachi R, et al: Cloned mice have an obese phenotype not transmitted to their offspring. Nat Med. 2002, 8: 262-7. 10.1038/nm0302-262.
Alberio R, Campbell KH: Epigenetics and nuclear transfer. Lancet. 2003, 361: 1239-40. 10.1016/S0140-6736(03)13029-2.
Gibbons J, Arat S, Rzucidlo J, Miyoshi K, Waltenburg R, Respess D, Venable A, Stice S: Enhanced survivability of cloned calves derived from roscovitine-treated adult somatic cells. Biol Reprod. 2002, 66: 895-900.
Enright BP, Kubota C, Yang X, Tian XC: Epigenetic Characteristics and Development of Embryos Cloned from Donor Cells Treated by Trichostatin A or 5-aza-2'-deoxycytidine. Biol Reprod. 2003, 14: 14-
Polejaeva IA, Campbell KH: New advances in somatic cell nuclear transfer: application in transgenesis. Theriogenology. 2000, 53: 117-26. 10.1016/S0093-691X(99)00245-9.
Clark AJ, Burl S, Denning C, Dickinson P: Gene targeting in livestock: a preview. Transgenic Res. 2000, 9: 263-75. 10.1023/A:1008974616402.
Clark AJ, Ferrier P, Aslam S, Burl S, Denning C, Wylie D, Ross A, De Sousa P, Wilmut I, Cui W: Proliferative lifespan is conserved after nuclear transfer. Nat Cell Biol. 2003, 5: 535-8. 10.1038/ncb992.
Kubota C, Yamakuchi H, Todoroki J, Mizoshita K, Tabara N, Barber M, Yang X: Six cloned calves produced from adult fibroblast cells after long-term culture. Proc Natl Acad Sci U S A. 2000, 97: 990-5. 10.1073/pnas.97.3.990.
Arat S, Rzucidlo SJ, Gibbons J, Miyoshi K, Stice SL: Production of transgenic bovine embryos by transfer of transfected granulosa cells into enucleated oocytes. Mol Reprod Dev. 2001, 60: 20-6. 10.1002/mrd.1057.
Denning C, Burl S, Ainslie A, Bracken J, Dinnyes A, Fletcher J, King T, Ritchie M, Ritchie WA, Rollo M, et al: Deletion of the alpha(1,3)galactosyl transferase (GGTA1) gene and the prion protein (PrP) gene in sheep. Nat Biotechnol. 2001, 19: 559-62. 10.1038/89313.
Piedrahita JA: Targeted modification of the domestic animal genome. Theriogenology. 2000, 53: 105-16. 10.1016/S0093-691X(99)00244-7.
Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE: Extension of life-span by introduction of telomerase into normal human cells. Science. 1998, 279: 349-52. 10.1126/science.279.5349.349.
Morales CP, Holt SE, Ouellette M, Kaur KJ, Yan Y, Wilson KS, White MA, Wright WE, Shay JW: Absence of cancer-associated changes in human fibroblasts immortalized with telomerase. Nat Genet. 1999, 21: 115-8. 10.1038/5063.
Jiang XR, Jimenez G, Chang E, Frolkis M, Kusler B, Sage M, Beeche M, Bodnar AG, Wahl GM, Tlsty TD, et al: Telomerase expression in human somatic cells does not induce changes associated with a transformed phenotype. Nat Genet. 1999, 21: 111-4. 10.1038/5056.
Rubio MA, Kim SH, Campisi J: Reversible manipulation of telomerase expression and telomere length. Implications for the ionizing radiation response and replicative senescence of human cells. J Biol Chem. 2002, 277: 28609-17. 10.1074/jbc.M203747200.
Schnieke AE, Kind AJ, Ritchie WA, Mycock K, Scott AR, Ritchie M, Wilmut I, Colman A, Campbell KH: Human factor IX transgenic sheep produced by transfer of nuclei from transfected fetal fibroblasts. Science. 1997, 278: 2130-3. 10.1126/science.278.5346.2130.
Arat S, Gibbons J, Rzucidlo SJ, Respess DS, Tumlin M, Stice SL: In vitro development of bovine nuclear transfer embryos from transgenic clonal lines of adult and fetal fibroblast cells of the same genotype. Biol Reprod. 2002, 66: 1768-74.
Bordignon V, Keyston R, Lazaris A, Bilodeau AS, Pontes JH, Arnold D, Fecteau G, Keefer C, Smith LC: Transgene expression of green fluorescent protein and germ line transmission in cloned calves derived from in vitro-transfected somatic cells. Biol Reprod. 2003, 68: 2013-23.
Chen SH, Vaught TD, Monahan JA, Boone J, Emslie E, Jobst PM, Lamborn AE, Schnieke A, Robertson L, Colman A, et al: Efficient production of transgenic cloned calves using preimplantation screening. Biol Reprod. 2002, 67: 1488-92.
Roh S, Shim H, Hwang WS, Yoon JT: In vitro development of green fluorescent protein (GFP) transgenic bovine embryos after nuclear transfer using different cell cycles and passages of fetal fibroblasts. Reprod Fertil Dev. 2000, 12: 1-6. 10.1071/RD00021.
Zakhartchenko V, Mueller S, Alberio R, Schernthaner W, Stojkovic M, Wenigerkind H, Wanke R, Lassnig C, Mueller M, Wolf E, et al: Nuclear transfer in cattle with non-transfected and transfected fetal or cloned transgenic fetal and postnatal fibroblasts. Mol Reprod Dev. 2001, 60: 362-9. 10.1002/mrd.1098.
Miyoshi K, Rzucidlo SJ, Pratt SL, Stice SL: Improvements in cloning efficiencies may be possible by increasing uniformity in recipient oocytes and donor cells. Biol Reprod. 2003, 68: 1079-86.
Wells DN, Laible G, Tucker FC, Miller AL, Oliver JE, Xiang T, Forsyth JT, Berg MC, Cockrem K, L'Huillier PJ, et al: Coordination between donor cell type and cell cycle stage improves nuclear cloning efficiency in cattle. Theriogenology. 2003, 59: 45-59. 10.1016/S0093-691X(02)01273-6.
Band MR, Larson JH, Rebeiz M, Green CA, Heyen DW, Donovan J, Windish R, Steining C, Mahyuddin P, Womack JE, et al: An ordered comparative map of the cattle and human genomes. Genome Res. 2000, 10: 1359-68. 10.1101/gr.145900.
Kappes SM: Utilization of gene mapping information in livestock animals. Theriogenology. 1999, 51: 135-47. 10.1016/S0093-691X(98)00237-4.
Harris A: Towards an ovine model of cystic fibrosis. Hum Mol Genet. 1997, 6: 2191-4. 10.1093/hmg/6.13.2191.
Gatti RA, Boder E, Vinters HV, Sparkes RS, Norman A, Lange K: Ataxia-telangiectasia: an interdisciplinary approach to pathogenesis. Medicine (Baltimore). 1991, 70: 99-117.
Woods CG, Taylor AM: Ataxia telangiectasia in the British Isles: the clinical and laboratory features of 70 affected individuals. Q J Med. 1992, 82: 169-79.
Savitsky K, Sfez S, Tagle DA, Ziv Y, Sartiel A, Collins FS, Shiloh Y, Rotman G: The complete sequence of the coding region of the ATM gene reveals similarity to cell cycle regulators in different species. Hum Mol Genet. 1995, 4: 2025-32.
Savitsky K, Bar-Shira A, Gilad S, Rotman G, Ziv Y, Vanagaite L, Tagle DA, Smith S, Uziel T, Sfez S, et al: A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science. 1995, 268: 1749-53.
Barlow C, Hirotsune S, Paylor R, Liyanage M, Eckhaus M, Collins F, Shiloh Y, Crawley JN, Ried T, Tagle D, et al: Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell. 1996, 86: 159-71.
Elson A, Wang Y, Daugherty CJ, Morton CC, Zhou F, Campos-Torres J, Leder P: Pleiotropic defects in ataxia-telangiectasia protein-deficient mice. Proc Natl Acad Sci U S A. 1996, 93: 13084-9. 10.1073/pnas.93.23.13084.
Xu Y, Ashley T, Brainerd EE, Bronson RT, Meyn MS, Baltimore D: Targeted disruption of ATM leads to growth retardation, chromosomal fragmentation during meiosis, immune defects, and thymic lymphoma. Genes Dev. 1996, 10: 2411-22.
Kuljis RO, Xu Y, Aguila MC, Baltimore D: Degeneration of neurons, synapses, and neuropil and glial activation in a murine Atm knockout model of ataxia-telangiectasia. Proc Natl Acad Sci U S A. 1997, 94: 12688-93. 10.1073/pnas.94.23.12688.
Kambadur R, Sharma M, Smith TP, Bass JJ: Mutations in myostatin (GDF8) in double-muscled Belgian Blue and Piedmontese cattle. Genome Res. 1997, 7: 910-6.
McWhir J: Biomedical and agricultural applications of animal transgenesis. Methods Mol Biol. 2002, 180: 3-23. 10.1385/1-59259-178-7:003.
Bueler H, Aguzzi A, Sailer A, Greiner RA, Autenried P, Aguet M, Weissmann C: Mice devoid of PrP are resistant to scrapie. Cell. 1993, 73: 1339-47.
Robl JM, Kasinathan P, Sullivan E, Kuroiwa Y, Tomizuka K, Ishida I: Artificial chromosome vectors and expression of complex proteins in transgenic animals. Theriogenology. 2003, 59: 107-13. 10.1016/S0093-691X(02)01262-1.
Hubner K, Fuhrmann G, Christenson LK, Kehler J, Reinbold R, De La Fuente R, Wood J, Strauss JF, Boiani M, Scholer HR: Derivation of oocytes from mouse embryonic stem cells. Science. 2003, 300: 1251-6. 10.1126/science.1083452.
Acknowledgments
C.A. Hodges is supported by a grant from the AT Children's Project
Author information
Authors and Affiliations
Corresponding author
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Hodges, C.A., Stice, S.L. Generation of bovine transgenics using somatic cell nuclear transfer. Reprod Biol Endocrinol 1, 81 (2003). https://doi.org/10.1186/1477-7827-1-81
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1477-7827-1-81